Abstract
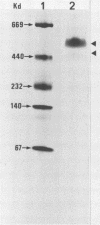
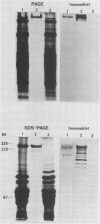
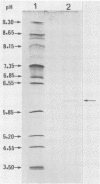
Hemorrhagic toxin (toxin HT) was purified from Clostridium sordellii culture filtrate. The purification steps included ultrafiltration through an XM-100 membrane filter and immunoaffinity chromatography, using a monoclonal antibody to toxin A of Clostridium difficile as the ligand. Toxin HT migrated as a major band with a molecular weight of 525,000 and a minor band at 450,000 on nondenaturing gradient polyacrylamide gel electrophoresis. The molecular weight was estimated at 300,000 by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Isoelectric focusing indicated an apparent pI of 6.1. Toxin HT was cytotoxic for cultured cells and lethal for mice by intraperitoneal injection, and it elicited an accumulation of hemorrhagic fluid in rabbit ileal loops. Immunodiffusion analysis revealed a reaction of partial identity between toxins A and HT. Immunological cross-reactivity between these toxins was further demonstrated by immunoblotting and by neutralization of toxin HT biological activity with antibodies to toxin A. A sensitive indirect enzyme-linked immunosorbent assay was used to examine the affinity involved in homologous and heterologous antigen-antibody interactions. Our findings show that toxin HT has biological activities and immunological properties similar to those of toxin A; however, the toxins are not identical.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Mashat R. R., Taylor D. J. Bacteria in enteric lesions of cattle. Vet Rec. 1983 Jan 1;112(1):5–10. doi: 10.1136/vr.112.1.5. [DOI] [PubMed] [Google Scholar]
- Al-Mashat R. R., Taylor D. J. Production od diarrhoea and enteric lesions in calves by the oral inoculation of pure cultures of Clostridium sordellii. Vet Rec. 1983 Feb 12;112(7):141–146. doi: 10.1136/vr.112.7.141. [DOI] [PubMed] [Google Scholar]
- Allo M., Silva J., Jr, Fekety R., Rifkin G. D., Waskin H. Prevention of clindamycin-induced colitis in hamsters by Clostridium sordellii antitoxin. Gastroenterology. 1979 Feb;76(2):351–355. [PubMed] [Google Scholar]
- Arseculeratne S. N., Panabokké R. G., Wijesundera S. The toxins responsible for the lesions of Clostridium sordelli gas gangrene. J Med Microbiol. 1969 Feb;2(1):37–53. doi: 10.1099/00222615-2-1-37. [DOI] [PubMed] [Google Scholar]
- Bartlett J. G., Chang T. W., Gurwith M., Gorbach S. L., Onderdonk A. B. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978 Mar 9;298(10):531–534. doi: 10.1056/NEJM197803092981003. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chang T. W., Gorbach S. L., Bartlett J. B. Neutralization of Clostridium difficile toxin by Clostridium sordellii antitoxins. Infect Immun. 1978 Nov;22(2):418–422. doi: 10.1128/iai.22.2.418-422.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrich M., Van Tassell R. L., Libby J. M., Wilkins T. D. Production of Clostridium difficile antitoxin. Infect Immun. 1980 Jun;28(3):1041–1043. doi: 10.1128/iai.28.3.1041-1043.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George R. H., Symonds J. M., Dimock F., Brown J. D., Arabi Y., Shinagawa N., Keighley M. R., Alexander-Williams J., Burdon D. W. Identification of Clostridium difficile as a cause of pseudomembranous colitis. Br Med J. 1978 Mar 18;1(6114):695–695. doi: 10.1136/bmj.1.6114.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivan H. C., Wilkins T. D. Purification of Clostridium difficile toxin A by affinity chromatography on immobilized thyroglobulin. Infect Immun. 1987 Aug;55(8):1873–1877. doi: 10.1128/iai.55.8.1873-1877.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larson H. E., Price A. B. Pseudomembranous colitis: Presence of clostridial toxin. Lancet. 1977 Dec 24;2(8052-8053):1312–1314. doi: 10.1016/s0140-6736(77)90363-4. [DOI] [PubMed] [Google Scholar]
- Libby J. M., Jortner B. S., Wilkins T. D. Effects of the two toxins of Clostridium difficile in antibiotic-associated cecitis in hamsters. Infect Immun. 1982 May;36(2):822–829. doi: 10.1128/iai.36.2.822-829.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby J. M., Wilkins T. D. Production of antitoxins to two toxins of Clostridium difficile and immunological comparison of the toxins by cross-neutralization studies. Infect Immun. 1982 Jan;35(1):374–376. doi: 10.1128/iai.35.1.374-376.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Krivan H. C., Wilkins T. D. Clostridium difficile: its disease and toxins. Clin Microbiol Rev. 1988 Jan;1(1):1–18. doi: 10.1128/cmr.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Lockwood D. E., Richardson S. H., Wilkins T. D. Biological activities of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1147–1150. doi: 10.1128/iai.35.3.1147-1150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Phelps C. J., Toth J., Wilkins T. D. Characterization of toxins A and B of Clostridium difficile with monoclonal antibodies. Infect Immun. 1986 Oct;54(1):70–76. doi: 10.1128/iai.54.1.70-76.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Phelps C. J., Wilkins T. D. Monoclonal and specific polyclonal antibodies for immunoassay of Clostridium difficile toxin A. J Clin Microbiol. 1985 Jan;21(1):12–14. doi: 10.1128/jcm.21.1.12-14.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Saum K. E., MacDonald D. K., Wilkins T. D. Effects of Clostridium difficile toxins given intragastrically to animals. Infect Immun. 1985 Feb;47(2):349–352. doi: 10.1128/iai.47.2.349-352.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyerly D. M., Sullivan N. M., Wilkins T. D. Enzyme-linked immunosorbent assay for Clostridium difficile toxin A. J Clin Microbiol. 1983 Jan;17(1):72–78. doi: 10.1128/jcm.17.1.72-78.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Ogura H., Tanaka J., Tanabe N., Yamakawa K., Hatano M., Nishida S. Difference in susceptibility of various cell cultures to cytotoxic culture filtrates of Clostridium sordellii. Microbiol Immunol. 1984;28(4):493–497. doi: 10.1111/j.1348-0421.1984.tb00700.x. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Tanabe N., Yamakawa K., Nishida S. Cytotoxin production by Clostridium sordellii strains. Microbiol Immunol. 1983;27(6):495–502. doi: 10.1111/j.1348-0421.1983.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Popoff M. R. Bacteriological examination in enterotoxaemia of sheep and lamb. Vet Rec. 1984 Mar 31;114(13):324–324. doi: 10.1136/vr.114.13.324. [DOI] [PubMed] [Google Scholar]
- Popoff M. R. Purification and characterization of Clostridium sordellii lethal toxin and cross-reactivity with Clostridium difficile cytotoxin. Infect Immun. 1987 Jan;55(1):35–43. doi: 10.1128/iai.55.1.35-43.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehg J. E. Cecal toxin(s) from guinea pigs with clindamycin-associated colitis, neutralized by Clostridium sordellii antitoxin. Infect Immun. 1980 Feb;27(2):387–390. doi: 10.1128/iai.27.2.387-390.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards K. L., Douglas S. D. Pathophysiological effects of Vibrio cholerae and enterotoxigenic Escherichia coli and their exotoxins on eucaryotic cells. Microbiol Rev. 1978 Sep;42(3):592–613. doi: 10.1128/mr.42.3.592-613.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin G. D., Fekety F. R., Silva J., Jr Antibiotic-induced colitis implication of a toxin neutralised by Clostridium sordellii antitoxin. Lancet. 1977 Nov 26;2(8048):1103–1106. doi: 10.1016/s0140-6736(77)90547-5. [DOI] [PubMed] [Google Scholar]
- Stiles B. G., Wilkins T. D. Clostridium perfringens iota toxin: synergism between two proteins. Toxicon. 1986;24(8):767–773. doi: 10.1016/0041-0101(86)90101-7. [DOI] [PubMed] [Google Scholar]
- Sullivan N. M., Pellett S., Wilkins T. D. Purification and characterization of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1032–1040. doi: 10.1128/iai.35.3.1032-1040.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor N. S., Thorne G. M., Bartlett J. G. Comparison of two toxins produced by Clostridium difficile. Infect Immun. 1981 Dec;34(3):1036–1043. doi: 10.1128/iai.34.3.1036-1043.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa K., Nakamura S., Nishida S. Separation of two cytotoxins of Clostridium sordellii strains. Microbiol Immunol. 1985;29(6):553–557. doi: 10.1111/j.1348-0421.1985.tb00857.x. [DOI] [PubMed] [Google Scholar]
- Yamakawa K., Tanabe N., Okada Y., Nishida S., Nakamura S. Rabbit ileal loop responses to Clostridium sordellii strains. Microbiol Immunol. 1983;27(9):807–809. doi: 10.1111/j.1348-0421.1983.tb00646.x. [DOI] [PubMed] [Google Scholar]