Abstract
Apoptosis is an important mechanism during the immunopathogenesis of sepsis. Early programmed cell death of lymphocytes substantially impairs innate and adaptive immunity reducing the capacity to ward off the invading pathogen. Apoptosis of parenchymal cells (e.g. in the lung, liver and gut) may also promote organ failure and death. Several experimental therapeutic strategies have now been developed to beneficially influence these mechanisms; however, their potential clinical benefit is yet to be evaluated.
Introduction
Sepsis is a clinical entity with high morbidity and mortality. About 750,000 people suffer from sepsis in the US annually [1]. The incidence of sepsis is on rise with the general aging of the population and emergence of microorganisms that are more and more resistant to antibiotic therapies [2]. Among more than 6.5 million hospital admissions 2.9% develop a severe form of sepsis for which the mortality associated can range around 30% [1;3]. Sepsis has been defined as a “systemic inflammatory response syndrome that occurs during infection”. Thus, work in this field has long focused on inflammation as the leading pathogenic mechanism. However, a variety of therapeutic approaches, mainly anti-inflammatory in nature, have mostly failed to cure human sepsis (e.g. studies involving interleukin (IL)-1β, tumor necrosis factor (TNF)-α, prostaglandins and leukotrienes, bradykinin, platelet-activating factor and nitric oxide). So far only a few strategies such as intensive insulin therapy, low dose corticosteroids and activated protein C have beneficially altered mortality from sepsis in the clinical setting (reviewed in [4]).
Apoptosis in Sepsis - Another Mechanism?
The failure of anti-inflammatory strategies leaves some doubts as to whether inflammation is truly the leading death causing factor in sepsis. It appears that it might actually be the minority of patients that show substantially elevated TNF-α and/or IL-1β levels during sepsis. Interestingly, there is evidence that patients suffering from sepsis show symptoms of immunosuppression. They are predisposed for nosocomial infections and have difficulty clearing the invading pathogen. In this respect, a shift towards anti-inflammatory cytokines can be observed during the course of sepsis. CD4+ T cells are shifted towards a Th2 profile, secreting large amounts of interleukin-4 and interleukin-10 for example. Furthermore, increased IL-10 plasma levels have been shown to predict mortality in septic patients [5]. Thus, developing evidence in sepsis research suggests not only that increased apoptosis may play a crucial role in the outcome of experimental sepsis, but possibly, also in patients. This increased apoptosis during sepsis resulting in a loss of immune and/or non-immune cell populations may contribute to immunosuppression and/or subsequent organ failure. Here we attempt to clarify the role immune cell apoptosis plays in the development of sepsis, with an aim at identifying which strategies are currently under consideration to counteract apoptotic cell death during sepsis. We will also briefly discuss parenchymal cell apoptosis in different organs, cells whose demise may also contribute to organ failure and death.
Mechanisms of Apoptosis – Many Ways to Die
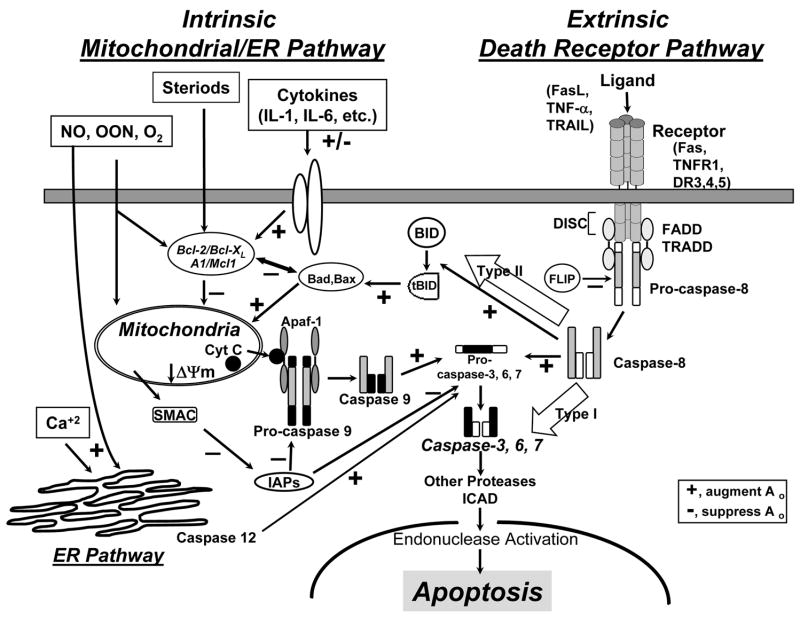
Apoptosis represents an energy consuming and organized process of cell death; however it is not the only way for a cell to die, necrosis, and intermediate processes of cell death have also been described [6]. In apoptosis though, a deformation of the cell membrane, shrinkage of the cell, condensation of the nuclear chromatin and activation of endonucleases cleaving DNA are characteristic. There are at least three pathways described by which apoptosis can be initiated (see Figure 1): The extrinsic pathway is triggered through death receptors, including Fas (CD95), tumor necrosis factor (TNF) receptor 1 (p55), TNF receptor apoptosis-mediating protein (TRAMP, DR3), TNF related apoptosis-inducing ligand receptor 1 (TRAIL-R1) or receptor-2 (DR5). These receptors contain an 80 amino acid sequence in their cytoplasmic tail, the so-called “death domain”. Upon ligation, these receptors are clustered and the Fas-associated death domain (FADD), the TNF-receptor I associated death domain (TRADD), procaspase-8, FADD-like IL-1-converting enzyme (FLICE), etc. are recruited forming the death-inducing signaling complex (DISC) [7–9]. Subsequently, caspase-8 can cleave caspase-3 or indirectly activate caspase-3 via tBID (truncated BH-3 interacting domain death agonist), resulting in the aggregation of Bax/Bak and the release of the apoptosome (Cytochrome-C, Apaf-1 [apoptotic protease activating factor-1], procaspase-9). Activated endonucleases then induce the cleavage of DNA bringing about the death of the cell.
Figure 1.
Schematic representation of the three signaling pathways of apoptosis: The death receptor pathway through ligation of TNFR or Fas (extrinsic signaling), the mitochondrial pathway through Bcl-2 family members (intrinsic signaling), and the ER stress pathway. The two-pathway model of Fas/FasL signaling (Type I and Type II) is also illustrated. Bcl- 2 (B-cell CLL/lymphoma 2), Mcl-1 (myeloid cell leukemia sequence 1), A1 (B-cell leukemia/lymphoma 2 related protein A1), Bad (Bcl-2/Bcl-XL-associated death promotor), Bax (Bcl-2-associated X protein), Bim (Bcl-2 interacting mediator of cell death), Bid (BH3 interacting domain death agonist), ΔΨm (mitochondrial membrane potential); Apaf-1 (apoptosis-activating factor-1), FADD (Fas-associated death domain), TRADD (TNFR1-associated death domain), TRAF (TNFR associated factor), IAP (inhibitor of apoptosis proteins), FLIP (FADD-like interleukin-1-converting enzyme (FLICE)-inhibitory protein), ICAD, CAD ((inhibitor of) caspase-activated DNase)
The intrinsic mitochondrial pathway (See Figure 1) is activated by diverse stimuli such as reactive oxygen species, radiation, chemotherapeutic agents, etc. These stimuli drive changes in the interaction of B-cell CCl/lymphoma 2 (Bcl-2) family members including anti-apoptotic Bcl-2, Bcl-extra long (Bcl-xL), myeloid cell leukemia sequence 1 (Mcl-1), and pro-apoptotic Bcl-2 associated X (Bax), Bcl/Bcl-xL-associated death promoter (Bad), Bcl-2 interacting mediator of cell death (Bim), BH3 interacting domain death agonist (Bid), Bcl-2 antagonistic killer (Bak), etc. In response to apoptotic stimuli, BH3-only members are activated and Bax-like factors then insert into the outer mitochondrial membrane where they provoke permeability transition (PT) (the mitochondrial membrane potential (ΔΨm) collapses) and the release of apoptogenic factors (e.g. cytochrome c) into the cytosol. Cytosolic cytochrome c binds to Apaf-1, which oligomerizes to assemble the apoptosome that activates procaspase-9 and initiates a downstream caspase cascade resulting in cell death [10–12].
Other apoptotic pathways have been identified, such as the endoplasmic reticulum pathway mediated by caspase-12 [13;14] (see Figure 1). There is evidence that caspase-12 deficiency in mice renders them resistant to peritonitis and septic shock, though the mechanisms appear more to be anti-inflammatory in nature [15]. The relevance of this pathway as a disease mechanism during sepsis still needs to be elucidated.
Regulatory Effects of Apoptosis on the Immune System and Organ Dysfunction
It is important to understand apoptosis as an upstream point of immune regulation. In this regard, there are several mechanisms by which apoptotic cells impact the immune system.
Lymphocyte Apoptosis Suppresses the Immune System
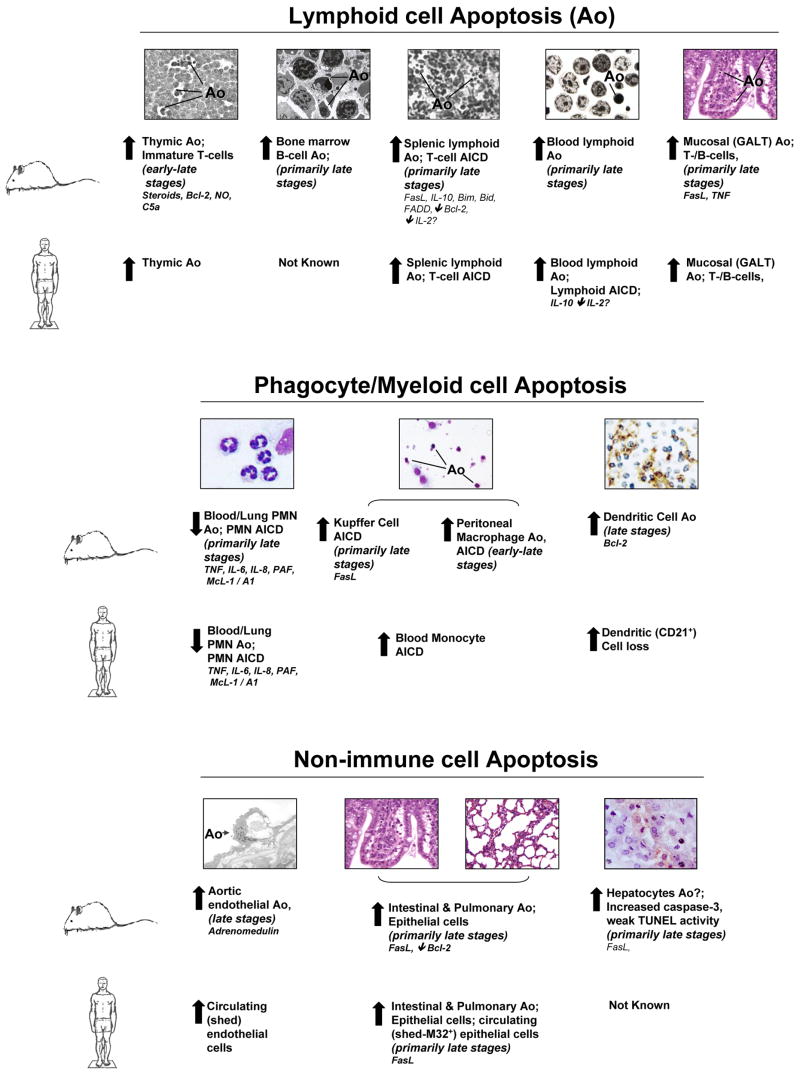
During sepsis, inflammation and apoptosis appear to be closely linked. It is hypothesized that the anti-inflammatory response induces apoptosis in cells of the innate and adaptive immune system rendering the host immunosuppressed. In this regard, apoptosis of lymphocytes was discovered to be an important mechanism in the immune regulatory process in response to experimental sepsis (see Figure 2). Apoptosis was not only seen in the thymus [16;17] but also in the bone marrow [18] and in mucosal lymphocytes [19] and appeared to involve T- [20] as well as B-lymphocytes [21], though with different kinetics. Thymocyte apoptosis during polymicrobial sepsis was independent from the early pro-inflammatory response mediated by TNF-α [16] and was neither mediated through FasL nor through endotoxin, but through corticosteroids acting on Bcl-2 [20;22] whereas mucosal lymphocyte [19] and CD4+-T-lymphocyte deaths [20] were mediated by FasL. In addition, complement C5a has been reported to contribute to thymocyte apoptosis in early sepsis [23]. Subsequently it was demonstrated that anti-inflammatory agents such as IL-10 participated in Th1 lymphocyte apoptosis during sepsis [24]. It has also been documented that mice lacking functional lymphocytes showed higher mortality when subjected to sepsis as compared to their background controls [25;26].
Figure 2.
A summary of the general changes in the levels of apoptosis (Ao) and/or activation induced cell death (AICD) reported both in experimental sepsis (filled arrows) in mice and septic-critically ill patients, as well as the mediators reported to affect the onset and frequency of Ao in various immune as well as non-immune cell types. Bcl-2 (B-cell CLL/lymphoma 2), NO (Nitric oxide), C5a (activated complement factor a), GALT (Gut associated lymphoid tissue), FasL (Fas-Ligand), TNF (Tumor necrosis factor), IL (Interleukin), Mcl-1 (myeloid cell leukemia sequence 1), A1 (B-cell leukemia/lymphoma 2 related protein A1), TUNEL (Terminal deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling)
Hotchkiss and colleagues demonstrated in septic patients that immune-cell death and depletion was also mediated through apoptosis [27]. Autopsies of patients having died from sepsis vs. non-septic etiologies revealed extensive apoptosis of lymphocytes (B-cells and CD4+-T-cell in the spleen) and gastrointestinal epithelial cells [27]. Le Tulzo et al. concluded that lymphocyte apoptosis was rapidly increased in the blood of patients in septic shock and that this development of lymphocyte apoptosis led to a profound and persistent lymphopenia associated with poor outcome [28]. Hotchkiss’s group observed that the frequency of apoptosis was substantially increased in CD4+ and CD8+ T-cells, B cells (CD20+), and NK cells (CD56+) in septic individuals when compared to non-septic patients and that the degree of lymphocyte apoptosis correlated with the severity of sepsis involving both (death receptor and mitochondrial) apoptotic pathways [29]. These experiments clearly supported the concept that lymphocyte apoptosis induced immune suppression during sepsis, mainly through a mechanism of direct depletion of cells of the adaptive immune system.
Parenchymal Cell Apoptosis - Driving Organ Failure
The pathophysiologic role of parenchymal cell apoptosis during sepsis is less clear. Studies have indicated that non-immune cells such as gut mucosal epithelial cells [30;31], lung epithelial cells [32;33], hepatocytes [34;35] and potentially endothelial cells [36;37] exhibit increased apoptosis in clinical or experimental sepsis (see Figure 2). In this regard gut mucosal epithelial cell apoptosis has been shown to be increased in animals [31] as well as humans [27] in response to sepsis. Decreasing gut epithelial cell death in septic mice markedly increased survival [30;38]. We have previously indicated that CD8+ T cells are able to induced hepatocyte apoptosis during sepsis by a Fas-FasL dependent mechanism [35]. Most interestingly, the Fas-FasL mechanism also appears to be crucial in lung epithelial cell death during hemorrhage induced septic acute lung injury: In the absence of a functional Fas-FasL pathway, lung epithelial apoptosis was markedly reduced and was associated with reduced lung injury and increased survival [33].
Phagocytosis of Apoptotic Cells Modulates the Immune System – Manipulating the Immune System beyond Death
Phagocytosis of apoptotic material can be seen as a three step process of interaction between the phagocyte and the apoptotic cell. In the first step, the cell is recognized as apoptotic through the expression of specific proteins or membrane components, CD31, SHPS [SH2 domain-containing tyrosine phosphatase substrate]-1 and phosphatidylserine, among others. Next, a tight interaction with the phagocyte takes place modulated by CD14 and CD47. Initiation of phagocytosis also modulates the inflammatory response of the phagocyte (e.g. mediated by integrins [αvβ3] and phosphatidylserine). In the final step engulfment of the apoptotic cell by the phagocyte takes place (reviewed in [39]).
With respect to the immunosuppressive capacity of apoptotic material, Fadok et al. demonstrated that the uptake of apoptotic cells, but not opsonized control cells, induced macrophages to produce transforming growth factor (TGF)-β1, prostaglandin (PG) E2 and platelet-activating factor (PAF) and, via para-/autocrine effects, induce a marked inhibition of classic inflammatory mediators [40]. TGF-β and PAF have immunosuppressive potential as they have been reported to inhibit TLR-4 mediated cytokine production [41]. The nature of the anti-inflammatory effect appears to be critically dependent on the nature of the receptor(s) engaged on the cell surface of the macrophage. CD36, a co-receptor for integrin αvβ3 cross-linking, or the phosphatidylserine receptor, appear to be the primary mediators of the anti-inflammatory effect of apoptotic cells [42;43]. In this respect, we recently reported that during polymicrobial sepsis the capacity of splenic macrophages to engulf apoptotic lymphocytes was actually enhanced [44]. These data suggest a mechanism by which the release of anti-inflammatory/immunosuppressive mediators might be potentiated during polymicrobial sepsis. The adoptive transfer of apoptotic cells five days prior to the induction of polymicrobial sepsis markedly reduced septic survival when compared to necrotic cell material or buffer [45]. Mechanistically, this alteration in survival was linked to a decreased production of IFN-γ by splenocytes [45]. In addition, apoptotic cells might alter the immune system not only by modulating inflammation but also by direct influence on the adaptive immunity by providing a variable source of antigen for dendritic cell antigenic processing [46;47].
Inhibition of Neutrophil Apoptosis – Helpful or Harmful?
Neutrophils, once mature, exhibit a constitutive form of programmed cell death with a life span between 6–12 hours in circulation. Several inflammatory agents, which are released during sepsis, such as LPS, TNF, IL-8, IL-6, IL-1, GM-CSF, etc., inhibit neutrophil apoptosis. This phenomenon was associated with a decrease in caspase-3 and -9 activities and a prolonged maintenance of the mitochondrial membrane potential [48]. The delayed apoptotic response provides neutrophils with a longer life span, which in turn allows them to accumulate at the local tissue sites. In this respect, neutrophils derived from patients following major surgery, burn injury, sepsis, and acute respiratory distress syndrome (ARDS), as well as from mice subjected to sepsis, showed evidence of decreased apoptosis [48;49] (see Figure 2). However, controversy persists as to whether this actually contributes to organ injury. Using a model of hemorrhagic shock followed by polymicrobial sepsis, Perl et al. found that prolonging the lifespan of neutrophils did not exacerbate acute lung injury but provided an initial survival benefit in response to sepsis [50]. This is in line with previous studies indicating that during polymicrobial sepsis the presence of neutrophils with prolonged lifespan actually proved beneficial to outcome [51]; this stands in direct contrast to having a detrimental impact on the animals’ survival in an inflammatory/ non-infectious environment [50]. Together these data imply that the tissue environment, which the neutrophil encounters, plays a major role in determining if the neutrophil mediates organ damage or not.
Inhibiting Apoptosis Improves Septic Survival – Therapeutic Options
Based on the results presented above it would appear reasonable that the concept of inhibiting apoptosis could be developed into a potential therapeutic strategy for sepsis (see Table). In this respect Hotchkiss et al. [52] observed early on that when administered one hour after experimental sepsis, the pan-caspase inhibitor N-benzyloxycarbonyl-Val-Ala-Asp(O-methyl) fluoromethyl ketone (z-VAD) significantly reduced apoptosis in the thymus and spleen, and z-VAD administration (6mg/kg) immediately following and 24 hours after sepsis resulted in 100% survival as compared to 40% observed in controls. Inhibition of caspase-3 by injection of M-791 also markedly improved survival in septic mice, reducing thymocyte and splenocyte apoptosis [53] while the adoptive transfer of apoptosis-resistant T cells also markedly enhanced the survival rate of the septic animals [53]. Studies with C3H/HeJ (endotoxin-tolerant) and C3H/HeJ-FasLgld (endotoxin-tolerant/FasL-deficient) mice showed that the Fas/FasL pathway was a mediator of apoptosis in B220+ B cells of the Peyer’s patches as well as CD4+ and CD8+ T cells of the intestinal intraepithelial lymphocyte (IEL) population or splenic CD4+ T cells during sepsis [19–21]. When Fas-receptor fusion protein (FasFP), which blocks the activation of Fas pathway, was injected 12 hours after the induction of sepsis, a significant decrease in septic mortality, Kupffer cell apoptosis and liver injury as well as an improvement of blood flow in various organs were observed [54;55].
Small interfering RNA (siRNA) have also been applied as another potential reversible inhibitor of the Fas/FasL pathway (see Table). SiRNA consists of short segments (20–25 nucleotides) of double-stranded RNA targeted to a specific genetic sequence, with the effect of disrupting gene expression. Given up to 12 hours after the induction of sepsis, the administration of Fas siRNA reduced the levels of active caspase-3 in the liver and spleen and improved survival in murine sepsis [56]. Similar results were obtained with caspase-8 siRNA [56]. Moreover, the instillation of Fas-siRNA into the lungs of animals was taken up by lung epithelial cells and this markedly reduced Fas expression. During septic acute lung injury this approach substantially reduced lung epithelial apoptosis and ameliorated the degree of lung injury [32].
With respect to the inhibition of lymphocyte apoptosis, it has also been shown that activating CD40, a potent anti-apoptotic member of the TNF receptor family, provides extensive protection against sepsis-induced lymphocyte apoptosis and improves survival in sepsis by a mechanisms involving Bcl-xL [57]. The cell membrane permeable TAT-Bcl-XL peptide construct as well as a TAT-conjugated peptide of the anti-apoptotic BH4 domain of Bcl-XL (TAT-BH4) decreased sepsis-induced splenocyte T- and B-cell apoptosis to near sham levels in experimental murine sepsis. TAT-Bcl-XL prevented Escherichia coli-induced human lymphocyte apoptosis ex vivo and markedly decreased lymphocyte apoptosis in an in vivo mouse model of sepsis [58].
Summary and Conclusions – Towards Anti-apoptotic Strategies in Sepsis?
Studies over the last decade revealed that apoptosis of immune, particularly lymphocytes, and non-immune cells, is an important pathophysiological event during experimental sepsis as well as in patients, involving both the extrinsic and intrinsic apoptotic pathways. The depletion of such a relevant component of the adaptive immune system renders the host immunosuppressed and thus susceptible to the fatal consequences of sepsis. In addition, there is considerable evidence that apoptosis of parenchymal cells in the lung, the gut and the liver substantially contributes to the failure of these organs in response to sepsis. On a more experimental level evidence suggests that inhibition of these apoptotic processes can restore necessary immune functions and improve the probability of surviving the septic insult. Nevertheless, the necessary translation of these anti-apoptotic strategies into the clinical setting has not yet been performed, leaving open the question of degree to which these anti-apoptotic therapeutic approaches will have utility in the treatment of human sepsis. Thus, the rapid translation of such approaches into the clinical setting is now urgently required and should be given high priority in the next few years.
Table 1.
Experimental treatment approaches that have been reported to change survival benefit in experimental sepsis#*.
| Strategic approach to target | Outcome of intervention at target
(primary effect on apoptosis – effect on septic survival) |
Who is working on the target (et al.)/Refs | |
|---|---|---|---|
| Intrinsic and/or Extrinsic Apoptotic Pathway Targets | FasL gene deficiency | Prevents intestinal lymphoid apoptosis - ↑survival | Chung CS [19] |
| Dominant-negative FADD | Prevents lymphoid apoptosis - ↑survival | Chang KC [59] | |
| Bim or Bid gene deficiency | Prevents lymphoid apoptosis - ↑survival | Chang KC [59] | |
| Fas fusion protein | Given 12 hour after onset of sepsis prevents apoptosis and hepatic injury - ↑survival | Chung CS [54] | |
| Fas & caspase-8 siRNA | Prevent apoptosis in the liver, may suppress the development of MOF when given 0.5 or 12 hours after onset of sepsis - ↑survival | Wesche-Soldato DE [56] | |
| Bim siRNA | Prevent apoptosis in the thymus & spleen, may suppress the development of MOF when given as pre-treatment before onset of sepsis – ↑survival | Schwultz SJ [60] | |
| Fas-, FasL gene deficiency, Fas siRNA | Prevent epithelial apoptosis in the lung, suppressed indices of acute lung injury after shock but prior to sepsis - ↑survival | Perl M [32;33] | |
| Caspase inhibitors | Prevent lymphocyte apoptosis - ↑survival | Hotchkiss RS [52;53] | |
| Bcl-2 overexpression | Inhibits activation of downstream effector caspases, reducing lymphocyte/ neutrophil/ gut epithelial apoptosis - ↑survival | Hotchkiss RS [25;30;38;50;51] | |
| TAT-BclXL & TAT-BH4 | Inhibits activation of downstream effector caspases, preventing dysregulated apoptosis- ↑survival | Hotchkiss RS [58] | |
| p53 gene deficiency | Prevent thymic but not splenic septic apoptosis- ↓survival | Hotchkiss RS [61] | |
| Other Agents/Strategies Effecting Apoptotic Pathways | Adrenomedullin | Vasodilator, increases microvascular blood flow and cardiac output - ↑survival | Zhou M [62] |
| anti-C5a | Reduces thymic apoptosis - ↑survival | Guo RF [23] | |
| Non-caspase protease inhibitors | May prevent mitochondrial pore formation decreased lymphocyte apoptosis - ↑survival | Weaver JG [63] | |
| Akt overexpression | Inhibits activation/interaction of pro-apoptotic Bcl family members with mitochondria - ↑survival | Bommhardt U [64] | |
| iNOS gene deficiency | Decreased thymic apoptosis in sepsis - ↓survival | Cobb JP [65] | |
| SOCS1 gene deficiency | Increased thymic apoptosis in sepsis - ↓survival | Chung CS [66] |
, FasL (Fas Ligand), FADD (Fas associated death domain), Bcl-2 (B-cell CCl/lymphoma 2), Bim (Bcl-2 interacting mediator of cell death), Bid (BH3 interacting domain death agonist), MOF (multiple organ failure), siRNA (small interfering RNA), TAT-Bcl-XL (Bcl-extra long), C5a (activated complement factor 5), Akt (V-akt murine thymoma viral oncogene), iNOS (inducible NO synthase), SOCS (Suppressor of cytokine signaling)
, the studies above have all been done in experimental animal models. No clinical trials in humans with sepsis have yet been initiated with these agents.
Acknowledgments
We wish to thank Dr. Joanne Lomas-Neira for her scientific critique and editorial assistance with the preparation of this manuscript. This work was supported in part by funds from NIH-RO1s GM53209 and HL73525 (to A.A.), as well as fellowship support from NIH-T32 GM65085 (for R.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Angus DC, Wax RS. Epidemiology of sepsis: an update. Crit Care Med. 2001;29:S109–S116. doi: 10.1097/00003246-200107001-00035. [DOI] [PubMed] [Google Scholar]
- 4.Russell JA. Management of sepsis. N Engl J Med. 2006;355:1699–1713. doi: 10.1056/NEJMra043632. [DOI] [PubMed] [Google Scholar]
- 5.Opal SM, Huber CE. The role of interleukin-10 in critical illness. Curr Opin Infect Dis. 2000;13:221–226. doi: 10.1097/00001432-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Leist M, Jaattela M. Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol. 2001;2:589–598. doi: 10.1038/35085008. [DOI] [PubMed] [Google Scholar]
- 7.Krammer PH. CD95's deadly mission in the immune system. Nature. 2000;407:789–795. doi: 10.1038/35037728. [DOI] [PubMed] [Google Scholar]
- 8.Krammer PH. The CD95(APO-1/Fas)/CD95L system. Toxicol Lett. 1998:102–103. 131–137. doi: 10.1016/s0378-4274(98)00297-5. [DOI] [PubMed] [Google Scholar]
- 9.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 10.Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000;7:1166–1173. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- 11.Hockenbery D, Nuñez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 12.Borner C. The Bcl-2 protein family: sensors and checkpoints for life-or-death decisions. Mol Immunol. 2003;39:615–647. doi: 10.1016/s0161-5890(02)00252-3. [DOI] [PubMed] [Google Scholar]
- 13.Szegezdi E, Fitzgerald U, Samali A. Caspase-12 and ER-stress-mediated apoptosis: the story so far. Ann N Y Acad Sci. 2003;1010:186–194. doi: 10.1196/annals.1299.032. [DOI] [PubMed] [Google Scholar]
- 14.Shiraishi H, Okamoto H, Yoshimura A, Yoshida H. ER stress-induced apoptosis and caspase-12 activation occurs downstream of mitochondrial apoptosis involving Apaf-1. J Cell Sci. 2006;119:3958–3966. doi: 10.1242/jcs.03160. [DOI] [PubMed] [Google Scholar]
- 15.Saleh M, Mathison JC, Wolinski MK, Bensinger SJ, Fitzgerald P, Droin N, Ulevitch RJ, Green DR, Nicholson DW. Enhanced bacterial clearance and sepsis resistance in caspase-12-deficient mice. Nature. 2006;440:1064–1068. doi: 10.1038/nature04656. [DOI] [PubMed] [Google Scholar]
- 16.Ayala A, Herdon CD, Lehman DL, DeMaso CM, Ayala CA, Chaudry IH. The induction of accelerated thymic programmed cell death during polymicrobial sepsis: control by corticosteroids but not tumor necrosis factor. Shock. 1995;3:259–267. doi: 10.1097/00024382-199504000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Wang S-D, Huang K-J, Lin Y-S, Lei H-Y. Sepsis-induced apoptosis of the thymocytes in mice. J Immunol. 1994;152:5014–5021. [PubMed] [Google Scholar]
- 18.Ayala A, Herdon CD, Lehman DL, Ayala CA, Chaudry IH. Differential induction of apoptosis in lymphoid tissues during sepsis: variation in onset, frequency, and the nature of the mediators. Blood. 1996;87:4261–4275. [PubMed] [Google Scholar]
- 19.Chung CS, Xu YX, Wang W, Chaudry IH, Ayala A. Is Fas ligand or endotoxin responsible for mucosal lymphocyte apoptosis in sepsis? Arch Surg. 1998;133:1213–1220. doi: 10.1001/archsurg.133.11.1213. [DOI] [PubMed] [Google Scholar]
- 20.Ayala A, Chung CS, Xu YX, Evans TA, Redmond KM, Chaudry IH. Increased inducible apoptosis in CD4+ T lymphocytes during polymicrobial sepsis is mediated by Fas ligand and not endotoxin. Immunology. 1999;97:45–55. doi: 10.1046/j.1365-2567.1999.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayala A, Xin XY, Ayala CA, Sonefeld DE, Karr SM, Evans TA, Chaudry IH. Increased mucosal B-lymphocyte apoptosis during polymicrobial sepsis is a Fas ligand but not an endotoxin-mediated process. Blood. 1998;91:1362–1372. [PubMed] [Google Scholar]
- 22.Ayala A, Xu YX, Chung CS, Chaudry IH. Does Fas ligand or endotoxin contribute to thymic apoptosis during polymicrobial sepsis? Shock. 1999;11:211–217. doi: 10.1097/00024382-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Guo RF, Huber-Lang M, Wang X, Sarma V, Padgaonkar VA, Craig RA, Riedemann NC, McClintock SD, Hlaing T, Shi MM, Ward PA. Protective effects of anti-C5a in sepsis-induced thymocyte apoptosis. J Clin Invest. 2000;106:1271–1280. doi: 10.1172/JCI10793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayala A, Chung CS, Song GY, Chaudry IH. IL-10 mediation of activation-induced TH1 cell apoptosis and lymphoid dysfunction in polymicrobial sepsis. Cytokine. 2001;14:37–48. doi: 10.1006/cyto.2001.0848. [DOI] [PubMed] [Google Scholar]
- 25.Hotchkiss RS, Swanson PE, Knudson CM, Chang KC, Cobb JP, Osborne DF, Zollner KM, Buchman TG, Korsmeyer SJ, Karl IE. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J Immunol. 1999;162:4148–4156. [PubMed] [Google Scholar]
- 26.Chung CS, Watkins L, Funches A, Lomas-Neira J, Cioffi WG, Ayala A. Deficiency of gammadelta T lymphocytes contributes to mortality and immunosuppression in sepsis. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1338–R1343. doi: 10.1152/ajpregu.00283.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Le TY, Pangault C, Gacouin A, Guilloux V, Tribut O, Amiot L, Tattevin P, Thomas R, Fauchet R, Drenou B. Early circulating lymphocyte apoptosis in human septic shock is associated with poor outcome. Shock. 2002;18:487–494. doi: 10.1097/00024382-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Hotchkiss RS, Osmon SB, Chang KC, Wagner TH, Coopersmith CM, Karl IE. Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J Immunol. 2005;174:5110–5118. doi: 10.4049/jimmunol.174.8.5110. [DOI] [PubMed] [Google Scholar]
- 30.Coopersmith CM, Stromberg PE, Dunne WM, Davis CG, Amiot DM, Buchman TG, Karl IE, Hotchkiss RS. Inhibition of intestinal epithelial apoptosis and survival in a murine model of pneumonia-induced sepsis. JAMA. 2002;287:1716–1721. doi: 10.1001/jama.287.13.1716. [DOI] [PubMed] [Google Scholar]
- 31.Hotchkiss RS, Swanson PE, Cobb JP, Jacobson A, Buchman TG, Karl IE. Apoptosis in lymphoid and parenchymal cells during sepsis: findings in normal and T- and B-cell-deficient mice. Crit Care Med. 1997;25:1298–1307. doi: 10.1097/00003246-199708000-00015. [DOI] [PubMed] [Google Scholar]
- 32.Perl M, Chung CS, Lomas-Neira J, Rachel TM, Biffl WL, Cioffi WG, Ayala A. Silencing of fas, but not caspase-8, in lung epithelial cells ameliorates pulmonary apoptosis, inflammation, and neutrophil influx after hemorrhagic shock and sepsis. Am J Pathol. 2005;167:1545–1559. doi: 10.1016/S0002-9440(10)61240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perl M, Chung CS, Perl U, Lomas-Neira J, de Paepe M, Cioffi WG, Ayala A. Fas Induced Pulmonary Apoptosis and Inflammation During Indirect Acute Lung Injury. Am J Respir Crit Care Med. 2007;176:591–601. doi: 10.1164/rccm.200611-1743OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim YM, Kim TH, Chung HT, Talanian RV, Yin XM, Billiar TR. Nitric oxide prevents tumor necrosis factor alpha-induced rat hepatocyte apoptosis by the interruption of mitochondrial apoptotic signaling through S-nitrosylation of caspase-8. Hepatology. 2000;32:770–778. doi: 10.1053/jhep.2000.18291. [DOI] [PubMed] [Google Scholar]
- 35.Wesche-Soldato DE, Chung CS, Gregory SH, Salazar-Mather TP, Ayala CA, Ayala A. CD8+ T cells promote inflammation and apoptosis in the liver after sepsis: role of Fas-FasL. Am J Pathol. 2007;171:87–96. doi: 10.2353/ajpath.2007.061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mutunga M, Fulton B, Bullock R, Batchelor A, Gascoigne A, Gillespie JI, Baudouin SV. Circulating endothelial cells in patients with septic shock. Am J Respir Crit Care Med. 2001;163:195–200. doi: 10.1164/ajrccm.163.1.9912036. [DOI] [PubMed] [Google Scholar]
- 37.Gambim MH, Carmo AO, Marti L, Verissimo-Filho S, Lopes LR, Janiszewski M. Platelet-derived exosomes induce endothelial cell apoptosis through peroxynitrite generation: experimental evidence for a novel mechanism of septic vascular dysfunction. Crit Care. 2007;11:R107. doi: 10.1186/cc6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coopersmith CM, Chang KC, Swanson PE, Tinsley KW, Stromberg PE, Buchman TG, Karl IE, Hotchkiss RS. Overexpression of Bcl-2 in the intestinal epithelium improves survival in septic mice. Crit Care Med. 2002;30:195–201. doi: 10.1097/00003246-200201000-00028. [DOI] [PubMed] [Google Scholar]
- 39.Lauber K, Blumenthal SG, Waibel M, Wesselborg S. Clearance of apoptotic cells: getting rid of the corpses. Mol Cell. 2004;14:277–287. doi: 10.1016/s1097-2765(04)00237-0. [DOI] [PubMed] [Google Scholar]
- 40.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 42.Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 43.Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swan R, Chung CS, Albina J, Cioffi W, Perl M, Ayala A. Polymicrobial sepsis enhances clearance of apoptotic immune cells by splenic macrophages. Surgery. 2007;142:253–261. doi: 10.1016/j.surg.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hotchkiss RS, Chang KC, Grayson MH, Tinsley KW, Dunne BS, Davis CG, Osborne DF, Karl IE. Adoptive transfer of apoptotic splenocytes worsens survival, whereas adoptive transfer of necrotic splenocytes improves survival in sepsis. Proc Natl Acad Sci USA. 2003;100:6724–6729. doi: 10.1073/pnas.1031788100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 47.Schulz O, Reis e Sousa Cross-presentation of cell-associated antigens by CD8alpha+ dendritic cells is attributable to their ability to internalize dead cells. Immunology. 2002;107:183–189. doi: 10.1046/j.1365-2567.2002.01513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taneja R, Parodo J, Jia SH, Kapus A, Rotstein OD, Marshall JC. Delayed neutrophil apoptosis in sepsis is associated with maintenance of mitochondrial transmembrane potential and reduced caspase-9 activity. Crit Care Med. 2004;32:1460–1469. doi: 10.1097/01.ccm.0000129975.26905.77. [DOI] [PubMed] [Google Scholar]
- 49.Ayala A, Chung CS, Lomas JL, Song GY, Doughty LA, Gregory SH, Cioffi WG, LeBlanc BW, Reichner J, Simms HH, Grutkoski PS. Shock-induced neutrophil mediated priming for acute lung injury in mice: divergent effects of TLR-4 and TLR-4/FasL deficiency. Am J Pathol. 2002;161:2283–2294. doi: 10.1016/S0002-9440(10)64504-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perl M, Chung CS, Perl U, Biffl WL, Cioffi WG, Ayala A. Beneficial Versus Detrimental Effects of Neutrophils Are Determined by the Nature of the Insult. J Am Coll Surg. 2007;204:840–852. doi: 10.1016/j.jamcollsurg.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 51.Iwata A, Stevenson VM, Minard A, Tasch M, Tupper J, Lagasse E, Weissman I, Harlan JM, Winn RK. Over-expression of Bcl-2 provides protection in septic mice by a trans effect. J Immunol. 2003;171:3136–3141. doi: 10.4049/jimmunol.171.6.3136. [DOI] [PubMed] [Google Scholar]
- 52.Hotchkiss RS, Tinsley KW, Swanson PE, Chang KC, Cobb JP, Buchman TG, Korsmeyer SJ, Karl IE. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc Natl Acad Sci USA. 1999;96:14541–14546. doi: 10.1073/pnas.96.25.14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hotchkiss RS, Chang KC, Swanson PE, Tinsley KW, Hui JJ, Klender P, Xanthoudakis S, Roy S, Black C, Grimm E, Aspiotis R, Han Y, Nicholson DW, Karl IE. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat Immunol. 2000;1:496–501. doi: 10.1038/82741. [DOI] [PubMed] [Google Scholar]
- 54.Chung CS, Yang S, Song GY, Lomas J, Wang P, Simms HH, Chaudry IH, Ayala A. Inhibition of Fas signaling prevents hepatic injury and improves organ blood flow during sepsis. Surgery. 2001;130:339–345. doi: 10.1067/msy.2001.116540. [DOI] [PubMed] [Google Scholar]
- 55.Chung CS, Song GY, Lomas J, Simms HH, Chaudry IH, Ayala A. Inhibition of Fas/Fas ligand signaling improves septic survival: differential effects on macrophage apoptotic and functional capacity. J Leukoc Biol. 2003;74:344–351. doi: 10.1189/jlb.0102006. [DOI] [PubMed] [Google Scholar]
- 56.Wesche-Soldato DE, Chung CS, Lomas-Neira J, Doughty LA, Gregory SH, Ayala A. In vivo delivery of Caspase 8 or Fas siRNA improves the survival of septic mice. Blood. 2005;106:2295–2301. doi: 10.1182/blood-2004-10-4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwulst SJ, Grayson MH, DiPasco PJ, Davis CG, Brahmbhatt TS, Ferguson TA, Hotchkiss RS. Agonistic monoclonal antibody against CD40 receptor decreases lymphocyte apoptosis and improves survival in sepsis. J Immunol. 2006;177:557–565. doi: 10.4049/jimmunol.177.1.557. [DOI] [PubMed] [Google Scholar]
- 58.Hotchkiss RS, McConnell KW, Bullok K, Davis CG, Chang KC, Schwulst SJ, Dunne JC, Dietz GP, Bahr M, McDunn JE, Karl IE, Wagner TH, Cobb JP, Coopersmith CM, Piwnica-Worms D. TAT-BH4 and TAT-Bcl-xL peptides protect against sepsis-induced lymphocyte apoptosis in vivo. J Immunol. 2006;176:5471–5477. doi: 10.4049/jimmunol.176.9.5471. [DOI] [PubMed] [Google Scholar]
- 59.Chang KC, Unsinger J, Davis CG, Schwulst SJ, Muenzer JT, Strasser A, Hotchkiss RS. Multiple triggers of cell death in sepsis: death receptor and mitochondrial-mediated apoptosis. FASEB J. 2007;21:708–719. doi: 10.1096/fj.06-6805com. [DOI] [PubMed] [Google Scholar]
- 60.Schwulst SJ, Muenzer JT, Peck-Palmer OM, Chang KC, Davis CG, McDonough JS, Osborne DF, Walton AH, Unsinger J, McDunn JE, Hotchkiss RS. Bim siRNA decreases lymphocyte apoptosis and improves survival in sepsis. Shock 29. 2007 doi: 10.1097/shk.0b013e318162cf17. In press. [DOI] [PubMed] [Google Scholar]
- 61.Hotchkiss RS, Tinsley KW, Hui JJ, Chang KC, Swanson PE, Drewry AM, Buchman TG, Karl IE. p53-dependent and -independent pathways of apoptotic cell death in sepsis. J Immunol. 2000;164:3675–3680. doi: 10.4049/jimmunol.164.7.3675. [DOI] [PubMed] [Google Scholar]
- 62.Zhou M, Simms HH, Wang P. Adrenomedullin and adrenomedullin binding protein-1 attenuate vascular endothelial cell apoptosis in sepsis. Ann Surg. 2004;240:321–330. doi: 10.1097/01.sla.0000133253.45591.5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weaver JG, Rouse MS, Steckelberg JM, Badley AD. Improved survival in experimental sepsis with an orally administered inhibitor of apoptosis. FASEB J. 2004;18:1185–1191. doi: 10.1096/fj.03-1230com. [DOI] [PubMed] [Google Scholar]
- 64.Bommhardt U, Chang KC, Swanson PE, Wagner TH, Tinsley KW, Karl IE, Hotchkiss RS. Akt decreases lymphocyte apoptosis and improves survival in sepsis. J Immunol. 2004;172:7583–7591. doi: 10.4049/jimmunol.172.12.7583. [DOI] [PubMed] [Google Scholar]
- 65.Cobb JP, Hotchkiss RS, Swanson PE, Chang K, Qiu Y, Laubach VE, Karl IE, Buchman TG. Inducible nitric oxide synthase (iNOS) gene deficiency increases the mortality of sepsis in mice. Surgery. 1999;126:438–442. [PubMed] [Google Scholar]
- 66.Chung CS, Chen Y, Grutkoski PS, Doughty L, Ayala A. SOCS-1 is a central mediator of steroid-increased thymocyte apoptosis and decreased survival following sepsis. Apoptosis. 2007;12:1143–1153. doi: 10.1007/s10495-007-0059-7. [DOI] [PubMed] [Google Scholar]