Abstract
Variation in pacifier stiffness on non-nutritive suck (NNS) dynamics was examined among infants born prematurely with a history of respiratory distress syndrome. Three types of silicone pacifiers used in the NICU were tested for stiffness, revealing the Super Soothie™ nipple is 7 times stiffer than the Wee™ or Soothie™ pacifiers even though shape and displaced volume are identical. Suck dynamics among 20 preterm infants were subsequently sampled using the Soothie™ and Super Soothie™ pacifiers during follow-up at approximately 3 months of age. ANOVA revealed significant differences in NNS cycles/min, NNS amplitude, NNS cycles/burst, and NNS cycle periods as a function of pacifier stiffness. Infants modify the spatiotemporal output of their suck central pattern generator when presented with pacifiers with significantly different mechanical properties. Infants show a non-preference to suck due to high stiffness in the selected pacifier. Therefore, excessive pacifier stiffness may decrease ororhythmic patterning and impact feeding outcomes.
Keywords: Preterm birth, Non-nutritive suck, Respiratory distress syndrome
Introduction
Observation of infant's oromotor patterns during their routine NICU follow up visits revealed a pacifier preference between two different models of a popular silicone pacifier which have identical external mold profile geometries yielding an oral displacement volume of 4 cc. There was a tendency for infants to spit out the blue Super Soothie™ pacifier and infants who did retain the pacifier and latch did not appear to suck in a burst-pause pattern. However, infants offered the green colored Soothie™ silicone pacifier appeared to enjoy the oral experience and demonstrated the highly organized burst-pause pattern associated with non-nutritive suck. Subjectively, the Super Soothie™ pacifier felt stiffer than the Soothie™ pacifier; however, no objective data on materials stiffness was available from the manufacturer (Children's Medical Ventures, Inc). These observations at the NICU follow-up clinic prompted the following questions: What is the mechanical stiffness of these two popular silicone pacifiers? If significant differences exist in the mechanical properties of the two pacifiers, does this affect infant's preference and alter the neural activity of brain stem circuits which regulate the suck central pattern generator (sCPG)?
Non-nutritive suck has been widely studied; however, few researchers have examined the physical properties of pacifier nipples such as size and thickness, nor the effects of pacifier stiffness on the spatiotemporal patterning of the sCPG. Given the documented sensitivity of the sCPG to somatosensory inputs (Barlow & Estep, 2006; Finan & Barlow, 1996), it is hypothesized that varying pacifier stiffness will alter the spatiotemporal organization and patterning of the sCPG in human infants.
Background
Central pattern generators
Central pattern generators (CPGs) consist primarily of specialized networks of interneurons which produce rhythmic motor patterns (ex: walking, breathing, flying, swimming, sucking) (Marder & Bucher, 2001). The suck central pattern generator, or sCPG, consists of a bilateral circuit of interneurons located in the brain stem reticular formation (Finan & Barlow, 1996; Iriki et al., 1988). Animal studies have revealed that ororhythmic movements can be evoked from this neuronal network when the slice preparation is localized to a segment of the pons between the trigeminal motor nucleus and the facial nucleus (Chandler & Tal, 1986; Nozaki et al., 1986). Interneurons that compose the ororhythm-generating circuits have intrinsic burst generating capabilities (Del Negro et al., 1998; Tanaka et al., 1999) which are tonically inhibited from lower brain stem sites. Thus, brain stem transection has been shown to disinhibit the rhythm generating circuits (Tanaka et al., 1999) which demonstrates that descending inputs from cerebral cortex play a modulatory role in ororhythmic generation (Barlow & Estep, 2006).
The act of sucking on a pacifier produces a rich stream of sensory cues from cutaneous and deep afferents which serve to refine the timing and magnitude of the efferent code delivered to trigeminal, facial, and hypoglossal lower motor neurons (LMNs) (Barlow & Estep, 2006). The lip vermilion and the tip of the tongue are areas with high densities of low-threshold, rapidly conducting mechanoreceptive afferents (Trulsson & Essick, 2004). These oral mechanoreceptors encode important information which is used by the baby during development to modulate the timing and magnitude of sCPG output. This form of neural adaptation plays a critical role in ororythmic behaviors, and is important in the reconfiguration of the sCPG to meet changing task dynamics such as bolus volume and consistency or mechanical properties of the nipple (Finan & Barlow, 1996). Trigeminal sensory flow also modulates the sCPG by tuning the sensitivity of orofacial reflexes (Barlow & Estep, 2006; Barlow et al., 1993; Barlow et al., 2001). Unexpected disturbances or changes to the environment, such as a stiffer pacifier, are ultimately encoded by trigeminal primary afferents which play a key role in modification of lip, tongue, and jaw movements for ororhythmic activity (Lund & Kolta, 2006a; Lund & Kolta, 2006b).
Experience plays a significant role in modulating these sensory signals which influence sCPGs (Barlow & Estep, 2006; Barlow et al., 2004; Estep & Barlow, 2007). Frequent exposure to self-generated orosensory events produces neural activity along the trigeminal lemniscus which is presumed to exert trophic effects on the formation and strengthening of central projections for suck development (Barlow & Estep, 2006). A reduction or qualitative change in the type of sensory input to the infant's face, often associated with procedures that restrict orofacial movements such as nasal cannulation or endotracheal intubation, may disrupt neurogenesis during a critical period of development (Bosma, 1973; Pascual et al., 1998). Environmental (sensory) deprivation represents another factor which negatively impacts mechanisms of cortical and cerebellar differentiation during the early postnatal period. Therefore, sensorimotor enrichment during early life is highly beneficial for the developing brain and suck development (Barlow & Estep, 2006; Pascual et al., 1996).
Structure of the Non-nutritive Suck
The non-nutritive suck (NNS) produced by a term infant normally cycles at a frequency of approximately 2 Hz and is organized into discrete bursts, consisting of 6-12 suck cycles, separated by pause periods as shown in Figure 1 (Finan & Barlow, 1996; Wolff, 1968). During NNS, the infant coordinates the burst-pause pattern with respiration (Goldson, 1987). Sucking on a pacifier or feeding nipple is one of the first oromotor tasks an infant is engaged to perform soon after birth. An infant with a less mature or damaged central nervous system (CNS) will often manifest a less developed suck pattern. Thus, sucking ability is presumed to reflect integrity of the CNS (Barlow & Estep, 2006; Mizuno & Ueda, 2005;). Suck has been observed in utero as early as 15-18 weeks gestational age (GA) (Humphrey, 1964; Miller et al., 2003). As the infant matures the suck becomes more organized and manifests the classic burst-pause pattern around 32 weeks GA (Pickler & Reyna, 2004; Wolff, 1968). By 37 weeks GA, the infant is expected to suck at the same rate as a full term infant (Wolff, 1968).
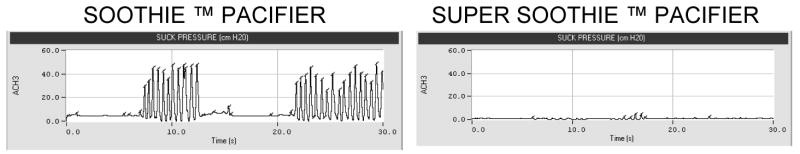
Figure 1.
Sample NNS compression waveforms from T45 while sucking on each of the pacifier types.
In contrast to full term babies, preterm infants often exhibit immature sucking skills (Lau & Schanler, 2000), delayed patterning (Estep & Barlow, 2007; Tamura et al., 1996), and generate fewer sucks characterized by shorter bursts, longer pauses between bursts, and a lower suck pressure (Medoff-Cooper et al., 1989). The physiological distress associated with prematurity translates to greater variability in suck patterns (Cowett et al., 1978; Dreier & Wolff, 1972; Estep & Barlow, 2007; Stumm et al., 2008).
The physical properties of the object (finger, pacifier, breast, etc) placed in a newborn's mouth has noticeable effects on suck patterning. Oral stimulation is a significant determinant of sucking development in the newborn. Infants who experience NNS stimulation daily tend to develop organized sucking behaviors earlier (Bernbaum et al., 1983). The physical characteristics of the nipple, including size, shape, and compressibility, have been shown to influence the frequency of NNS (Dubignon & Campbell, 1968; Lipsitt & Kaye, 1965). Wolff (1968) suggested that pacifier stiffness may affect the amplitude of sucking, however, ororhythmic patterning did not appear to change with variations in the shape of the nipple.
Rationale And Hypothesis
One of the earliest and most accessible forms of external oral stimulation which can influence the development of an infant's suck is their pacifier. The pacifier represents an accessible and convenient oral appliance which has the potential to facilitate or degrade oromotor skill development in infants. Given the sCPG's sensitivity to somatosensory cues from the oral sensorium, it is hypothesized that pacifier stiffness is one key variable encoded by the CNS during sCPG formation. Excessive nipple stiffness may retard suck, whereas a more compliant pacifier may promote ororhythmic activity and needed levels of trigeminal flow for suck development.
Pacifiers have become an essential part of early oromotor stimulation in preterm infants. However, little is known about the physical properties of pacifiers nor their effect on infants' NNS and the sCPG. One key factor to consider is the stiffness of the silicone pacifier and resultant effects on the infant's sCPG. The current study is organized along two experiments. The first was designed to determine the mechanical stiffness of three popular silicone pacifiers available in most NICUs today. The second experiment was designed to assess the effects of two popular silicone pacifiers, equivalent in size and volume with radically different stiffness profiles, on the dynamics of NNS production in a group of premature RDS infants tested at their 3 month NICU follow-up visit.
Experiment One: Pacifier Stiffness Measurement
Methods
Three popular one-piece silicone pacifiers used in the NICU, including the Wee Soothie™, Soothie™, and Super Soothie™, were measured for materials stiffness using a linear servo motor programmed to impose step-wise nipple compression in a repeated measures design as seen in Figure 2. Each pacifier was coupled to a Delrin pacifier receiver, vented to atmosphere at room temperature (∼72 degrees F), and securely mounted in a vise with the silicone nipple oriented up and positioned against a stationary platform on one side. A custom linear servo motor, operating under position feedback, was positioned on the opposite side of the nipple cylinder and programmed to impose an 8-step sequential compression of the silicone nipple. The resultant force (Newtons) and displacement (millimeters) generated by the linear motor against the pacifier were digitized in real time at 100 samples per second at 16-bits of vertical resolution. The change in force was divided by the change in displacement at each compression step, (ΔF/ΔX)STEPS 1 thru 7. The resulting stiffness coefficients were plotted as a function of imposed compression (millimeters). Stiffness was measured on each pacifier type at the cylinder of the nipple.
Figure 2.
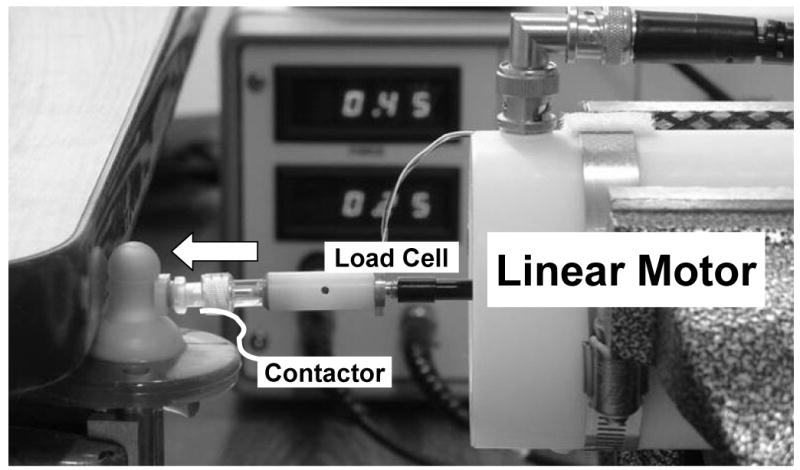
The linear servo motor used to introduce step-wise nipple compressions in a repeated measures design to estimate materials stiffness of each pacifier type.
Results
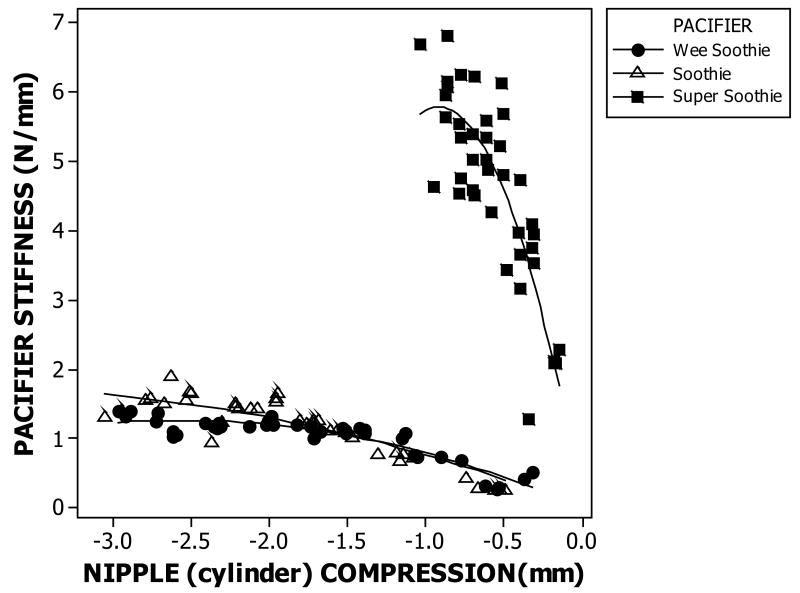
The results of this initial experiment revealed that the nipple cylinder of the Super Soothie™ pacifier is approximately 7 times stiffer over the first millimeter of compression than either the Wee Soothie™ or Soothie™ pacifiers shown in Figure 3. The Super Soothie™ yielded a stiffness value of nearly 6 N/mm following 1 mm of nipple compression levels, whereas, the stiffness coefficients for the Wee Soothie™ and Soothie™ pacifiers were only 0.8N/mm at similar compression levels. Under a full compression load, the difference in stiffness coefficients between the Super Soothie™ and the other two pacifiers is on the order of 7X. Increased wall thickness of the silicone nipple accounts for the significant increase in stiffness for the Super Soothie™ pacifier.
Figure 3.
Stiffness profiles as a function of pacifier type.
Experiment Two: Pacifier Preference And NNS Dynamics
Patients and Methods
The effects of pacifier stiffness on the dynamics of the sCPG was studied in 20 infants (11 Males: 9 Females) seen at the University of Kansas Medical Center NICU follow-up Clinic and the Stormont-Vail Regional Medical Center Premature Follow-up Clinic (Table 1). All infants had a history of RDS with an average corrected age of 2 months and 18 days and an average of 40.8 days on oxygen. This study was approved by the human subjects committees of the University of Kansas Medical Center (Kansas City, KS) and Stormont-Vail Regional Medical Center (Topeka, KS), and informed consent was obtained from the parents prior to the study.
Table I. Patient information.
| Infant | Sex
(F:M) |
GA @birth
(wks;days) |
BW (gms) | Age @ Study
(months; days) |
O2 Hx
(days) |
|---|---|---|---|---|---|
| T36 | M | 29;0 | 1305 | 2;24 | 3 |
| T45 | M | 31;0 | 1725 | 2;23 | 4 |
| T46 | F | 28;1 | 1125 | 3;7 | 23 |
| T47 | M | 27;5 | 765 | 2;19 | 65 |
| T50 | F | 29;0 | 1150 | 2;23 | 44 |
| T51 | M | 29;0 | 1178 | 2;23 | 56 |
| T54 | F | 31;1 | 1290 | 2;18 | 12 |
| T57 | F | 31;1 | 1405 | 2;18 | 15 |
| T59 | F | 26;3 | 1070 | 2;24 | 69 |
| T60 | M | 30;1 | 1085 | 3;16 | 54 |
| T61 | F | 27;5 | 1235 | 2;24 | 18 |
| T63 | F | 27;5 | 765 | 2;28 | 86 |
| T64 | F | 24;6 | 735 | 2;7 | 35 |
| T66 | M | 29;5 | 610 | 3;9 | 91 |
| T89 | F | 27;1 | 750 | 2;24 | 43 |
| T90 | M | 26;6 | 795 | 2;21 | 94 |
| T93 | M | 26;6 | 918 | 2;24 | 64 |
| T98 | M | 30;0 | 1190 | 3;2 | 13 |
| T102 | M | 31;3 | 1685 | 2;28 | 6 |
| T116 | M | 32;1 | 1325 | 2;24 | 22 |
|
| |||||
| MEAN | 9:11 | 28;2 | 1105.3 | 2;18 | 40.8 |
|
| |||||
| S.D. | 2;3 | 310.9 | 0;15 | 31.1 | |
NNS dynamics were sampled in real time from infants using the ACTIFIER technology (Finan & Barlow, 1996). All pacifiers and receivers were gas sterilized with ethylene oxide (EtO). Presentation order for pacifier type (Soothie™ versus Super Soothie™) was counterbalanced and a 2-minute sample of NNS behavior was digitized for each infant. Testing was typically completed 10 minutes prior to the NICU Follow-up Clinic ensuring that the family had ample time for the study and was not late for the clinic. Each infant was held in a developmentally supportive position by an experienced member of the research staff or the infant's caretaker and testing was initiated once the infant achieved a quiet alert state (Als, 1995). Five dependent variables were calculated from the digitized records of ororhythmic activity for each pacifier type using the software algorithm NEOSUCK RT. These included Mean NNS Cycle Amplitude (cmH2O), Mean NNS Cycle Periods (ms), NNS Cycles/Burst, and minute-rates for NNS Burst and NNS Cycle production.
Results: Experiment Two
Infant's tested at the 3-month NICU Follow-up Clinic reorganize their sCPG when faced with silicone pacifiers of varying mechanical properties. An example of this NNS reorganization is shown in Figure 1 for infant T45 who was tested at 2 months and 23 days corrected age. The panel on the left show high levels of NNS behavior when presented with the Soothie™ pacifier. The 2 Hz cycling of nipple compression occurs at nearly 40 cmH2O. A switch to the stiffer Super Soothie™ pacifier resulted in significantly diminished ororhythmic activity characterized by low-amplitude, short duration NNS bursts. Over the 2 minute analysis window, the average NNS burst length was only 5 cycles in length with the Super Soothie pacifier compared to 13 suck cycles per burst produced on the more compliant Soothie™ pacifier. These sample results of oromotor output clearly illustrate the negative performance effect with use of the stiffer Super Soothie™ pacifier.
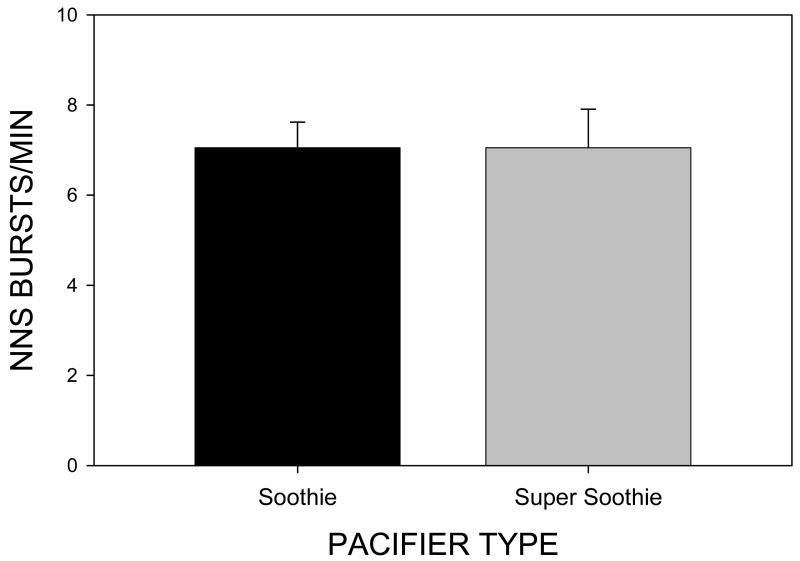
ANOVA (v. SPSS 14.0) completed on the five dependent variables among the 20 test infants revealed several significant differences as a function of pacifier type. The first dependent variable, NNS bursts/min was not significant [F(1,39)=.00, p =1.00] with a Soothie™ mean of 7.05 (SE=.56) bursts/min and a Super Soothie™ mean of 7.05 (SE=.85) bursts/min (Figure 4). Thus, the stiffer pacifier did not deter infants from making attempts to engage the sCPG. However, the fine structure of the resulting NNS burst was significantly altered with the stiffer pacifier as described in the following sections.
Figure 4.
NNS Busts/min
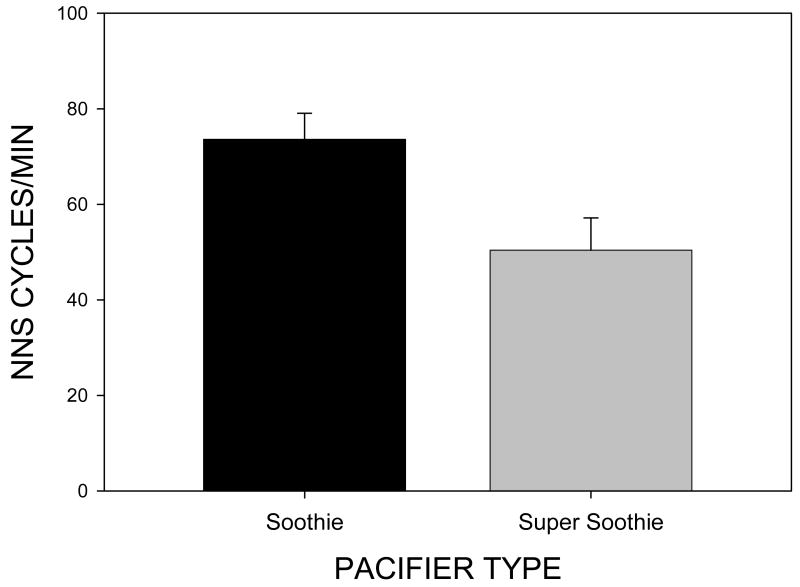
NNS cycles/min was a significant main effect [F(1,39)=9.2, p=.004] with oromotor output on the Soothie™ pacifier yielding an average 74.85 (SE=5.22) cycles/min but only 49.15 (SE=6.66) cycles/min on the Super Soothie™ pacifier (see Figure 5). Infants were able to generate more NNS cycles per minute on the more compliant Soothie™ pacifier than on the Super Soothie™ pacifier.
Figure 5.
NNS Cycles/min
NNS mean amplitude (Figure 6) was significantly different for pacifier type [F(1,39)=44.79, p=.000] with Soothie™ use yielding a mean of 25.22 (SE=2.35) cmH20 and Super Soothie™ use only 8.30 (SE=.91) cmH20 of nipple compression pressure.
Figure 6.
Mean NNS Amplitude
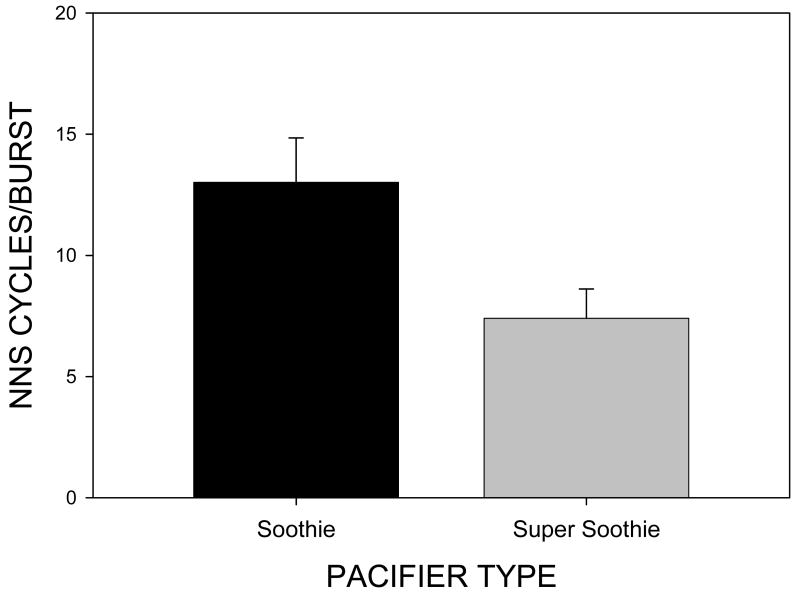
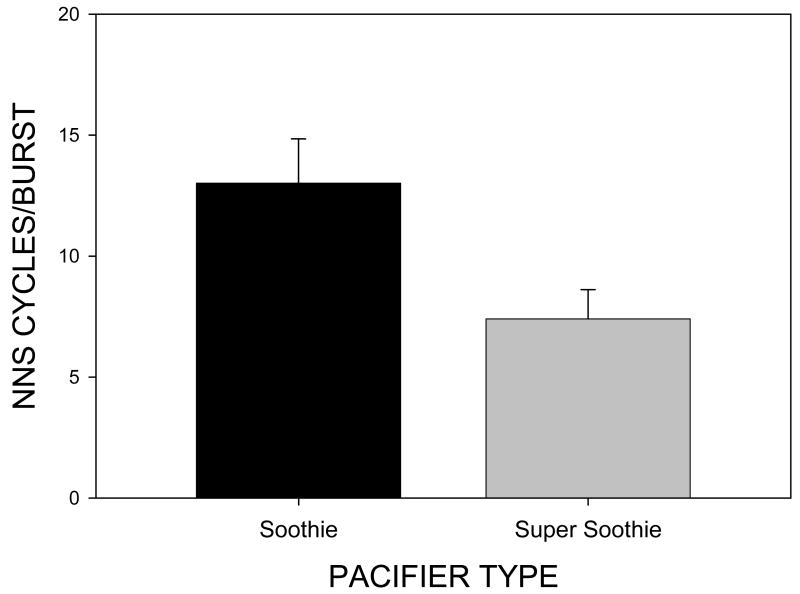
NNS cycles/burst which reflects the complexity of suck burst structure was significantly different for pacifier type [F(1,39)=6.53, p=.015] with a mean of 13.01(SE=8.17) cycles/burst on the Soothie™ pacifier and a mean of 7.40 (SE=1.21) cycles/burst on the Super Soothie™ pacifier (see Figure 7). Thus, the complexity of NNS burst structure is greater when an infant uses the more compliant Soothie™ pacifier.
Figure 7.
Mean Cycles/burst
NNS cycle periods were also different depending on pacifier type [F (1, 37) =4.57, p=.039]. As shown in Figure 8, NNS cycle periods associated with the use of the Soothie pacifier averaged 507.53 (SE=12.12) milliseconds and increased significantly to a mean of 550.48 (SE=16.32) milliseconds when infants switched to the Super Soothie™ pacifier. This is an especially interesting finding since it demonstrates that the intrinsic properties and temporal structure of the sCPG can be modified by changing the local environment.
Figure 8.
Mean NNS Periods
Discussion
Children's Medical Ventures, the manufacturer of the silicone pacifiers used in this study, has never completed an objective examination of the mechanical properties of the pacifiers they market. This is somewhat surprising since infant's preference will ultimately determine the success of their marketed product. After testing each pacifier type for nipple stiffness and discovering the Super Soothie™ pacifier is 7 times stiffer than either the Wee Soothie™ or the Soothie™ pacifiers, it was hypothesized that the NNS dynamics would be altered because of the vastly different mechanical environments these pacifiers present to the infant's orofacial system. Objective testing of the pacifiers for their stiffness levels confirmed the need for physiological study of sCPG dynamics in the developing infant.
There are many different pacifier manufacturers and yet little is known about the mechanical properties and potential effects of their pacifiers on the central patterning of ororhythmic movements in preterm and term infants. Similarly, there are many manufacturers of feeding nipples which are utilized during nutritive suck, and still, little is known about the mechanics of these nipples and their effect on nutritive suck dynamics. Infants who manifest poor feeding skills are often those who are faced with poor patterns of intraoral stimulation (Dubignon & Campbell, 1968). Christensen, Dubignon and Campbell (1976) found that infants respond less frequently to a large nipple but still did not include a complete description of the morphometrics and mechanical properties of this nipple. The mechanical and physical properties of the pacifiers and presumably, feeding nipples, are an important piece of information to attain from manufacturers since these factors can significantly impact ororhythmic patterning in infants. Without careful consideration of these factors, poor sucking/feeding performance observed in infants may be exacerbated.
The sCPG is a precocial neural network that is modified by activity-dependent mechanisms and oral experience. The present study has clearly demonstrated that changing the stiffness environment significantly modifies the dynamics of NNS along several dimensions. Ororhythmic activity on the Soothie™ pacifier was associated with more NNS cycles/min and a more elaborate NNS burst structure when compared to the stiffer Super Soothie™ pacifier. Wolff (1968) described the NNS as having 6-12 suck cycles per burst. In the present group of infants tested at approximately 3 months of age, the mean NNS cycles/burst for the Soothie™ pacifier was 13.01, whereas, use of the Super Soothie™ pacifier decreased output to 7.40 cycles/burst. Since the external shape and displaced volume of the two pacifier nipples are identical (∼4 cc), it logically follows that the observed difference in ororhythmic output between the two pacifiers (Soothie™ vs. Super Soothie™) was due to the large difference in cylinder wall stiffness.
Wolff (1968) suggested that the amplitude of sucking may vary with pacifier stiffness, however, this variation was not measured objectively. In the present study, NNS amplitude was found to vary with pacifier stiffness. It is logical to assume the infant will have reduced amplitude when sucking on a stiffer pacifier but the question still remains whether or not the same amount of muscle force was used by the infant on both pacifiers. The significant difference between the two pacifiers on amplitude represents the intraluminal pressure between the two pacifiers. More research is needed to fully understand the relation between pacifier mechanics and infant applied compression force during suck.
NNS production on the Super Soothie™ pacifier significantly increased within-burst suck cycle periods reflecting a change in the temporal characteristics of the sCPG. Previous research found that rhythmic features were not influenced by the variation in the shape of the nipple (Wolff, 1968). However, the present study reveals that the rhythmic or temporal features of NNS do change systematically when nipple stiffness is increased. The infants modulated their sCPG by slowing down the production of nipple compression cycles. Suck cycle periods increased by 7.8% when infants used the stiffer Super Soothie™ pacifier. This reflects a form of neural adaptation to modify the fine structure of the NNS burst neural generator to accommodate the more challenging mechanics associated with the Super Soothie™ pacifier.
In summary, the results of the present investigation have demonstrated that young infants modify the spatiotemporal dynamics of the sCPG as a function of changes in local oral environment (i.e., changes in nipple stiffness with shape/size held constant). The activity encoded by peri- and intraoral mechanoreceptors is central to modulation of the sCPG in both the time and frequency domain. Based on the current findings, pacifier/nipple manufacturers should pay special attention to the mechanical properties of their products and carefully monitor the influence of these properties on infant oromotor behavior. Further research in the area of pacifier mechanics and their effect on NNS dynamics and the spatiotemporal characteristics of the sCPG is needed.
Acknowledgments
This study was supported by grants NIH R01 DC03311-06 (SM Barlow), NIH P30 HD02528, and NIH P30 DC005803. The authors would like to thank the parents who allowed their children to participate in this study and the NICU medical teams at the University of Kansas Medical Center and Stormont-Vail Regional Health Center for their support. Special gratitude towards Mr. Rajesh Vantipalli, MSCS, for software design., and Mr. Jaehoon Lee, M.A., biostatistician, Advanced Statistical Methods Core, Center for Biobehavioral Neuroscience Communication Disorders.
Supported by: NIH R01 DC03311-06, NIH P30 HD02528, and NIH P30 DC005803
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Emily Zimmerman, Graduate Research Associate, Communication Neuroscience Laboratories, Program in Speech, Language Hearing and Neuroscience, University of Kansas, Lawrence, Kansas USA.
Steven M. Barlow, Professor, SPLH, Programs in Neuroscience, Human Biology, and Bioengineering Director Communication Neuroscience Laboratories, University of Kansas, Lawrence, Kansas USA.
References
- Als H. A manual for naturalistic observation of the newborn (preterm and full term infants) In: Goldson E, editor. Nurturing the premature infant, Developmental Interventions in the Neonatal Intensive Care Nursery. Oxford University Press; New York: 1995. pp. 77–85. [Google Scholar]
- Barlow SM, Dusick A, Finan DS, Coltart S, Biswas A. Mechanically evoked perioral refleces in preterm and term human infants. Brain Res. 2001;899:251–254. doi: 10.1016/s0006-8993(01)02239-9. [DOI] [PubMed] [Google Scholar]
- Barlow SM, Estep M. Central pattern generation and the motor infrastructure for suck, respiration, and speech. J Com Dis. 2006;39:366–380. doi: 10.1016/j.jcomdis.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Barlow SM, Finan DS, Bradford PT, Andreatta R. Transitional properties of the mechanically evoked perioral reflex from infancy through adulthood. Brain Res. 1993;623:181–188. doi: 10.1016/0006-8993(93)91425-r. [DOI] [PubMed] [Google Scholar]
- Barlow SM, Finan DS, Park SY. Central pattern generation and sensorimotor entrainment of respiratory and orofacial systems in humans. In: Maassen B, Hulstijn W, Kent R, Peters HFM, vLieshout PHMM, editors. Speech Motor Control in Normal and Disordered Speech. Oxford University Press; 2004. pp. 211–224. [Google Scholar]
- Bernbaum JC, Pereira GR, Watkins JB, Peckham GJ. Nonnutritive sucking during gavage feeding enhances growth and maturation in premature infants. Pediatrics. 1983;71(1):41–45. [PubMed] [Google Scholar]
- Bosma JF. Prologue to the symposium. In: Bosma JF, editor. Fourth Symposium on Oral Sensation and Perception; Bethesda: Charles C. Thomas; 1973. p. 7. [Google Scholar]
- Chandler SH, Tal M. The effects of brain stem transection on the neuronal networks responsible for rhythmical jaw muscle activity in the guinea pig. J Neurosci. 1986;6:1831–1842. doi: 10.1523/JNEUROSCI.06-06-01831.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen S, Dubignon J, Campbell D. Variations in intra-oral stimulation and nutritive sucking. Child Dev. 1976;47:39–542. [PubMed] [Google Scholar]
- Cowett R, Lispsitt L, Vohr B, Oh W. Aberrations in sucking behaviors of low birth weight infants. Dev Med Child Neurol. 1978;20:701–709. doi: 10.1111/j.1469-8749.1978.tb15300.x. [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Hsiao CF, Garfinkel A, Chandler SH. Evidence for a novel bursting mechanism in rodent trigeminal neurons. Bio-phys. 1998;75:174–182. doi: 10.1016/S0006-3495(98)77504-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubignon J, Campbell D. Intraoral stimulation and sucking in the new born. J Exp Child Psych. 1968;6:154–166. doi: 10.1016/0022-0965(68)90080-5. [DOI] [PubMed] [Google Scholar]
- Dreier T, Wolff PH. Sucking, state, and perinatal distress in newborns. A preliminary report. Biol Neonate. 1972;21:16–24. doi: 10.1159/000240491. [DOI] [PubMed] [Google Scholar]
- Estep M, Barlow SM, Vantipalli R, Finan D, Lee J. Non-Nutritive Suck Parameters in Preterm Infants with RDS. Journal of Neonat Nurs. 2007 doi: 10.1016/j.jnn.2007.12.005. Under review at. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan DS, Barlow SM. The Actifier and neurophysiological studies of orofacial control in human infants. J Speech Hear Res. 1996;39:833–838. [PubMed] [Google Scholar]
- Goldson E. Non-nutritive sucking in the sick infant. Journal Perinatol. 1987;7:30–34. [PubMed] [Google Scholar]
- Humphrey T. Some correlations between the appearance of human fetal reflexes and the development of the CNS. Progress Brain Res. 1964;4:93–135. [Google Scholar]
- Iriki A, Nozaki S, Nakamura Y. Feeding behavior in mammals: Corticobulbar projection is reorganized during conversion from sucking to chewing. Dev Brain Res. 1988;44:189–196. doi: 10.1016/0165-3806(88)90217-9. [DOI] [PubMed] [Google Scholar]
- Lau C, Schanler RJ. Oral feeding in premature infants: advantage of a self-paced milk flow. Acta Paediatrica. 2000;89(7):453–459. doi: 10.1080/080352500750028186. [DOI] [PubMed] [Google Scholar]
- Lipsitt L, Kaye H. Change in neonatal response to optimizing and nonoptimising sucking stimulation. Psychonomic Med. 1965;20:221–222. [Google Scholar]
- Lund JP, Kolta A. Generation of the central masticatory pattern and its modification by sensory feedback. Dysphagia. 2006a:167–174. doi: 10.1007/s00455-006-9027-6. [DOI] [PubMed] [Google Scholar]
- Lund JP, Kolta A. Brain stem circuits that control mastication: Do they have anything to say during speech? J Com Dis. 2006b;39:381–390. doi: 10.1016/j.jcomdis.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D. Central pattern generators and the control of rhythmic movements. Curr Biol. 2001;11:986–996. doi: 10.1016/s0960-9822(01)00581-4. [DOI] [PubMed] [Google Scholar]
- Medoff-Cooper B, Weininger S, Zukowsky K. Neonatal sucking as a clinical assessment tool: preliminary findings. Nursing Res. 1989;38:162–165. [PubMed] [Google Scholar]
- Miller JL, Sonies BC, Macedonia C. Emergence of oropharyngeal, laryngeal, and swallowing difficulty in the developing fetal upper aerodigestive tract: an ultrasound evaluation. Early Hum Dev. 2003;71:61–87. doi: 10.1016/s0378-3782(02)00110-x. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Ueda A. Neonatal feeding performance as a predictor of neurodevelopmental outcome at 18 months. Dev Med Child Neurol. 2005;47:299–304. doi: 10.1017/s0012162205000587. [DOI] [PubMed] [Google Scholar]
- Nowak J, Smith W, Erenberg A. Imaging evaluation of artificial nipples during bottle feeding. Arch Ped Adol Med. 1994;148:40–42. doi: 10.1001/archpedi.1994.02170010042008. [DOI] [PubMed] [Google Scholar]
- Nozaki S, Iriki A, Nakamura Y. Role of corticobulbar projection neurons in cortically induced rhythmical masticatory jaw-opening movement in the guinea pig. J Neurophysiol. 1986;55:806–825. doi: 10.1152/jn.1986.55.4.826. [DOI] [PubMed] [Google Scholar]
- Pascual R, Figueroa H. Effects of preweaning sensorimotor stimulation on behavioral and neuronal development in motor and visual cortex of the rat. Biol Neonate. 1996;69:399–404. doi: 10.1159/000244337. [DOI] [PubMed] [Google Scholar]
- Pascual R, Hervias MC, Toha ME, Valero A, Figueroa HR. Purkinje cell impairment induced by early movement restriction. Biol Neonate. 1998;73:47–51. doi: 10.1159/000013959. [DOI] [PubMed] [Google Scholar]
- Pickler R, Reyna B. Effects of non-nutritive sucking on nutritive sucking, breathing, and behavior during bottle feedings of preterm infants. Adv Neonat Care. 2004;4:226–234. doi: 10.1016/j.adnc.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Pinelli J, Symington A. How rewarding can a pacifier be? Neonat Network. 2000;19:41–48. doi: 10.1891/0730-0832.19.8.41. [DOI] [PubMed] [Google Scholar]
- Rocha A, Moreira M, Pimenta H, Ramos J, Lucena S. A randomized study of the efficacy of sensory-motor-oral stimulation and non-nutritive sucking in very low birth weight infant. Early Hum Dev. 2007;83(6):385–389. doi: 10.1016/j.earlhumdev.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Stumm S, Barlow SM, Estep M, Lee J, Cannon S, Carlson J, Finan D. Respiratory Distress Syndrome Degrades the Fine Structure of the Non-nutritive Suck in Preterm Infants. Journal of Neonat Nurs. 2008 February; doi: 10.1016/j.jnn.2007.11.001. in press. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura Y, Horikawa Y, Yoshida S. Co-ordination of tongue movements and peri-oral muscle activities during nutritive sucking. Dev Medicine Child Neurol. 1996;38:503–510. doi: 10.1111/j.1469-8749.1996.tb12111.x. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kogo M, Chandler SH, Matsuya T. Localization of oral-motor rhythmogenic circuits in the isolated rat brain stem preparation. Brain Res. 1999;821:190–199. doi: 10.1016/s0006-8993(99)01117-8. [DOI] [PubMed] [Google Scholar]
- Trulsson M, Essick GK. Mechanosensation. In: Miles T, Nauntofte B, Svensson P, editors. Clinical oral physiology. Copenhagen: Quintessence; 2004. pp. 165–97. [Google Scholar]
- Wolff PH. The serial organization of sucking in the young infant. Pediatrics. 1968;42:943–956. [PubMed] [Google Scholar]
- Zimmerman E, Barlow SM. Pacifier stiffness alters the dynamics of the suck central pattern generator. Soc Pediatric Research. 2006;5571:393. doi: 10.1016/j.jnn.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]