Abstract
As the “artificial pancreas” becomes closer to reality, automated insulin delivery based on real-time glucose measurements becomes feasible for people with diabetes. This paper is concerned with the development of novel feedforward-feedback control strategies for real-time glucose control and type 1 diabetes. Improved post-meal responses can be achieved by a pre-prandial snack or bolus, or by reducing the glucose setpoint prior to the meal. Several feedforward-feedback control strategies provide attractive alternatives to the standard meal insulin bolus and are evaluated in simulations using a physiological model.
Keywords: diabetes, control, glucose
1 Introduction
Diabetes is a metabolic disease characterized by elevated concentrations of blood glucose for prolonged periods of time, i.e., hyperglycemia. Glucose is the main sugar in the blood and is the body's main source of energy. For healthy individuals, glucose is regulated within narrow limits by insulin secretions from the pancreas. Diabetes is the result of defects in insulin production, insulin action, or both [1]. Chronic, untreated hyperglycemia can lead to serious complications that include cardiovascular diseases, blindness, kidney failure, and stroke. Furthermore, very low values of blood glucose (hypoglycemia) for even a short duration can result in loss of consciousness and coma. Thus, the maintenance of normal glucose concentrations (euglycemia) is of critical importance for both diabetic and non-diabetic individuals [2].
Approximately 21 million people in the United States have diabetes, according to a 2005 U.S. Government estimate [1]. In 2002, diabetes was the sixth leading cause of death and had an estimated total cost of $132 billion [3]. There are two basic types of diabetes mellitus, type 1 and type 2. Type 1 diabetes mellitus is characterized by failure of the pancreas to secrete insulin secondary to the auto-immune destruction of the islet-cells. It was formerly referred to as juvenile onset diabetes but can occur at any age. Type 1 diabetes accounts for approximately 5-10% of all cases while type 2 diabetes accounts for 90-95% [1]. In order to survive, persons with type 1 diabetes require exogenous insulin administration delivered by injection or by continuous subcutaneous insulin infusion (CSII) via a pump. An extensive, long term study [4] has demonstrated that intensive diabetes therapy aimed at achieving near normoglycemia reduces the complications of type 1 diabetes. It also significantly reduces the long-term incidence of cardiovascular disease [5]. Intensive therapy for diabetes was also associated with an increase in the frequency of severe hypoglycemia.
Type 2 diabetes is a disease characterized by a dual defect: 1) by insulin resistance which prevents cells from using insulin properly, and 2) degrees of reduced pancreatic insulin secretion. Type 2 diabetes is associated with certain ethnic groups, obesity, family history of diabetes, and physical inactivity, among other factors.
Whether the treatment modality for type 1 diabetes is multiple daily insulin injections or continuous subcutaneous insulin infusion (CSII) delivered via a pump, both modalities necessitate blood glucose (fingerstick) measurements throughout the day in order to determine insulin doses for meal times; in addition, one to three injections or programmed continuous infusion of short–acting or rapid–acting insulin via CSII are required to maintain euglycemia between meals. For type 1 diabetes, euglycemia has been difficult to achieve due to the infrequent blood glucose measurements, patient variability, and changes in insulin sensitivity due to exercise or stress (psychological, physical or hormonal). Consequently, there has been considerable interest in developing an artificial pancreas, a portable (or implantable) automated insulin delivery system that consists of three components: a glucose sensor which provides frequent measurements (e.g., every five minutes), an insulin infusion pump, and a control algorithm which calculates the appropriate insulin dosage for the current conditions.
During the past 30 years, a variety of glucose control strategies based on continuous and intermittent glucose sensing has been reported. Since this literature has been reviewed in recent survey articles [6–12], we will not present a detailed review here. Most of the relevant literature has been concerned with variations of proportional–integral–derivative (PID) control algorithms that are used in conjunction with an insulin injection (or bolus) at meal time. The magnitude of the insulin bolus is typically based on the individual's estimate of the carbohydrate content (CHO) of the meal. A PID control strategy is attractive for glucose control because it mimics the first and second phase responses that the pancreas beta cells use to secrete insulin in response for non-diabetic individuals to the continuously sensed glucose [9]. In recent years, model–based control strategies such as model predictive control (MPC), adaptive control [6,13–18], optimal control [19], neural networks [20,21], and H-infinity control [22,23], have been proposed and evaluated in simulation studies. A few experimental applications of MPC have also been reported [16,17,24]. Glucose control strategies can be updated on an infrequent basis (e.g., daily) using a “run–to–run” control approach [25,26]. A grading system for glucose control has also been proposed [27].
This paper is concerned with the development of several novel feedforward-feedback glucose control strategies for type 1 diabetes. Controller performance is evaluated in a simulation study for a physiological model and realistic conditions that include inaccurate estimates of the carbohydrate content of the meal, changes in insulin sensitivity, and different meal sizes. Measurement noise and delay, and sensor dynamics can also be accommodated, as demonstrated in related studies [28,29].
2 Physiological model
Many physiological models have been proposed that describe glucose and/or insulin dynamics [24,30–34]. In this paper, the simulation studies are based on the model developed by Hovorka et al. [24] and the modifications reported by Wilinska et al. [33]. This model will be referred to as the “Hovorka model”. It represents the relationship between input variables, subcutaneous insulin infusion rates (basal and bolus), and the output variable, intravenous glucose concentration. This model also includes a submodel for meal ingestion. The Hovorka model is a nonlinear compartmental model with three subsystems for glucose, insulin, and insulin action. The model consists of three compartments: plasma glucose, subcutaneous and plasma insulin, and insulin action. Both insulin and glucose compartments are divided into two subsystems. The insulin action describes the physiological effect of insulin on glucose transport, removal and endogenous production. The values of the model parameters were determined from experimental data for both normal and diabetic subjects [35]. Model constants were defined to be those quantities difficult to identify, while model parameters were a priori identifiable.
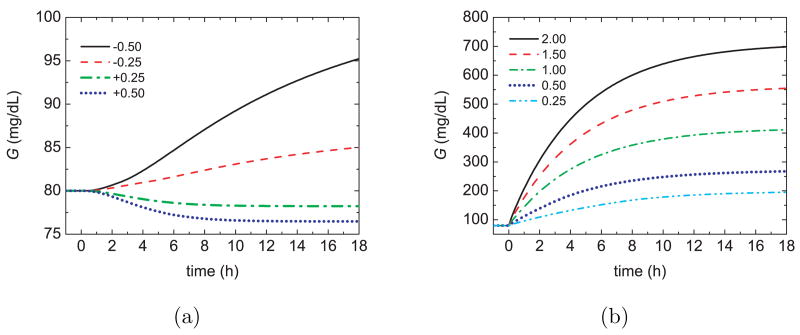
The nonlinear behavior of the dynamic model to changes in basal insulin and gut glucose absorption is indicated by the responses for a 75 kg person in Figure 1. Although this model has been effectively used in several studies [6], [24], it can result in non-physiological responses for some conditions. For example, large (but reasonable) basal infusion rates can produce an extended postprandial phase and meaningless negative G values [36].
Fig. 1.
Open–loop glucose responses to step changes in (a) the basal insulin (mU/min) and (b) the gut glucose absorption rate (mmol/min).
3 A PID switching control strategy
A variety of PID control strategies have been developed for diabetes and described in survey papers and articles [7–9,11,37–39]. Most of the related evaluations have been based on simulation studies but experimental applications to dogs and humans have also been reported. Several authors have considered PD controllers with heavy weighting on the derivative action [40,41]. Integral action is often omitted in order to reduce potential over-dosing and hypoglycemia. However, integral action is also desirable to accommodate unanticipated patient changes (e.g., insulin sensitivity). PID controllers have also been used in conjunction with “sliding scales” that partition blood glucose levels into several ranges [42]. The PID controller calculates the insulin infusion rate that is released by the pump into subcutaneous tissues.
In a recent study [28,29] the authors proposed and evaluated a novel PID plus meal bolus control strategy for type 1 diabetes. The key features are:
A switching strategy for determining when to initiate PID control action after a meal and insulin bolus;
A novel time-varying trajectory for the glucose setpoint;
A limit on the integral control action that greatly reduces the possibility of insulin over-dosing and hypoglycemia.
The new control strategy was compared to four alternatives in a simulation study and was shown to be superior for both insulin sensitivity changes and meal challenges with poor CHO estimates [28,29]. In the present paper, novel feedforward control strategies are considered for use with feedback control strategies. Thus, it is appropriate to briefly summarize the new PID control strategy here. Additional details are available elsewhere [28].
The PID control algorithm is expressed in velocity form:
| (1) |
where Δu(k) = u(k) − u(k − 1), k [min] is the sampling instant, and the derivative term is:
| (2) |
Constants Kc, τD and τI are the controller parameters, and Δt = 5 min is the sampling period. The controller error is defined as:
| (3) |
where Gsp is the desired glucose setpoint.
Next, we indicate why a switching strategy for the PID control algorithm is desirable. Improved glucose control for combined meal challenges and poor CHO estimates can be achieved by starting the PID controller after the meal and bolus occur. However, the specification of the switching time is important. If the PID controller is started too early, hypoglycemia (G < 60 mg/dL) can occur. On the other hand, if the PID controller is switched on too late, the postprandial glucose peak may be very large and slowly decrease to the setpoint value. In this study, the desired glucose range is considered to be 60 to 150 mg/dL with a target value of 80 mg/dL. Simulation studies for the Hovorka model in Section 2 have indicated that an effective switching strategy is obtained if the PID controller is started when one of two criteria is satisfied [28]:
G reaches its peak value;
G > 150 mg/dL and dG/dt > 1.5 mg/dL min.
The 150 mg/dL threshold was selected because it is the peak value of G for a 60 g CHO meal and a correct bolus. The rate-of-change limit of 1.5 mg/dL min was chosen to be greater than the maximum rate of change for this same situation. The threshold values in criterion (ii) are patient and meal size dependent.
Next, we consider the rationale for using a time-varying setpoint. During the postprandial period, the blood glucose concentration is expected to increase, and then decrease. Consequently, it is appropriate to have a time-varying setpoint, Gsp, that reflects this expected behavior [9,42]. The following strategy has been devised. When the PID controller is initiated, Gsp should be set equal to the current measurement, G, and then eventually decrease to the desired value of 80 mg/dL. However, for the case of a CHO under-estimate, G is still increasing when the PID controller is switched on. Thus, it would be inappropriate to force Gsp to decrease right away. Extensive simulations have demonstrated that an appropriate setpoint trajectory is:
| (4) |
where k* = k − ksw, ksw [min] is the switching instant, and τsp [min] is a time constant that serves as a design parameter. For k* < 0, Gsp = G. In order to avoid unexpected hypoglycemia, a lower limit of 80 mg/dL was specified for Gsp. The time-varying setpoint trajectory defined in (4) has the following properties:
It is affected by the actual value of G for every k*;
It varies from G(k* = 0) to 80 mg/dL;
As τsp → 0, Gsp(k*) → 80 mg/dL;
As τsp → ∞, Gsp(k*) → G(k*).
4 Pre-prandial insulin adjustments
The conventional approach for regulating postprandial glucose profiles is for the subject to introduce an insulin bolus at meal time via injection or insulin infusion pump. The magnitude of the insulin bolus is based on the estimated CHO meal content and a specified insulin-to-CHO (I/C) ratio. On the other hand, studies [43–47] have demonstrated that postprandial glucose profiles can be improved by pre-prandial administration of a portion of the meal insulin bolus, i.e., a pre-bolus. In this section, we consider alternative strategies for administration of a pre-bolus in conjunction with feedback control.
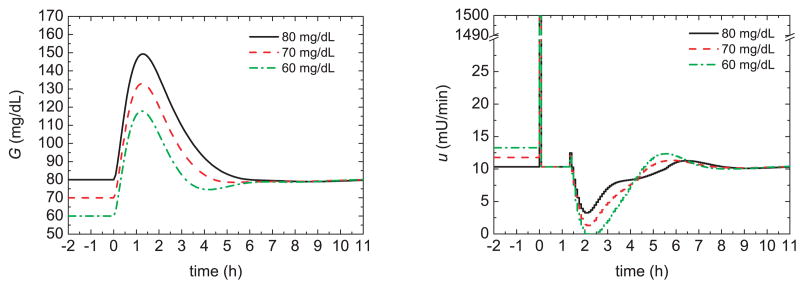
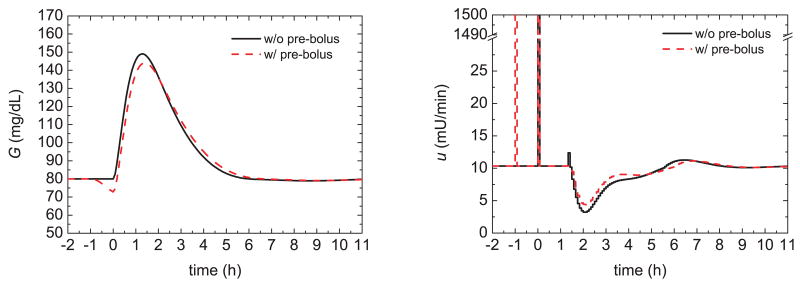
As motivation for the proposed pre-prandial strategies, we first consider some simulation results for the Hovorka model and the conditions in the previous section. The glucose response of a 75 kg patient was simulated for a nominal meal with 60 g of CHO and an insulin bolus of 3.16 U. Figure 2 shows the glucose responses (G) and insulin profiles (u) for the feedback control strategy of the previous section and initial glucose steady-state values of 80, 70, and 60 mg/dL, respectively. For the same meal uptake, low initial glucose values result in lower peak values, as would be expected. The peak magnitude, defined to be the difference between the maximum and initial glucose values, was also somewhat reduced for low initial values. Figure 3 demonstrates that administering 20% of the insulin bolus 60 minutes prior to the meal, i.e., a pre-bolus, slightly reduces the peak magnitude. Although the strategies depicted in Figs. 2 and 3 improve the postprandial glucose profiles, very low initial glucose levels are not desirable because they can lead to postprandial hypoglycemia for unforeseen conditions.
Fig. 2.
Glucose responses and insulin profiles for different initial steady states.
Fig. 3.
Glucose responses and insulin profiles with and without a 20% pre-bolus 60 min prior to the meal.
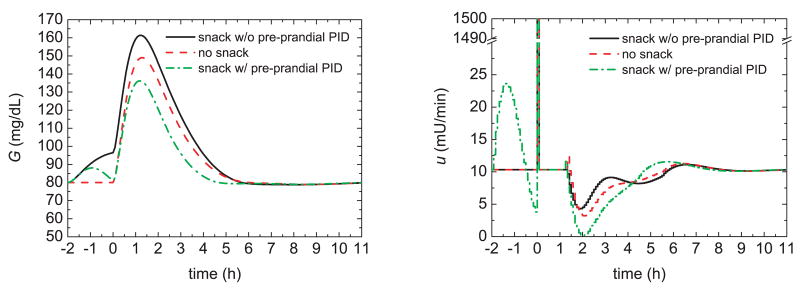
The effect of a pre-prandial snack, with and without feedback control, is illustrated in Figure 4 where a 5 g CHO meal snack occurs at time t = −2 h. After the snack, the glucose concentration increases and the feedback controller takes corrective action by releasing more insulin. At t = 0, the regular 60 g meal and the corresponding insulin bolus occur, and the PID controller is temporarily shut off. The controller is started again at t = 1.5 h when the second switching criterion of the previous section is satisfied. It is somewhat surprising that the pre-prandial snack and PID control reduce the peak magnitude by 20% compared to the no snack situation. This improvement is a result of the induced insulin that is delivered by the PID controller. These simulation results suggest that improved postprandial glucose profiles can be achieved if the subject has a small snack before the main meal, and feedback control is utilized. Because it is not always convenient or feasible for the subject to take a snack prior to a meal, alternative approaches will be considered next.
Fig. 4.
Glucose responses and insulin profiles for a pre-prandial glucose increase induced by a snack at t = −2 h.
The preliminary results in Figs. 2–4 demonstrate that improved postprandial glucose profiles can be obtained by reducing the pre-prandial glucose level or by pre-prandial insulin delivery (e.g., the result of a small snack and feedback control). Based on these insights, two methods are proposed for effective pre-prandial insulin delivery.
Method (i) A pre-prandial glucose setpoint change is made during PID control;
Method (ii) Both a pre-prandial setpoint change and an insulin pre-bolus are introduced.
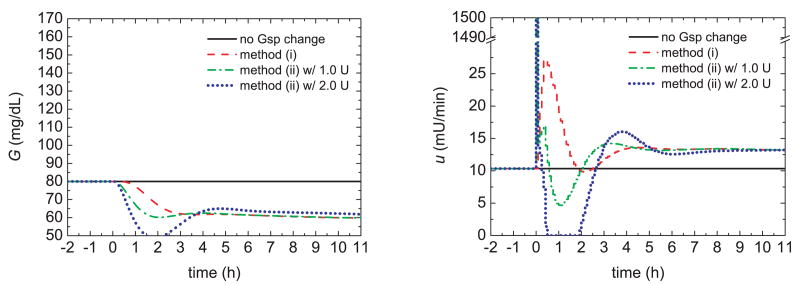
The rationale for these proposed two methods is as follows. A gradual pre-prandial setpoint change and conservative controller settings reduce the glucose level by increasing the insulin infusion, while minimizing the possibility of hypoglycemia. For example, 60 minutes prior to a meal the glucose setpoint could be ramped down from the nominal value of 80 mg/dL to a pre-prandial value of 60 mg/dL over a period of 20 minutes. If a pre-bolus were introduced, the meal insulin bolus would be reduced accordingly so that the total insulin (pre-bolus plus meal bolus) is still based on the estimated meal CHO content. In order to determine a reasonable magnitude for the pre-bolus, different values were evaluated for use with the ramp setpoint change. The results in Fig. 5 represent a worst case scenario where a pre-bolus is introduced but no meal is actually consumed. A pre-bolus of 2 U produces a hypoglycemic condition, while a value of 1 U can be safely used.
Fig. 5.
Glucose responses and insulin profiles for pre-prandial insulin delivery methods without meal uptake.
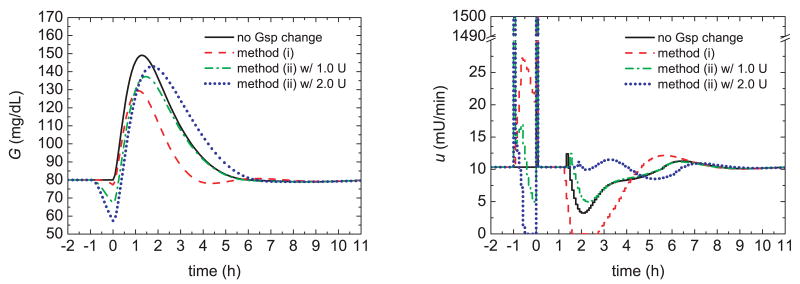
The meal challenge results for Methods (i) and (ii) are shown in Fig. 6. The best results are achieved when the setpoint change is introduced without a pre-bolus (i.e., Method (i)). It results in the largest glucose peak reduction, the smallest area under the postprandial glucose curve, and the shortest settling time. These improvements are due to the larger pre-prandial insulin delivery provided by Method (i) at meal time, as indicated in Fig. 7. In summary, Method (i) improves the postprandial glucose response by reducing the pre-prandial glucose setpoint in a safe, controlled manner. A pre-prandial bolus of insulin is not required. Even if the anticipated meal is postponed or not consumed, the subject's glucose concentration is still maintained at a safe level.
Fig. 6.
Glucose responses and insulin profiles for pre-prandial insulin delivery methods and meal uptake. For method (ii), an insulin pre-bolus is introduced at time t = −1.
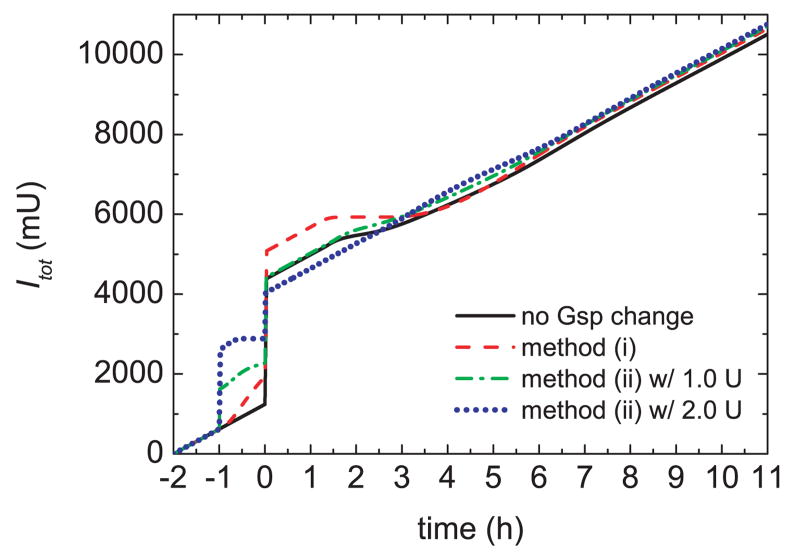
Fig. 7.
Total insulin Itot released for a meal uptake and pre-prandial insulin delivery.
5 Gain scheduling of the insulin–to–CHO ratio (I/C)
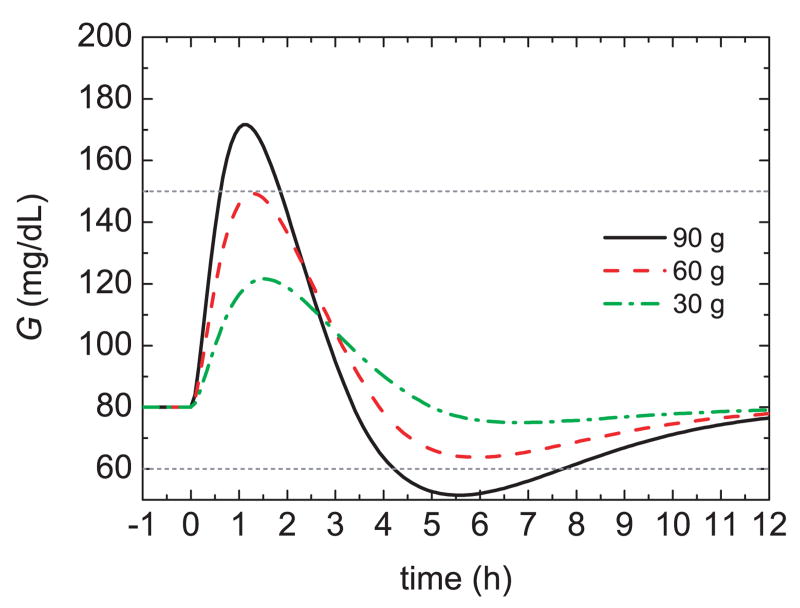
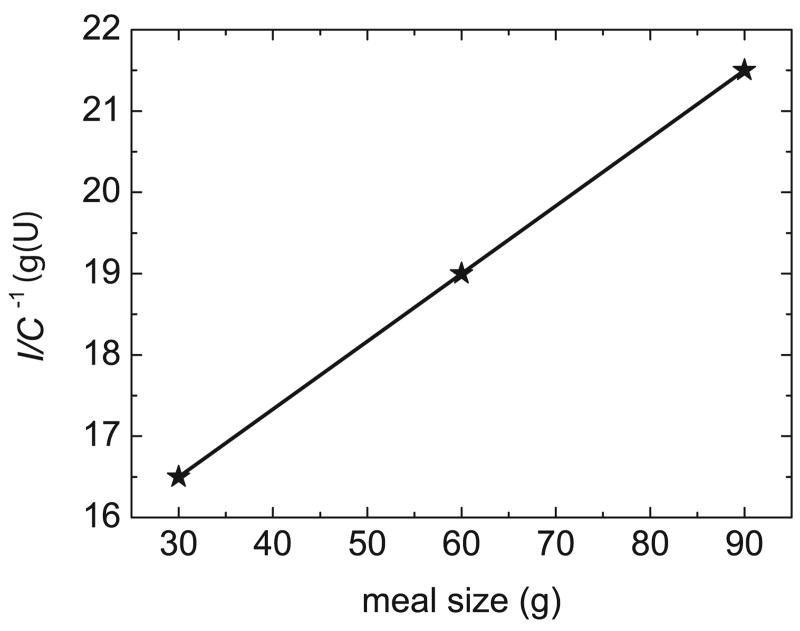
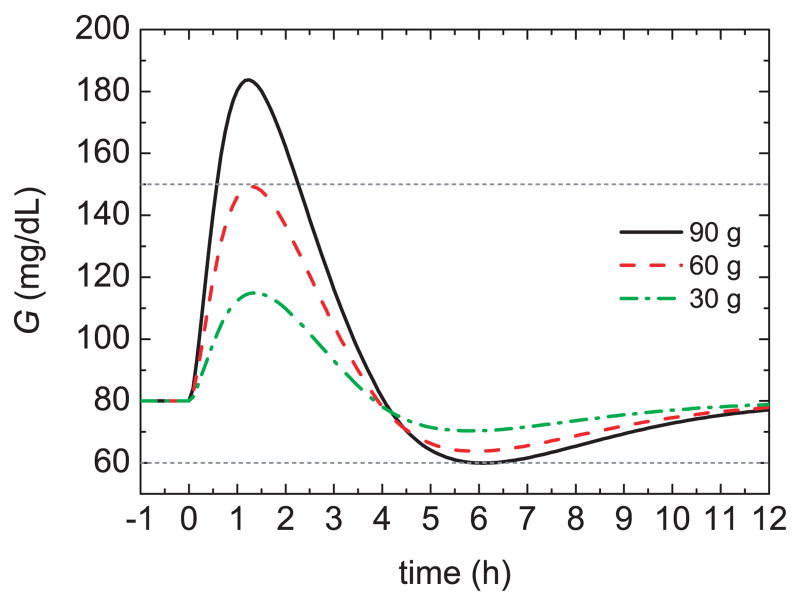
As indicated in Section 3, the insulin bolus for a meal is typically calculated by multiplying the estimated CHO content of the meal, by a specified insulin-to-CHO ratio (I/C). For example, in order to obtain an acceptable glucose profile (60 mg/dL < G(t) < 150 mg/dL) for the Hovorka model, a 75 kg subject, and a standard 60 g CHO meal, an I/C of 1 U/19 g is satisfactory. But for different meal sizes, this I/C value can result in poor responses, as shown in Figure 8. For the 90 g CHO meal, the bolus is too large, causing hypoglycemia, while for the 30 g CHO meal the bolus is too small, yielding a sluggish response. These results suggests that the I/C ratio should be modified for different meal sizes, in effect, a gain scheduling approach [48].
Fig. 8.
Glucose responses for three meal sizes and a standard bolus.
In order to develop an appropriate gain scheduling algorithm, an appropriate I/C value was determined by trial and error for each of the three meal sizes. Figure 9 shows that these I/C values are inversely related to meal size for the simulated patient. The regression equation for Figure 9 is given by,
Fig. 9.
Gain scheduling of the I/C ratio for different meal sizes.
| (5) |
where I/C has units of U/g and X is the CHO content of the meal in grams. Figure 10 shows the glucose responses for different meal sizes and insulin boluses based on (5). A comparison of Figures 8 and 10 demonstrates that the gain-scheduled I/C approach improves the postprandial glucose responses.
Fig. 10.
Glucose responses for three meal sizes with gain scheduling of the I/C ratio.
6 Feedforward controller design
In Sections 4 and 5, a simple type of feedforward control for disturbance compensation, ratio control, was used to determine the meal bolus. However, the ratio control strategy has two disadvantages. First, because the glucose dynamics are different for meal uptake and insulin boluses, a “dynamic mismatch” occurs for the two inputs. Second, if the insulin bolus is too large (e.g., due to an over-estimated CHO meal content), it is difficult to avoid hypoglycemia using only feedback control. In this section, a model-based feedforward controller is developed that avoids these disadvantages, to a large extent. The feedforward controller consists of a lead-lag model, a common feedforward control form for industrial process control [48].
For process control applications, feedforward controller design is typically based on two types of information [48]: (i) the effect of the manipulated input, u, on the controlled variable, y, and (ii) the effect of the measured disturbance variable d on y. In this paper, y is the glucose concentration, u is the insulin infusion rate, and d is the gut absorption rate of the CHO content of the meal. The latter was determined from the gut absorption sub-model by Hovorka et al. [24].
Figure 1 shows the glucose responses for step changes in the manipulated variable and the disturbance variable. The disturbance response suggests that an appropriate disturbance model is a first-order transfer function:
| (6) |
where Y and D are the Laplace transforms of y and d, which are expressed as deviation variables. The average values of the transfer function parameters for the four step responses are Kd = 375 mg min/mmol dL and τd = 313 min.
Because the step responses in Fig. 1a exhibit both nonlinear dynamic and steady-state behavior, specifying a form for the process transfer function, Gp(s), is somewhat problematic. In view of the overdamped nature of these step responses, a second-order transfer function with a time delay was employed:
| (7) |
The average values of the model parameters for the four step responses in Fig. 1a are: K = −24 mg min/mU dL, τ1 = 366 min, τ2 = 151 min, and θ = 25 min.
The feedforward control law can be expressed as:
| (8) |
where Gf (s) is the transfer function for the feedforward controller. The ideal design equation for feedforward control has the form [48]:
| (9) |
Although this controller is physically unrealizable, it can be approximated by a lead-lag unit, which is physically realizable:
| (10) |
where Kf, τ3, and τ4 are the tuning parameters. As a first approach, the values of the tuning parameters can be specified as Kf = Kd/K, τ3 = τ1 + τ2 + θ, and τ4 = τd. Alternatively, other tuning techniques can be employed [48].
The tuning strategy used in this research was based on minimization of the discrete version of the integral absolute error for a meal challenge:
| (11) |
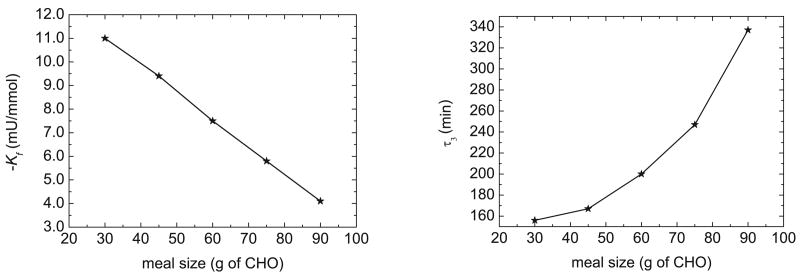
where IAE (mg min/dL) is the integral absolute error, t∞ is the duration of the simulation, e = Gsp− G where Gsp is the setpoint (mg/dL), and Δt is the sampling period (5 min). Based on extensive simulations for different meal sizes, the optimal value of τ4 appeared to be quite constant, and a value of τ4 = 0.3 min was satisfactory in all cases. Thus, τ4 was specified to be 0.3 min for all simulations. The optimal values for Kf and τ3 for different meal sizes are shown in Figure 11.
Fig. 11.
Feedforward controller parameters for different meal sizes.
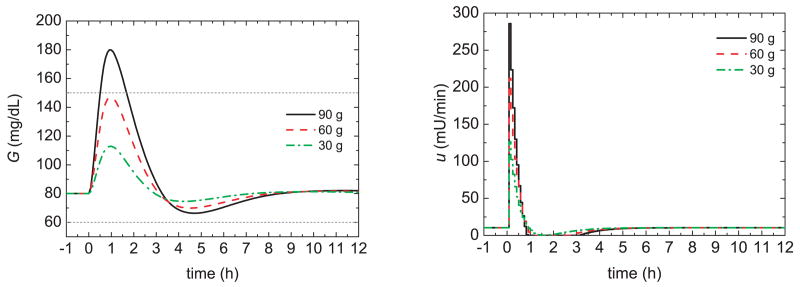
Figure 12 shows the glucose responses for different meal sizes and feedforward control only (i.e., without an insulin bolus or feedback control). For each meal size, the optimal feedforward controller settings in Figure 11 were employed. A comparison of Figures 8, 10, and 12 demonstrates that a feedforward controller can be a valid alternative to a standard meal bolus and to a gain-scheduled bolus, because the feedforward controller results in lower peak values, shorter settling times, and smaller values of the area under the curve.
Fig. 12.
Glucose responses and insulin profiles for model–based feedforward control.
Another advantage of the model-based feedforward controller is that the meal bolus is released more gradually (e.g., over a period of one hour) while the traditional meal bolus over is injected very quickly. This gradual insulin release allows the feedback controller to compensate for a poor CHO estimate before the meal bolus has been completely released. An appropriate feedforward-feedback control strategy will be developed in the next section.
7 Feedforward-feedback control
The main features of the proposed feedforward-feedback control strategy are:
The model-based feedforward controller of Section 6;
A PID feedback controller;
The option of generating the setpoint trajectory for the feedback controller using a model-reference approach.
The rationale for the model-reference approach will now be discussed.
In the previous sections, the feedback control employed the time-varying setpoint trajectory in (4). Next, we consider an alternative approach for generating the time-varying setpoint, namely, model reference control. This model reference approach is well-known technique in the field of adaptive control [49]; the basic idea is as follows. If a reasonably accurate model of a diabetes subject is available, then the expected postprandial response for a specified meal size can be easily calculated. The model response can then be used as the time-varying setpoint for the PID controller. The feedback controller merely compensates for departures from the expected postprandial response due to modeling errors, poor CHO estimates, instrumentation problems, etc. In this paper, we consider an open-loop model response for a meal and a bolus as the glucose setpoint; alternatively, a desired closed-loop response could be used.
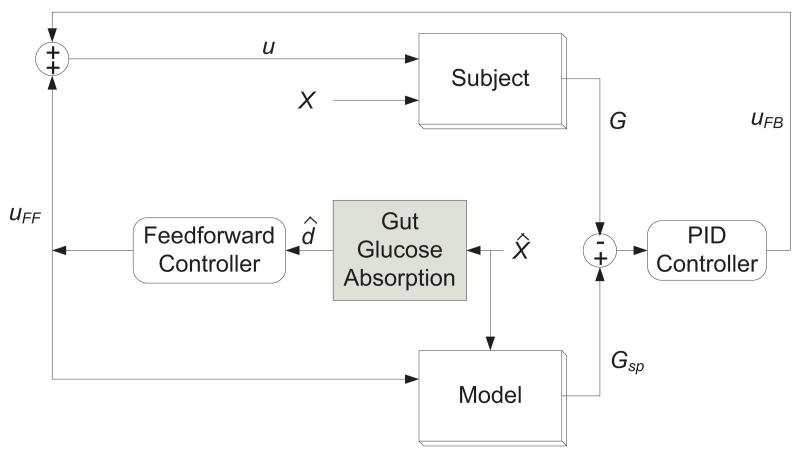
A block diagram of the proposed feedforward-feedback control strategy with the model-reference option is shown in Figure 13. The outputs of the feedback controller uFB and the feedforward controller uFF are added together to generate the insulin infusion rate, u. The feedforward controller calculates uFF based on the estimated gut absorption rate d̂, which is calculated from the estimated CHO content X̂ and the gut absorption model by Hovorka et al. [24]. The model block generates the setpoint Gsp for the PID controller based on uFF and X̂. In this paper, both the “Model” and the “Subject” blocks employ the Hovorka model of Section 2. However, for incorrect meal estimates and insulin sensitivity changes, the models for the two blocks are not identical, as explained below.
Fig. 13.
Block diagram of the feedforward–feedback control strategy.
The controller tuning procedure of Marchetti et al. [28] is also used in this paper, in order to facilitate controller comparison. Limits were placed on the integral term in the control law. Initially, the reset time was specified to be τI = 100 min and the controller gain Kc and derivative time τD were determined by minimizing the IAE index for postprandial responses with insulin boluses for three cases: the correct bolus or boluses that were 50% too large or too small.
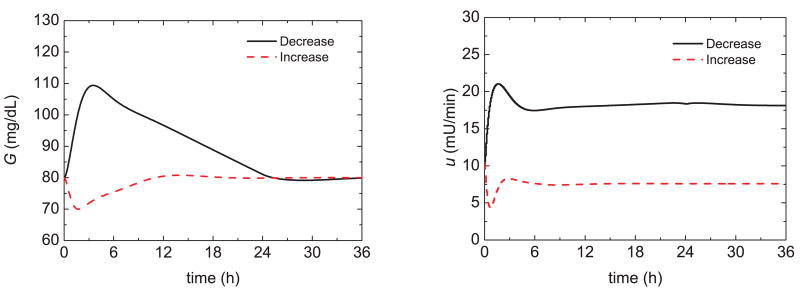
Figure 14 shows the postprandial responses of the novel model-reference feedback control strategy (plus a meal bolus) and three meal challenges. Figure 15 shows glucose responses for the same meal challenges and the switching control strategy of Marchetti et al. [28] described in Section 3. The model-reference controller provides superior postprandial responses. Next, the reset time was optimized for insulin sensitivity changes, as suggested by Marchetti et al. [28]. Figure 16 shows that the proposed model-reference control strategy is able to accommodate changes in insulin sensitivities during fasting (i.e., non-meal conditions). Changes in insulin sensitivities means that all the three insulin sensitivities of the Hovorka model are increased/reduced by the specified amount.
Fig. 14.
Glucose responses and insulin profiles for three meal challenges and feedforward–feedback control strategy.
Fig. 15.
Glucose responses and insulin profiles for three meal challenges and the switching control strategy with time–varying setpoint trajectory described by Marchetti et al. [28].
Fig. 16.
Glucose responses and insulin profiles for insulin sensitivity 50% decrease/increase and the feedforward–feedback control strategy.
8 Meal challenges and insulin sensitivity changes
In Section 7 the proposed feedforward-feedback control strategy performed well for meal challenges, and for basal (non-meal) conditions after an insulin sensitivity change occurred. In this section, we consider controller performance for insulin sensitivity changes, followed by meal challenges. This is a difficult and important diabetes control problem, because insulin sensitivity changes can be unknown and persist for long time periods (e.g., hours to days). In the simulation results of this section, postprandial responses are considered after insulin sensitivity changes of +/- 50% of the nominal value. The basal insulin infusion rates were adjusted so that at t = 0, the glucose concentration was at a steady-state value of G = 80 mg/dL.
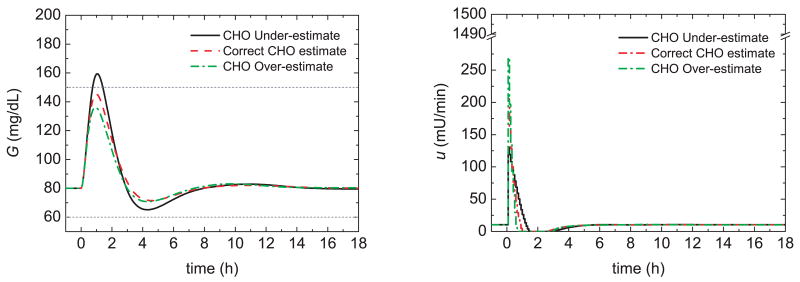
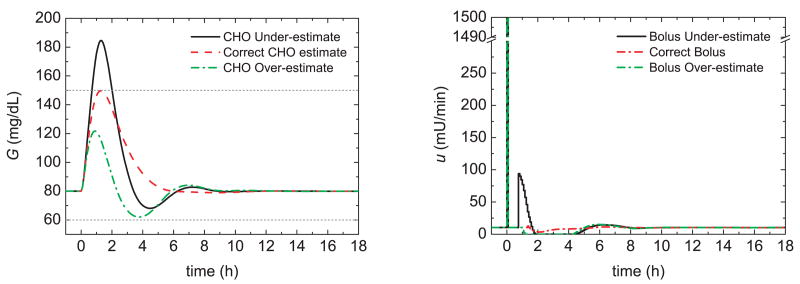
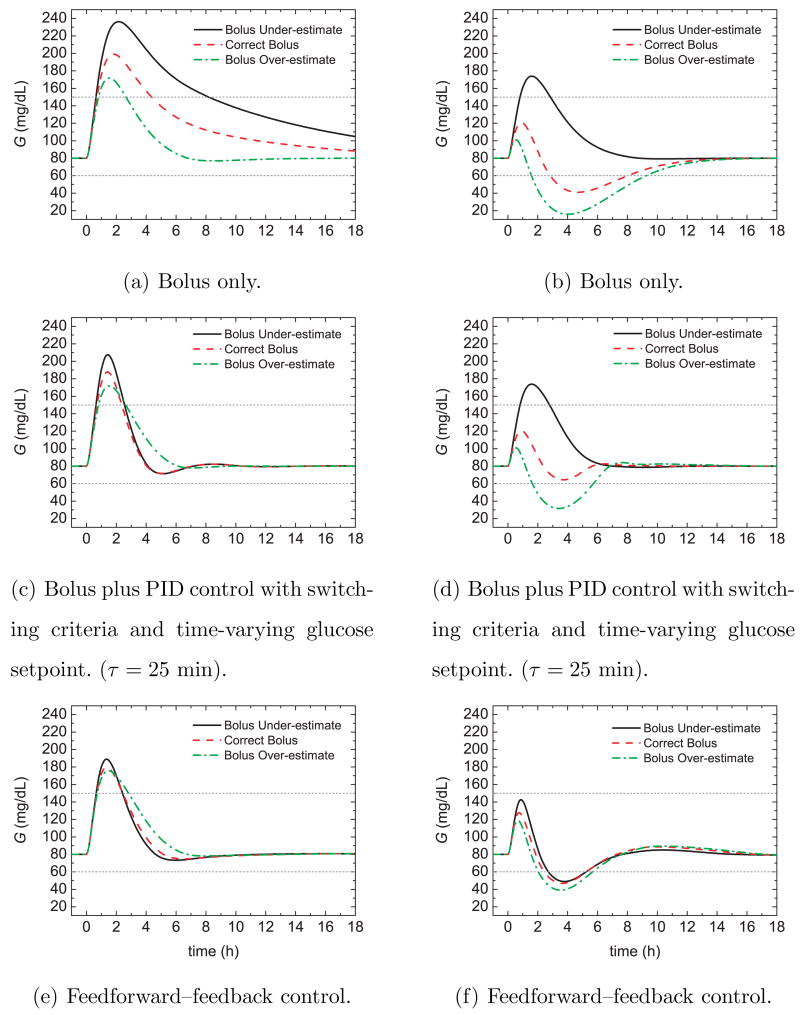
Figure 17 compares the postprandial glucose responses for 50% insulin sensitivity changes and three control strategies: bolus only, the PID switching control strategy of Section 3, and the feedforward-feedback control strategy of Section 7. The controller settings developed in Sections 3 and 7 were employed. Thus, the controller setting were based on the nominal insulin sensitivity while the meal bolus was calculated from the nominal meal size and a CHO ratio of 1 U:19 g CHO.
Fig. 17.
Postprandial glucose profiles for meal challenges and a 50% insulin sensitivity (left) decrease and (right) increase.
Figure 17a,b demonstrates that for bolus-only control, the glucose responses are quite sluggish for the decrease in insulin sensitivity and too aggressive for the increase. In particular, the 50% increase in insulin sensitivity results in hypoglycemia when the bolus is either correct or over-estimated. The PID switching control strategy in Fig. 17c,d provides a significant improvement over bolus-only control but hypoglycemia occurs for the bolus over-estimate in Fig. 17d. The feedforward-feedback control strategy in Fig. 17e,f provides consistent responses with small glucose peaks. However, hypoglycemia occurs for the insulin sensitivity increase. The results in Fig. 17 suggest that the three controllers should be tuned to accommodate insulin sensitivity increases.
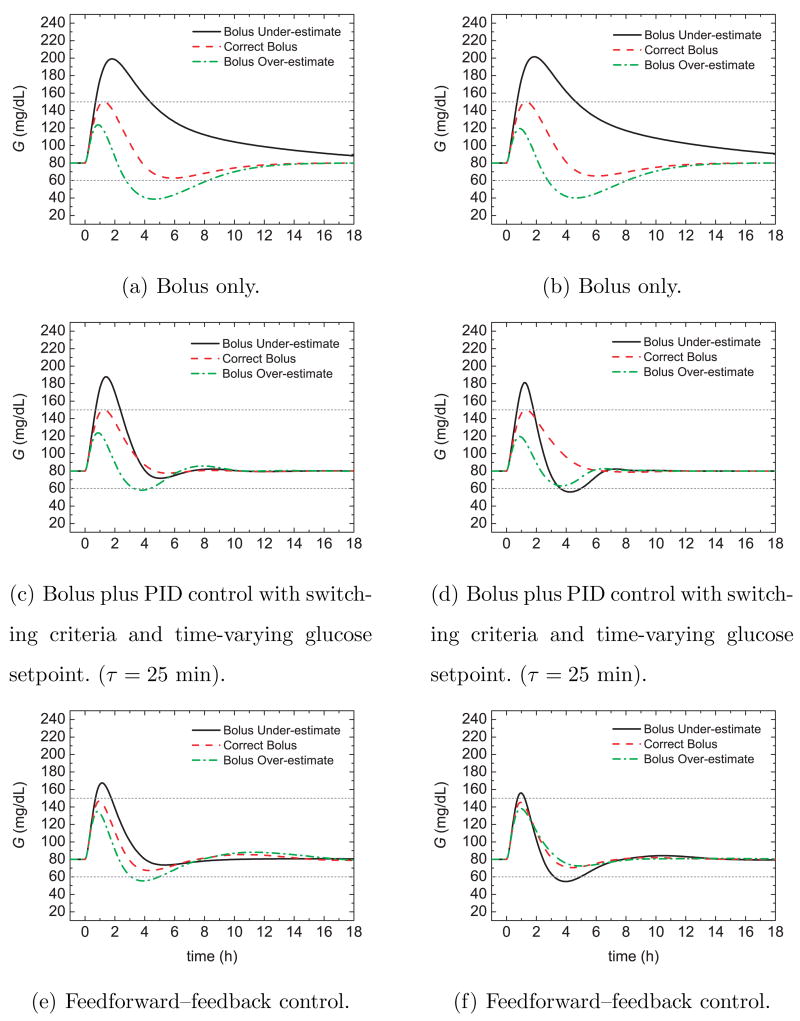
Figure 18 shows the glucose responses for the conditions of Figure 17 and the ideal situation where the insulin-to-CHO ratio (I/C) and the feedforward controller gain Kf were tuned by trial and error for each insulin sensitivity change and the correct CHO meal estimate. The feedforward controller gain was multiplied by a constant, c, as discussed below. The other controller settings were not changed. A comparison of Figs. 17 and 18 indicates that the new I/C values improved the postprandial responses for bolus-only control and PID control. For the feedforward-feedback controller, adjusting Kf reduced the glucose peaks with only slight excursions beyond the 60 mg/dL limit for two of the six cases.
Fig. 18.
Postprandial glucose profiles for meal challenges and an insulin sensitivity (left) decrease and (right) increase with adjustments of the I/C ratio and feedforward controller gain.
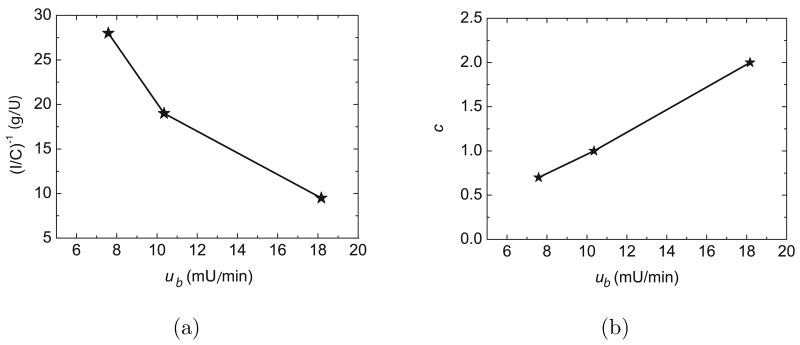
It can be argued that the simulation results in Fig. 18 are unrealistic because the controller settings were determined based on the value of the insulin sensitivity, which is often unknown. But for PID control, the insulin sensitivity changes are reflected in the steady-state insulin infusion rate that is required for basal (non-meal) conditions. As the insulin sensitivity decreases, the basal insulin infusion rate ub required to achieve the glucose setpoint increases, and vice versa. Consequently, insulin sensitivity changes can be inferred from the observed value of ub. The relationship between the tuned control parameters, c and I/C, and ub is shown in Fig. 19. Because these relationships are quite linear, they can be well-approximated by linear regression equations,
Fig. 19.
Relationship between the I/C ratio, the corrective coefficient, c, for the feedforward controller gain and the basal insulin required to achieve a steady state of G = 80 mg/dL.
| (12) |
| (13) |
where Kf (mU/mmol) is the feedforward controller gain, X (g) is the CHO content of the meal, c is a corrective coefficient, and ub (mU/min) is the basal insulin infusion rate.
In summary, insulin sensitivity changes can be accommodated for the three control schemes in Figs. 17 and 18 by a novel gain scheduling approach. The insulin-to-carbohydrate ratio I/C and the feedforward controller gain Kf are adjusted based on the current basal insulin infusion rate, ub. With this new gain scheduling feature, the feedforward-feedback control strategy yielded satisfactory results for meal challenges and/or insulin sensitivity changes.
9 Conclusions
Several important issues concerning the development of an effective feedforward-feedback control strategy for type 1 diabetes have been considered. Improved postprandial responses can be achieved by a pre-prandial snack or bolus, or by reducing the glucose setpoint prior to the meal. The simulation studies for the Hovorka model have demonstrated that setpoint reduction is the most effective approach.
Several new feedforward control strategies have been proposed as alternatives to the standard insulin bolus approach. The proposed strategies include a feedforward controller design based on simple transfer function models and a ratio controller in which the I/C ratio is adjusted based on the estimated meal CHO. Furthermore, the feedforward controller gain can be gain scheduled based on the steady-state insulin infusion rate. Simulation results indicate that the new approaches provide improved postprandial responses over a wide range of conditions.
It is also demonstrated that a model-following approach for the feedback controller can be very effective when a reasonably accurate dynamic model is available.
Acknowledgments
The authors would like to thank the Education Abroad Program (EAP), a program for exchange students and faculty between the University of Padova and the University of California, that made this research possible. Financial support from the National Institutes of Health (Grant R21DK069833) is gratefully acknowledged. The research was performed in collaboration with Francis J. Doyle III, Cesar C. Palerm and Daniel A. Finan at UCSB. Their advice and experience are greatly appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention. National diabetes fact sheet, (Atlanta, GA) 2005 available as: www.cdc.gov/diabetes/pubs/ndfs_2005.pdf.
- 2.Gerich JE. The importance of tight glycemic control. Am J Med. 2005;118(9A):7–11. doi: 10.1016/j.amjmed.2005.07.051. URL http://dx.doi.org/10.1016/j.amjmed.2005.07.051. [DOI] [PubMed]
- 3.Hogan P, Dall T, Nikolov P. Economic costs of diabetes in the us in 2002. Diabetes Care. 2003;26(3):917–932. doi: 10.2337/diacare.26.3.917. [DOI] [PubMed] [Google Scholar]
- 4.Diabetes Control and Complications Trials Research Group. The effect of intensive treatment of diabetes on the development and progression of long–term complications in insulin–dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 5.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hovorka R. Management of diabetes using adaptive control. Int J Adapt Control Signal Process. 2005;19(5):309–325. URL http://dx.doi.org/10.1002/acs.851.
- 7.Bellazzi R, Nucci G, Cobelli C. The subcutaneous route to insulin–dependent diabetes therapy. IEEE Eng Med Biol Mag. 2001;20(1):54–64. doi: 10.1109/51.897828. URL http://dx.doi.org/10.1109/51.897828. [DOI] [PubMed]
- 8.Parker RS, Doyle FJ, III, Peppas NA. The intravenous route to blood glucose control. IEEE Eng Med Biol Mag. 2001;20(1):65–73. doi: 10.1109/51.897829. [DOI] [PubMed] [Google Scholar]
- 9.Steil GM, Panteleon AE, Rebrin K. Closed-loop insulin delivery–the path to physiological glucose control. Adv Drug Deliv Rev. 2004;56(2):125–144. doi: 10.1016/j.addr.2003.08.011. URL http://dx.doi.org/10.1016/j.addr.2003.08.011. [DOI] [PubMed]
- 10.Steil GM, Rebrin K. Closed–loop insulin delivery — what lies between where we are and where we are going? Expert Opin Drug Deliv. 2005;2(2):353–362. doi: 10.1517/17425247.2.2.353. URL http://dx.doi.org/10.1517/17425247.2.2.353. [DOI] [PubMed]
- 11.Bequette BW. A critical assessment of algorithms and challenges in the development of a closed–loop artificial pancreas. Diabetes Technol Ther. 2005;7(1):28–47. doi: 10.1089/dia.2005.7.28. URL http://www.liebertonline.com/doi/abs/10.1089/dia.2005.7.28. [DOI] [PubMed]
- 12.Hovorka R. Continuous glucose monitoring and closed–loop systems. Diabet Med. 2006;23:1–12. doi: 10.1111/j.1464-5491.2005.01672.x. URL http://dx.doi.org/10.1111/j.1464-5491.2005.01672.x. [DOI] [PubMed]
- 13.Bergman RN, Bowden CR, Cobelli C. The minimal model approach to quantification of factors controlling glucose disposal in man. In: Cobelli C, Bergman RN, editors. Carbohydrate Metabolism, Ch 13. John Wiley & Sons Ltd; 1981. pp. 269–293. [Google Scholar]
- 14.Parker RS, Doyle FJ, III, Peppas NA. A model–based algorithm for blood glucose control in type I diabetic patients. IEEE Trans Biomed Eng. 1999;46(2):148–157. doi: 10.1109/10.740877. [DOI] [PubMed] [Google Scholar]
- 15.Lynch SM, Bequette BW. Model predictive control of blood glucose in type I diabetics using subcutaneous glucose measurements. Proc. of the 2002 Amer. Control Conf.; Anchorage, Alaska. May, 2002; pp. 4039–4043. [Google Scholar]
- 16.Hovorka R, Chassin LJ, Wilinska ME, Canonico V, Akwi JA, Federici MO, Massi-Benedetti M, Hutzli I, Zaugg C, Kaufmann H, Both M, Vering T, Schaller HC, Schaupp L, Bodenlenz M, Pieber TR. Closing the loop: the ADICOL experience. Diabetes Technol Ther. 2004;6(3):307–18. doi: 10.1089/152091504774197990. URL http://dx.doi.org/10.1089/152091504774197990. [DOI] [PubMed]
- 17.Schaller HC, Schaupp L, Bodenlenz M, Wilinska ME, Chassin LJ, Wach P, Vering T, H R, Pieber TR. On-line adaptive algorithm with glucose prediction capacity for subcutaneous closed loop control of glucose: evaluation under fasting conditions in patients with type 1 diabetes. Diabetic Medicine. 2006;23(1):90–93. doi: 10.1111/j.1464-5491.2006.01695.x. [DOI] [PubMed] [Google Scholar]
- 18.Candas B, Radziuk J. An adaptive plasma glucose controller based on a nonlinear insulin/glucose model. IEEE Trans Biomed Eng. 1994;41(2):116–124. doi: 10.1109/10.284922. [DOI] [PubMed] [Google Scholar]
- 19.Ibbini M, Masadeh M, Amer MMB. A semiclosed-loop optimal control system for blood glucose level in diabetics. J Med Eng Technol. 2004;28(5):189–96. doi: 10.1080/03091900410001662332. URL http://dx.doi.org/10.1080/03091900410001662332. [DOI] [PubMed]
- 20.Trajanoski Z, Wach P. Neural predictive controller for insulin delivery using the subcutaneous route. IEEE Trans Biomed Eng. 1998;45(9):1122–1134. doi: 10.1109/10.709556. [DOI] [PubMed] [Google Scholar]
- 21.Trajanoski Z, Regittnig W, Wach P. Simulation studies on neural predictive control of glucose using the subcutaneous route. Comput Methods Programs Biomed. 1998;56(2):133–139. doi: 10.1016/s0169-2607(98)00020-0. [DOI] [PubMed] [Google Scholar]
- 22.Parker RS, Doyle FJ, III, Ward JH, Peppas NA. Robust H∞ glucose control in diabetes using a physiological model. AIChE J. 2000;46(12):2537–2549. [Google Scholar]
- 23.Ruiz-Velzquez E, Femat R, Campos-Delgado DU. Blood glucose control for type I diabetes mellitus: A robust tracking H∞ problem. Control Engineering Practice. 2004;12(9):1179–1195. URL http://dx.doi.org/10.1016/j.conengprac.2003.12.004.
- 24.Hovorka R, Canonico V, Chassin LJ, Haueter U, Massi-Benedetti M, Federici MO, Pieber TR, Schaller HC, Schaupp L, Vering T, Wilinska ME. Nonlinear model predictive control of glucose concentration in subjects with type 1 diabetes. Physiol Meas. 2004;25(4):905–20. doi: 10.1088/0967-3334/25/4/010. URL http://dx.doi.org/10.1088/0967-3334/25/4/010. [DOI] [PubMed]
- 25.Zisser H, Jovanovič L, Doyle F, III, Ospina P, Owens C. Run–to–run control of meal–related insulin dosing. Diabetes Technol Ther. 2005;7(1):48–57. doi: 10.1089/dia.2005.7.48. URL http://www.liebertonline.com/doi/abs/10.1089/dia.2005.7.48. [DOI] [PubMed]
- 26.Palerm CC, Zisser H, Jovanovič L, Doyle FJ., III Flexible run–to–run strategy for insulin dosing in type 1 diabetic subjects. Proc. of the IFAC International Symposium on Advanced Control of Chemical Processes (ADCHEM); Gramado, Brazil. 2006. pp. 521–526. [Google Scholar]
- 27.Chassin LJ, Wilinska ME, Hovorka R. Grading system to assess clinical performance of closed-loop glucose control. Diabetes Technol Ther. 2005;7(1):72–82. doi: 10.1089/dia.2005.7.72. URL http://dx.doi.org/10.1089/dia.2005.7.72. [DOI] [PubMed]
- 28.Marchetti G, Barolo M, Jovanovic L, Zisser H, Seborg DE. An improved PID switching control strategy for type 1 diabetes. IEEE Trans Biomedical Eng. 2008 Mar;55(3):857–65. doi: 10.1109/TBME.2008.915665. [DOI] [PubMed] [Google Scholar]
- 29.Marchetti G. Graduation Thesis in Chemical Engineering, Department of Chemical Engineering Priciples and Practice. University of Padova; Italy: 2006. Improved glucose control strategies for subjects with type 1 diabetes mellitus. [Google Scholar]
- 30.Cobelli C, Nucci G, Del Prato S. A physiological simulation model of the glucose–insulin system in type I diabetes. Diabetes Nutr Metab. 1998;11(1):78. [Google Scholar]
- 31.Bergman RN. The minimal model: yesterday, today, and tomorrow. In: Bergman RN, Lovejoy JC, editors. The minimal model approach and determinants of glucose tolerance, Vol 7 of Pennington Center Nutrition Series. Louisiana State University Press; Baton Rouge, LA: 1997. pp. 3–50. [Google Scholar]
- 32.Makroglou A, Li J, Kuang Y. Mathematical models and software tools for the glucose-insulin regulatory system and diabetes: an overview. Appl Num Math. 2006;56(3–4):559–573. URL http://dx.doi.org/10.1016/j.apnum.2005.04.023.
- 33.Wilinska ME, Chassin LJ, Schaller HC, Schaupp L, Pieber TR, Hovorka R. Insulin kinetics in type–1 diabetes: Continuous and bolus delivery of rapid acting insulin. IEEE Trans Biomed Eng. 2005;52(1):3–12. doi: 10.1109/TBME.2004.839639. URL http://dx.doi.org/10.1109/TBME.2004.839639. [DOI] [PubMed]
- 34.Dalla Man C, Camilleri M, Cobelli C. A system model of oral glucose absorption: validation on gold standard data. IEEE Trans Biomed Eng. 2006;53(12 Pt 1):2472–2478. doi: 10.1109/TBME.2006.883792. [DOI] [PubMed] [Google Scholar]
- 35.Hovorka R, Shojaee-Moradie F, Carroll PV, Chassin LJ, Gowrie IJ, Jackson NC, Tudor RS, Umpleby AM, Jones RH. Partitioning glucose distribution/transport, disposal, and endogenous production during IVGTT. Am J Physiol Endocrinol Metab. 2002;282(5):E992–1007. doi: 10.1152/ajpendo.00304.2001. URL http://dx.doi.org/10.1152/ajpendo.00304.2001. [DOI] [PubMed]
- 36.Finan DA, Zisser H, Jovanovič L, Bevier W, Seborg DE. Identfication of linear dynamic models for type 1 diabetes: a simulation study. Proc. of the International Symposium on Advanced Control of Chemical Processes (ADCHEM); Gramado, Brazil. 2006. pp. 503–508. [Google Scholar]
- 37.Steil G, Rebrin K, Darwin C, Hariri F, Saad M. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes. 2006;55(12):2244–3350. doi: 10.2337/db06-0419. [DOI] [PubMed] [Google Scholar]
- 38.Steil G, Saad M. Automated insulin delivery for type 1 diabetes. Diabetes and the endocrine pancreas, Current Opinion in Endocrinology & Diabetes. 2006;13(2):205–211. [Google Scholar]
- 39.Wintergerst K, Deiss D, Buckingham B, Cantwell M, Kache S, Agarwal s, Wilson D, Steil G. Glucose control in pediatric intensive care unit patients using an insulin-glucose algorithm. Diabetes Technol Ther. 2007;9(3):211–222. doi: 10.1089/dia.2006.0031. [DOI] [PubMed] [Google Scholar]
- 40.Doran CV, Chase JG, Shaw GM, Moorhead KT, Hudson NH. Derivative weighted active insulin control algorithms and intensive care unit trials. Contr Eng Pract. 2005;13(9):1129–1137. URL http://dx.doi.org/10.1016/j.conengprac.2004.10.009.
- 41.Lam ZH, Hwang KS, Lee JY, Chase JG, Wake GC. Active insulin infusion using optimal and derivative–weighted control. Med Eng Phys. 2002;24(10):663–672. doi: 10.1016/s1350-4533(02)00147-9. URL http://dx.doi.org/10.1016/S1350-4533(02)00147-9. [DOI] [PubMed]
- 42.Chee F, Fernando TL, Savkin AV, van Heeden V. Expert PID control system for blood glucose control in critically ill patients. IEEE Trans Inform Technol Biomed. 2003;7(4):419–425. doi: 10.1109/titb.2003.821326. URL http://dx.doi.org/10.1109/TITB.2003.821326. [DOI] [PubMed]
- 43.Dimitriadis G, Gerich J. Importance of timing of preprandial subcutaneous insulin administration in the management of diabetes mellitus. Diabetes Care. 1983;6(4):374–377. doi: 10.2337/diacare.6.4.374. [DOI] [PubMed] [Google Scholar]
- 44.Jacobs M, Keulen E, Kanc K, Casteleijn S, Scheffer P, Deville W, Heine R. Metabolic efficacy of preprandial administration of Lys(B28), Pro(B29) human insulin analog in IDDM patients a comparison with human regular insulin during a three-meal test period. Diabetes Care. 1997;20(8):1279–1286. doi: 10.2337/diacare.20.8.1279. [DOI] [PubMed] [Google Scholar]
- 45.Danne T, Aman J, Schober E, Deiss D, Jacobsen JL, Friberg HH, Jensen LH. A comparison of postprandial and preprandial administration of insulin aspart in children and adolescents with type 1 diabetes. Diabetes Care. 2003;26(8):2359–2364. doi: 10.2337/diacare.26.8.2359. URL http://care.diabetesjournals.org/cgi/content/abstract/26/8/2359. [DOI] [PubMed]
- 46.Carr K, Idama T, Masson E, Ellis K, Lindow S. A randomised controlled trial of insulin lispro given before or after meals in pregnant women with type 1 diabetes–the effect on glycaemic excursion. J Obstet Gynaecol. 2004;24(4):382–386. doi: 10.1080/01443610410001685493. [DOI] [PubMed] [Google Scholar]
- 47.Jovanovič L, Giammattei J, Acquistapace M, Bornstein K, Sommermann E, Pettitt DJ. Efficacy comparison between preprandial and postprandial insulin aspart administration with dose adjustment for unpredictable meal size. Clin Ther. 2004;26(9):1492–1497. doi: 10.1016/j.clinthera.2004.09.001. URL http://dx.doi.org/10.1016/j.clinthera.2004.09.001. [DOI] [PubMed]
- 48.Seborg DE, Edgar TF, Mellichamp DA. Process Dynamics and Control. 2. John Wiley & Sons Ltd; New York: 2004. [Google Scholar]
- 49.Åström K, Wittenmark B. Adaptive Control. 2. Addison-Wesley; New York: 1995. [Google Scholar]