Abstract
An improvement of current method of selective hydride generation based on pre-reduction for differentiation of tri- and pentavalent arsenicals is described, applied for the oxidation state specific speciation analysis of inorganic, mono-, di- and trimethylated arsenicals with minimum sample pretreatment using atomic absorption spectrometry with the multiatomizer. The preconcentration and separation of arsine, methylarsine, dimethylarsine and trimethylarsine is then carried out by means of cryotrapping. Presented study shows that 2% (m/v) L-cysteine hydrochloride monohydrate (L-cys) currently used for off-line pre-reduction of pentavalent arsenicals can be substituted with 1% (m/v) thioglycolic acid (TGA). Much faster pre-reduction of pentavalent arsenicals at 25°C with equal sensitivities as in the case of L-cys has been achieved with TGA. A setup for on-line pre-reduction by TGA has been optimized, with the application of segmented flow analysis for suppression of axial dispersion in the pre-reduction coil. Standard calibrations measured with or without on-line pre-reduction indicate uniform and equal sensitivities for all As forms. The possibility of standardization by water standards of single species (e.g. iAs(III)) for quantification of all other As forms in urine is demonstrated in the recovery study. Limits of detection were 100 ng·l−1 for iAs(III), 135 ng·l−1 for iAs(V) and 30 to 50 ng·l−1 for methylated arsenicals.
Keywords: Methylated arsenic species, Speciation analysis, Hydride generation, Cryotrapping, Thioglycolic acid
1. Introduction
Tri- and pentavalent inorganic, mono-, di- and trimethylated arsenicals are products of animal and human metabolism of arsenic and are required to be determined even at ultra trace levels due to their high toxicity and mutagenic, teratogenic and carcinogenic effects [1–4]. Human metabolism of inorganic As (iAs) consists of reduction of pentavalent arsenicals and oxidative methylation of trivalent species that yields methylated arsenicals [5–9]. The conversion of species such as arsenite (iAs(III)), arsenate (iAs(V)), methylarsonite (MAs(III)), methylarsonate (MAs(V)), dimethylarsinite (DMAs(III)), dimethylarsinate (DMAs(V)) and trimethylarsine oxide (TMAs(V)O) to their corresponding arsines by means of reaction with tetrahydroborate is underlying technique of hydride generation (HG). Association of HG with cryotrapping and gas chromatography (HG-CT) using atomic absorption spectrometry (AAS) for sensitive detection is a very old method [10] and allows to separate arsines generated from iAs, MAs, DMAs and TMAs(V)O according to their boiling points and chromatographic properties of the trap. The main advantage of the HG-CT system as compared to widely used liquid chromatography separation of As species prior to detection by any method of analytical atomic spectrometry is that analysis can be carried out directly without sample pretreatment. This is particularly important in biological samples, where As species are bound to proteins and can be determined without extraction and when attempting the determination of very unstable MAs(III) and DMAs(III), which are quickly oxidized to MAs(V) and DMAs(V) even at temperatures below 0 °C [11,12]. That is why this method is suitable for the speciation analysis of all above-mentioned compounds [6,13,14].
Distinguishing between tri- and pentavalent arsenicals when using the HG-CT approach is provided by selective HG which is usually based on pH specific efficiency of generation [6,10,11,15]. Instead of the choice of pH, the selective HG in presence or absence of pre-reduction agent has been developed in our laboratory for the arsenic speciation analysis [13,14], overcoming the different sensitivity obtained for individual species observed for pH specific generation approach [6,11,13].
Originally pre-reductant of iAs(V), potassium iodide, usually in combination with ascorbic acid [16–19] or with very concentrated HCl [20], was commonly used. Due to low stability and neccessity of high concentration of KI and HCl for reaching complete pre-reduction, agents with –SH group, namely L-cysteine (L-cys) are more popular nowadays [13,16,21–24]. These agents reduce pentavalent arsenicals to trivalent and react with trivalent arsenicals forming arsinothiol derivates. It has also been suggested that L-cys is enhancing the HG performance by forming borane complexes with tetrahydroborate [25]. By means of L-cys treatment uniform signals can be obtained from iAs(V), MAs(V) and DMAs(V) [13,26]. Nevertheless, optimum conditions must be carefully maintained when HG is performed from HCl media, because HG is efficient in a very narrow range of acid concentration (0.01 – 0.1 M) only [13,27]. This problem has been solved by the use of buffered media (TRIS·HCl buffer) proposed and studied in our previous report [13]. At the same time, this buffer ensures selective HG only from trivalent As species without pre-reduction.
Except L-cys, thioglycolic acid (TGA) and other similar compounds with – SH group were tested as the pre-reducing agents [28]. TGA performed well, others such as 2-mercaptoethanol, 3-mercapropropionic acid reduced only one of the three species completely, thiourea commonly used for reduction of Se(VI) to Se(IV) could not reduce MAs(V) at all [28].
Nowadays, automatization of any analytical methods and minimum sample pretreatment are required. Therefore, replacement of the time-consuming off-line pre-reduction by a method with integrated pre-reduction step (on-line pre-reduction) is desirable. No loss of sensitivities, low consumption of all reagents and simplicity are the main requirements. The general limiting factor is reaction rate of pre-reductant with As species which mostly affects time of analysis and sample throughput. It has been proved [13,28] that L-cys needs about 1 hour to complete the reaction with iAs(V), MAs(V) and DMAs(V) at room temperature. The reactor must be heated to perform pre-reduction of pentavalent arsenicals on-line by means of L-cys [29,30] or iAs(V) by KI with ascorbic acid [31]. On-line pre-reduction by means of TGA was suggested by Howard and Salou [28] for its short reaction time with inorganic and methylated pentavalent As species, but its application has never been reported.
The general aims of this study have been to confirm the performance of TGA for off-line pre-reduction of As species in comparison to commonly used L-cys and to develop a setup for on-line pre-reduction integrated with the HG-CT system. Flow injection (FI) arrangement with batch separation and the multiatomizer for atomization of arsines were chosen being compatible with an automated system recently developed in our laboratory [13,14]. The validation of the method is presented for As speciation in human urine samples.
2. Experimental
2.1. Instrumentation
Perkin-Elmer 503 AAS spectrometer without background correction with arsenic electrodeless discharge lamp (EDL) System I (Perkin-Elmer) operated at 8 W was used as a detector for the speciation analysis. Analog strip-chart recorder output signal from the spectrometer was processed by an A/D converter and recorded in a personal computer. Signals were exported as text files into Microcal™ Origin® 6.0 (Microcal Software, USA) or Microsoft® Excel 2000 (Microsoft Corporation, USA) for futher processing.
2.2. Standards and reagents
Deionized water (Ultrapur, Watrex, USA) was used for preparation of all solutions. A 1000 mg·l−1 As AAS standard solution (Merck, Darmstadt, Germany) was used as iAs(V) stock standard solution. Stock solutions of 1000 mg·l−1 As were prepared for arsenic species iAs(III), MAs(V), DMAs(V) and TMAs(V)O in deionized water using following compounds: As2O3 (Lach-Ner, s.r.o., Neratovice, Czech Republic); Na2CH3AsO3·6H2O (Chem. Service, West Chester, USA); (CH3)2As(O)OH (Strem. Chemicals, USA); (CH3)3AsO (University of British Columbia, Vancouver, Canada) [32]. Working standards were prepared for individual species by serial dilution of stock solutions in deionized water. Mixed standards (100 μg.l−1 of iAs(V), MAs(V), DMAs(V) and iAs(III), TMAs(V)O, respectively) were used for final dilution to μg·l−1 level (fresh daily). Deionized water for preparation of iAs(III) standard was deaerated by purging with nitrogen for at least 1 hour to prevent oxidation. Human urine diluted with deionized water (1:1) spiked with As species was employed as a real biological matrix for the recovery study.
A reducing solution containing 1% (m/v) NaBH4 (FLUKA, Steinheim, Germany) in 0.1% (m/v) KOH (Lach-Ner, s.r.o., Neratovice, Czech Republic) was prepared fresh daily. For analysis of samples of human urine, 0.2 ml of 1% (m/v) solution of Antifoam B emulsion (Sigma Chemical Company, St. Louis, USA) per 100 ml of the reducing solution was added to prevent foaming. A 0.75 M TRIS·HCl buffer was prepared from reagent grade Trizma® hydrochloride (Tris(hydroxy-methyl)aminomethane hydrochloride, Sigma, Steinheim, Germany) and pH was adjusted to value 6 by 10% (m/v) KOH. Aqueous solutions of L-cysteine hydrochloride monohydrate (L-cys) (Merck, Darmstadt, Germany) for off-line pre-reduction and thioglycolic acid (TGA) (Fluka, Steinheim, Germany) for both off-line and on-line pre-reduction were used as pre-reduction agents. pH of buffer, solutions and reaction mixtures was measured by a S20 SevenEasy™ pH meter (Mettler Toledo, Schwerzenbach, Switzerland) with InLab®413 pH combination polymer electrodes (Mettler Toledo, Schwerzenbach, Switzerland).
2.3. Multiatomizer
The multiatomizer was identical to the one described previously (model MM5 in Ref. [33]). The inner tube of the optical bar of the atomizer was 120 mm long with 7mm i.d. and with fourteen holes of approximately 0.5mm diameter. The atomizer was heated electrically to 900 °C by an in-house made furnace controlled by a REX-C100 controller (Syscon, Indiana, USA) with a K-type thermocouple sensor (Omega Engineering, USA). 40 ml·min−1 of air as outer gas was employed for atomization.
2.4. Systems for HG
2.4.1. FI mode with off-line pre-reduction and cryotrapping
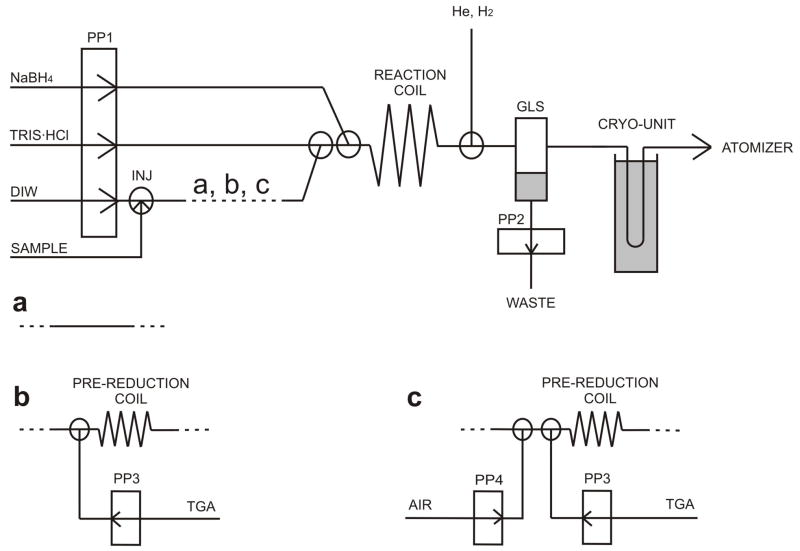
A scheme of the system is shown in Fig. 1a. NaBH4, TRIS·HCl buffer and deionized water were pumped by means of peristaltic pump (PP1) Reglo Digital (Ismatec, Switzerland) at the flow rates of 1 ml·min−1. The manifold was built from PTFE T-junctions (Cole Parmer, USA) and PTFE tubing of 0.75 mm i.d.-1/16″ o.d. except the reaction coil (1 mm i.d.-1/16″ o.d., 1000 mm long, total volume 0.79 ml), the He and H2 inlet (0.5 mm i.d.-1/16″ o.d), tubing between the gas-liquid separator (GLS) and the cryogennic trap (1.6 mm i.d.-1/8″ o.d., 260 mm long) and the cryogennic trap to the atomizer connection (1 mm-1/16″ o.d., 290 mm long). Sample was injected into flow of deionized water by a six-port injection valve (Rheodyne, California, USA) with 597μl sample loop volume. A plastic GLS with forced outlet, made from a 50ml polypropylene screw-cup with a home made acrylic lid [13] which is capable to handle overpressure caused by resistance of a U-tube was employed. The cryogennic trap device consisted of the 305 mm long glass U-tube with 2.5 mm i.d. wrapped with a wire Ni80-Cr20 (0.6 mm o.d.; 5.275 ωm−1; Omega Engineering, Inc., Stamford, USA) providing a total resistance of 15 ω for gradual heating and filled with 0.92 g Chromosorb WAW-DMCS 45/60, 15 % OV-3 (Supelco, Bellefonte, USA). After packing, the U-tube was treated with 50 μl REJUV-8 (hexamethyldisilazane, N,O-bis(trimethylsilyl)acetamide, n-trimethylsilyl-imidazole; Supelco, Bellefonte, USA) and flushed with He for 4 hours at the rate 50 ml·min−1. About ¾ of the U-tube was immersed into the 1.5 l Dewar flask (KGW-Isotherm, Karlsruhe, Germany) with a liquid nitrogen during trapping of hydrides, while in the release phase the U-tube was gradually heated with Trennstelltrafo LTS 604 (Thalheim, Germany) by means of current of 2 A (30 V). The flow rates of carrier He (75 ml·min−1) and H2 (20 ml·min−1) were controlled by mass flow controllers (FMA 2400 or FMA 2600 Series, Omega Engineering, Inc., Stamford, USA).
Fig. 1.
Experimental setups of HG-CT-AAS with off-line pre-reduction (a) and with on-line pre-reduction (b,c); INJ – injection (0.6ml loop); GLS – gas-liquid separator; PP1–4 – peristaltic pumps
2.4.2. CF mode
HG in conventional continuous flow (CF) arrangement was performed in some experiments. The previous manifold was used with some changes. The injection valve and the cryogennic trap were omitted, the plastic GLS was exchanged for a glass GLS with forced outlet (20 ml volume) and the waste liquid was pumped out constantly at the approximate rate of 3.5 ml·min−1. The flow rate of carrier gas Ar was controlled by the mass flow controller (FMA 2600 Series, Omega Engineering, Inc., Stamford, USA) at the rate of 50 ml·min−1.
2.4.3. FI mode with on-line pre-reduction and cryotrapping
For preliminary experiments, the FI system described in Sec. 2.4.1 was modified between the injection valve and the reaction coil as displayed in Fig. 1b. A peristaltic pump (PP3) was added to pump 5% (m/v) TGA at the rate of 0.25 ml·min−1 (1% in each liquid segment after mixing, see Sec. 3.1.1). To form segmented flow of liquid (SFA), another peristaltic pump (PP4) was added to pump air into the manifold at the rate 0.15 ml·min−1 (Fig. 1c). Both additional channels were built from PTFE T-junctions and 0.75 mm i.d.-1/16″ o.d. PTFE tubing. Various pre-reduction coils were made from 1/16″ o.d. PTFE tubing: 0.75 mm i.d. for 0.06 to 2.4ml coils and 1 mm i.d. for 2.9 and 3.5ml coils.
2.5. Procedure
Off-line pre-reduction
Pre-reducing agent was added to samples at least 1 hour prior to analysis. The U-tube was immersed into liquid N2 before beginning of the cycle. It was started with switching on the PP1 and after 3 s sample was manually injected by means of the injection valve into a carrier flow (deionized water). The PP1 was switched off after 90 s; another 90 s was allowed to complete the reaction and transport arsines from the GLS to the U-tube. Then, at the beginning of volatilization stage the Dewar flask with liquid nitrogen was manually removed and heating of the resistance wire was switched on. Recording of signal (60s read window) started after 10s delay. As the U-tube was gradually heated, arsines evaporated, separated and entered the atomizer. At the end of measurement, the PP2 was switched on and the waste liquid was removed from the GLS; the heating was left on for another 30 s to dry moisture from the U-tube. Total time of the procedure was 343 s.
On-line pre-reduction
The HG setup of Figs. 1b,c was used. The whole procedure was similar to to the procedure with off-line pre-reduction. Apart from experiment when TGA was being added for the whole time of HG the PP3 was pumping TGA solution for 60 s to cover sample plug only, while the PP4 had been running 3 s before and in the course of the whole HG step when SFA was performed (Fig. 1c). Duration of the HG step depends on the pre-reduction coil volume: time was 230 and 330 s necessary for 2.4 and 3.5ml pre-reduction coils, respectively, when on-line pre-reduction without SFA was employed, and 90; 90; 120; 150; 160; 190; 210; 240 and 270 s for 0; 0.06; 0.5; 1; 1.4; 1.9; 2.4; 2.9 and 3.5ml pre-reduction coils, respectively, for measurements with SFA. The rest of the cycle was identical to off-line pre-reduction.
3. Results and discussion
3.1. Off-line pre-reduction
3.1.1. Comparison of L-cys and TGA
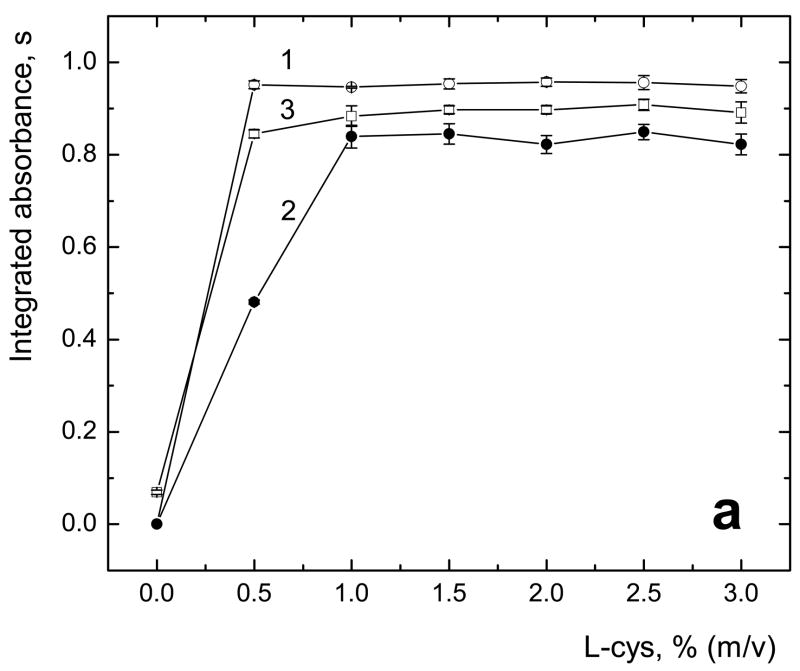
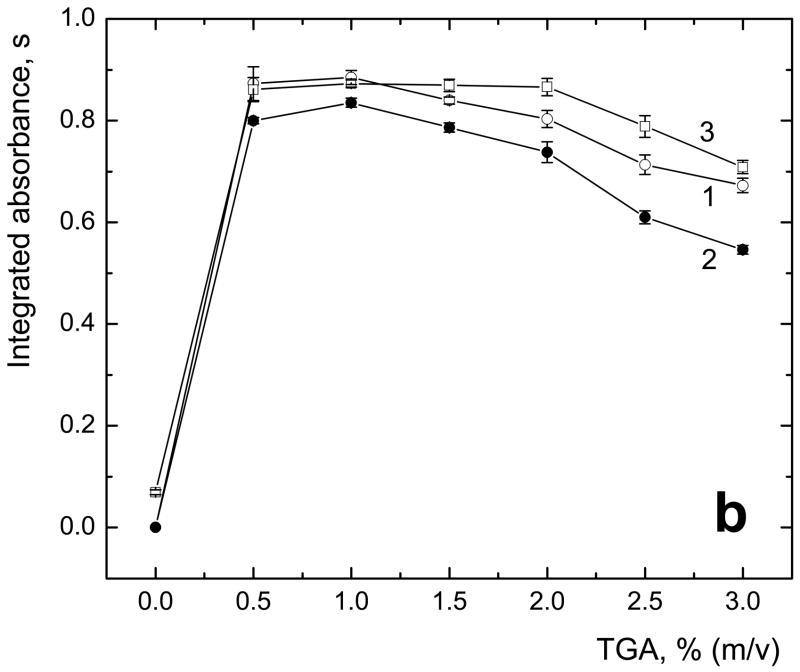
The performance of commonly used L-cys and proposed TGA as pre-reductants/reaction modifiers for HG of sum of both tri- and pentavalent arsenicals in terms of sensitivity and repeatibility was compared. Optimum concentration of both pre-reductants in TRIS·HCl buffer reaction media [13] was tested. In off-line mode of pre-reduction, solid L-cys or liquid TGA was added to final concentration to sample or standard solutions at least one hour prior to analysis because of long time of pre-reduction by L-cys reported for of all arsenicals [13,28,29]. The results are shown in Figs. 2a and 2b, respectively, for L-cys and TGA.
Fig. 2.
Optimal concentration of L-cys (a) and TGA (b) in sample solution measured with off-line pre-reduction; 1 – iAs(V); 2 – MAs(V); 3 – DMAs(V); sample loop volume 597 μl; 2 μg·l−1 As for each species; uncertainty expressed as standard deviations (SD) for n=4 measurements
As it is shown in Fig. 2a, L-cys can be used for off-line pre-reduction in concentration range between 1 % and 3 % (m/v) of L-cys added to sample, which provides complete pre-reduction of iAs(V), MAs(V) and DMAs(V). At the concentrations below 1 % (m/v), i.e. 0.057 M, longer time may be needed to complete the process of pre-reduction of all arsenicals [16,22], however, this was not tested in this study. 2 % (m/v) of L-cys was used for further experiments.
In the case of TGA, it was found that 0.5 % (m/v), i.e. 0.054 M, was sufficient for the complete pre-reduction (Fig. 2b). 1% (m/v) TGA in sample solution was found optimal and used in experiments with on-line pre-reduction. It was verified that pH of the reaction mixture in TGA modified samples behaves in the same way as with L-cys [13], it means that pH after pre-reduction by means of L-cys or TGA followed by NaBH4 reaction remained below 8.
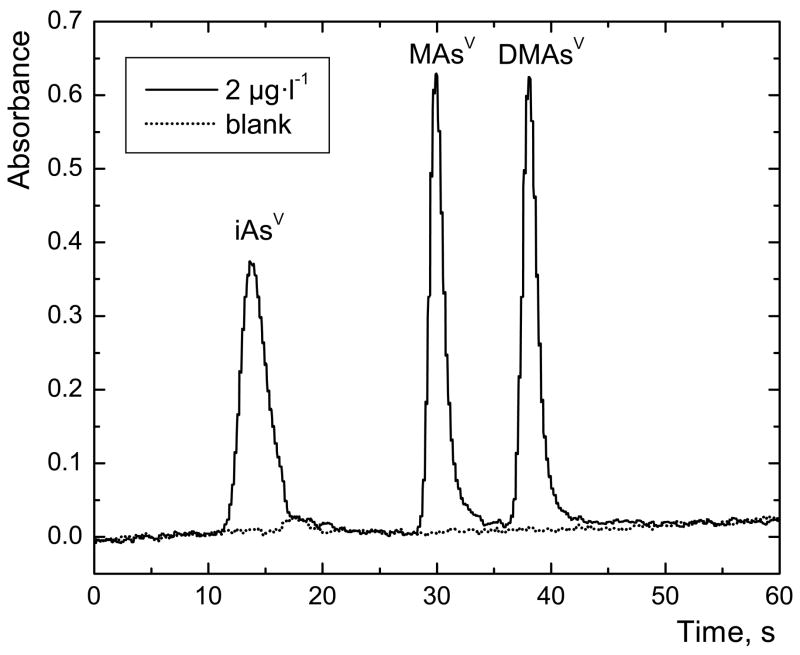
The typical chromatogram of mixed standard of pentavalent species obtained by means of off-line L-cys pre-reduction is ilustrated in Fig. 3. The peak area of blank in iAs(V) zone was subtracted from the peak area corresponding to a sample because of the measurement without background correction. The peak area of non-specific absorption (visible in blank signal) depends on concentration of the pre-reduction agent containing –SH group, concretely it is around 0.060 s at 2 % (m/v) of L-cys in sample or blank and also very similarly at 1 % (m/v) of TGA. It is interfering with arsine signal and had to be subtracted from the signal, as our instrument was not equipped with background correction.
Fig. 3.
Typical chromatogram of arsenic species measured with 2% (m/v) L-cys off-line pre-reduction; sample loop volume 597 μl; 2 μg·l−1 As for each species
No changes in sensitivities or repeatabilities (RSD) using TGA instead of commonly used L-cys were found. The relative peak area responses of arsenicals (2 μg·l−1 for each form) treated with TGA with respect to signals measured with L-cys pre-reduction (mean ± combined SD; n=8) were 98.9 ± 3.0 % for iAs(V); 100.2 ± 3.1 % for MAs(V); 101.0 ± 2.3 % for DMAs(V). More distinctly, the peak area responses corresponding to iAs(V), MAs(V) and DMAs(V) were 1.085; 1.022 and 1.019 s with relative standard deviations (RSD; n=8) 2.1; 2.4 and 1.6 % for L-cys and 1.074; 1.024 and 1.029 s with RSDs 2.0; 1.9 and 1.6 % for TGA pre-reduction.
The comparison of L-cys and TGA was also carried out by means of CF mode without cryotrapping at 20 μg·l−1 for each water standard. The absorbances corresponding to iAs(V), MAs(V) and DMAs(V) were 0.215; 0.213 and 0.206 with RSDs (n=3) 1.9; 0.4 and 1.8 % for L-cys and 0.216; 0.218 and 0.213 with RSDs 1.2; 0.8 and 0.2 % for TGA pre-reduction. The signals compared in the same way as above yielded steady state response ratios (mean ± combined SD; n=3) 100.5 ± 2.3 % for iAs(V); 102.3 ± 0.9 % for MAs(V); and 103.4 ± 1.3 % for DMAs(V), respectively.
3.1.2. Pre-reduction of TMAs(V)O
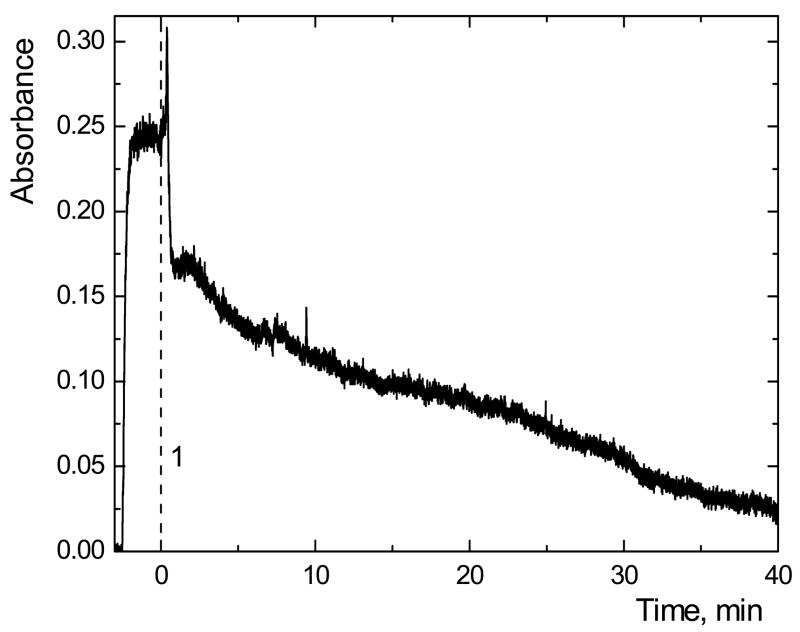
Pre-reduction of TMAs(V)O procedure by L-cys was reported to decrease greatly the response [13,29]. In our experiments, TMAs(V)O provided equal peak area response as other arsenic species from TRIS·HCl buffer medium when no pre-reduction was used, in agreement with previous report [13]. When off-line pre-reduction by TGA had been carried out one hour before measurement, the response was only approximately 7 % and further declining in time. Therefore CF mode was employed to scan the time dependence of the signal of TMAs(V)O after addition of TGA (Fig. 4). TMAs(V)O standard solution was introduced to the HG system. After reaching the steady state, TGA was added to the standard solution to final concentration 1 % (m/v) (1 in Fig. 4). The observed decrease of signal in time is presumably caused by formation of volatile trimethylarsine and its loss from the solution [32] before it is introduced into the HG system. Therefore, off-line pre-reduction should be avoided in TMAs(V)O determination. In an on-line pre-reduction setup the situation may be improved because the analyte should not be lost in the closed HG system (see Sec. 3.2.3).
Fig. 4.
Time dependence of the signal of TMAs(V)O in CF mode after addition of TGA to final concentration 1 % (m/v); 1 – TGA addition; 20 μg·l−1 of As
3.2. On-line pre-reduction
3.2.1. Preliminary investigation
To achieve an optimum concentration of 1 % (m/v) of TGA in a sample solution (see Section 3.1.1), the concentration of TGA 5 % (m/v) at the flow rate of 0.25 ml·min−1 in the separate channel was used. On-line pre-reduction was tested with 2.4 and 3.5ml pre-reduction coils (Fig. 1.b). The efficiencies of pre-reduction (mean ± combined SD; n=4) expressed as ratios of peak areas corresponding to species iAs(V), MAs(V) and DMAs(V) measured with on-line pre-reduction to off-line pre-reduction results were only 95.0 ± 2.6 %; 95.4 ± 2.4 %; 93.6 ± 1.8 % for 2.4 ml pre-reduction coil and 99.8 ± 3.3 %; 93.8 ± 2.2 %; 98.5 ± 2.7 %, respectively, for 3.5 ml pre-reduction coil. The disadvantages of this setup were incomplete pre-reduction of MAs(V) even in the larger coil, long time of pre-reduction, and increased content of total iAs in the blanks because of high consumption of all reagents. When TGA was being added over the whole time interval of HG, the non-specific absorption, proportional to TGA consumption, increased dramatically. This non-specific absorption is interfering with iAs signal, which necessitates the use of background correction [13] not available at the present instrument.
In the case when TGA is pumped only to the zone of the sample, the axial dispersion can cause that the whole zone may not contain optimum concentration of pre-reductant, as well as prolong time necessary for purging a sample from the pre-reduction coil. The suppression of an axial dispersion and better mixing can be achieved using SFA by means of air segments added to a sample and carrier flow (Fig. 1.c). Air segmentation enabled adding TGA solely to the sample zone. The efficiencies of pre-reduction (mean ± combined SD; n=4) by means of SFA with 3.5ml pre-reduction coil as compared with off-line pre-reduction results for iAs(V), MAs(V) and DMAs(V) were 97.4 ± 2.6 %; 100.1 ± 2.0 % and 99.4 ± 2.5 %. Therefore the setup with SFA was chosen for futher investigation (Fig. 5).
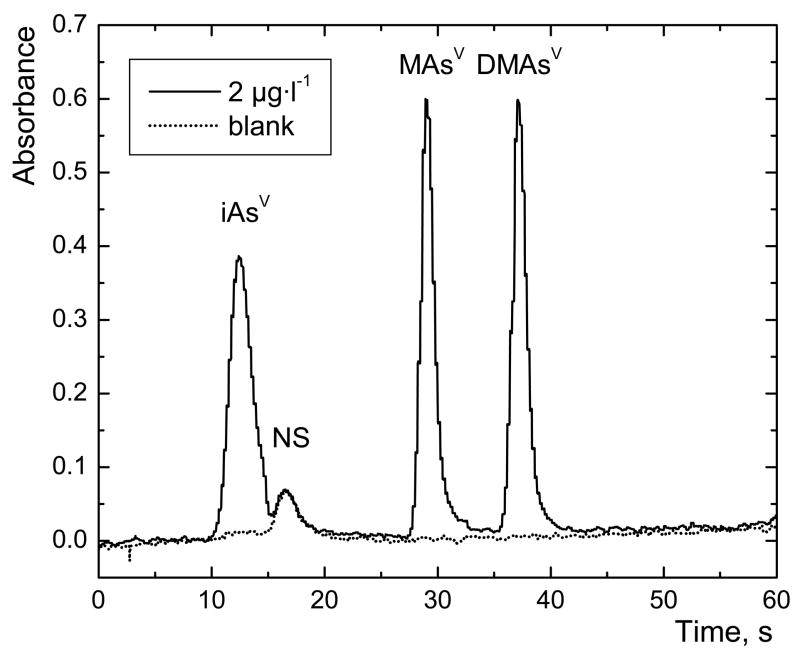
Fig. 5.
Typical chromatogram of arsenic species measured with on-line pre-reduction using SFA; NS – non-specific signal; sample loop volume 597 μl; 2 μg·l−1 As for each species; pre-reduction coil volume 2.4 ml
3.2.2. Pre-reduction coil volume
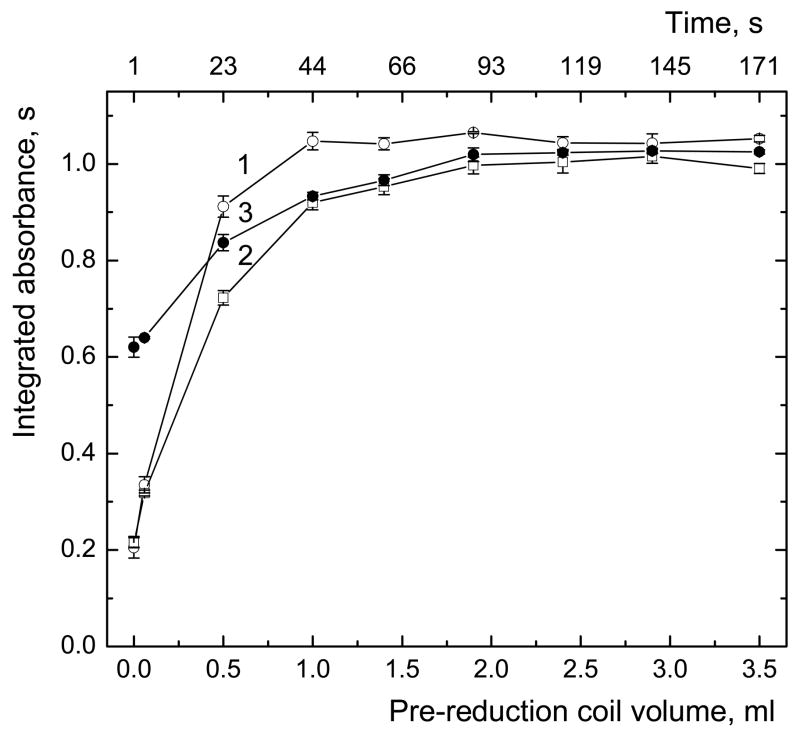
The optimal pre-reduction coil volume (and corresponding reaction times of iAs(V), MAs(V) and DMAs(V) with TGA) was studied (Fig 6). The time axis was calculated from volume of the pre-reduction coil and manifold and flow rates of reagents. It responds to time between mixing the analyte with TGA and with NaBH4. Fig. 6 indicates then that iAs(V) is fully reduced in about 45 s and complete pre-reduction of MAs(V) and DMAs(V) occurs in 115 s, therefore the 2.4ml coil was found sufficient for complete pre-reduction with SFA.
Fig. 6.
Dependence of the signal on the pre-reduction coil volume; 1 – iAs(V), 2 – MAs(V), 3 – DMAs(V); sample loop volume 597 μl; 2 μg·l−1 As for each species; pre-reduction coil volume within the range 0 - 3.5 ml; uncertainty expressed as SD (n=4)
3.2.3. Calibrations and limits of detection
Calibration graphs with cryotrapping were tested for all forms. When no pre-reduction required, the deionized water instead of TGA solution was pumped to assure identical reaction time. The comparison of slopes of calibrations shows good uniformity of sensitivities for iAs(III) with and without pre-reduction, and iAs(V), MAs(V), DMAs(V) after pre-reduction (Table 1). These results enable standardization on single species for quantification of all As forms [13] using on-line setup of pre-reduction. Lower sensitivity of TMAs(V)O with the pre-reduction is probably caused by production of trimethylarsine at earlier stage. An insufficient separation from liquid phase in the GLS and also transport losses of volatile compound before the GLS can play a role.
Table 1.
Slopes of calibrations, relative sensitivities and LODs; concentration 0.25; 0.5; 1; 2 μg·l−1 As each form
| As species | Slopec, l·s·μg−1 | Relative sensitivityd, % | LOD, ng·l−1 | LOD, pg |
|---|---|---|---|---|
| iAs(III)a | 0.420 ± 0.0040 | 91.9 ± 1.6 | 107 | 64 |
| iAs(III)b | 0.457 ± 0.0063 | 100.0 ± 1.4 | 131 | 78 |
| iAs(V)b | 0.452 ± 0.0026 | 98.9 ± 1.5 | 132 | 79 |
| MAs(V)b | 0.448 ± 0.0034 | 98.0 ± 1.6 | 44 | 26 |
| DMAs(V)b | 0.470 ± 0.0034 | 102.8 ± 1.6 | 48 | 28 |
| TMAs(V)Oa | 0.432 ± 0.0031 | 94.5 ± 1.5 | 30 | 18 |
| TMAs(V)Ob | 0.401 ± 0.0078 | 87.7 ± 2.2 | 45 | 27 |
Without pre-reduction
On-line pre-reduction
Uncertainty expressed as SD
Related to iAs(III) sensitivity measured with on-line pre-reduction; uncertainty expressed as combined SD
The limits of detection (LOD; 3σ, 0.6ml sample) for all methylated arsenicals range between 30 and 50 ng·l−1. LODs for iAs(III) and iAs(V) were 100 and 135 ng·l−1, respectively, because of non-specific absorption and iAs(III) content in the blanks. The relative LODs could be futher improved using a greater sample loop volume and in the case of iAs(III) and iAs(V) also by use of background correction and decreasing As content in the blanks.
3.2.4. Recoveries of As species in urine
A recovery study of the HG-CT method with on-line pre-reduction by TGA was tested in human urine samples (diluted 1:1 by deionized water) spiked with inividual As forms. pH of the reaction mixture after NaBH4 reaction was below 8 as in aqueous standards (see Sec. 3.1.1). The recoveries of arsenic species (Table 2) were between 96 and 105 %, which verifies the possible using of aqueous standards of single species for quantification of all studied As forms even in this matrix.
Table 2.
Recovery studya; standard addition concentration 0; 1; 2 μg·l−1 As each form
| iAs(III) | iAs(V) | MAs(V) | DMAs(V) | TMAs(V)O | |
|---|---|---|---|---|---|
| Without pre-reduction | 98.6 ± 2.9 | 0 | 0 | 0 | 95.7 ± 2.8 |
| On-line pre-reduction | 102.6 ± 2.0 | 104.6 ± 2.5 | 99.5 ± 2.1 | 96.3 ± 3.4 | – |
Relative sensitivities (%) of As species in biological matrix (diluted urine, 1:1) compared to aqueous standards; uncertainty expressed as combined SD
4. Conclusions
The selective HG procedure based on off-line pre-reduction by TGA is capable of resolution and quantification of inorganic and methylated arsenicals. TGA provides much faster reaction with all pentavalent arsenicals (1–2 min) compared to commonly used L-cys (30–60 min) [13,28], which makes it much more suitable for on-line pre-reduction at room temperature. The drawbacks of the TGA agent, its malodour and toxicity, were found quite tolerable as the handling is minimized in the on-line arrangement. Optimal arrangement of the HG setup associated with on-line pre-reduction step was accomplished by means of SFA which permits better mixing, suppresses the axial dispersion of the sample plug in the manifold and thus allows to shorten substantially the time of analysis. Standard calibrations measured with or without on-line pre-reduction indicated the uniform and equal sensitivities. The possibility of standardization by water standards of single species (e.g. iAs(III)) for quantification of all other toxicologically important As forms in urine was demonstrated in the recovery study. Despite the analysis being prolonged by 2 min as compared to off-line procedure, on-line pre-reduction simplifies sample pretreatment and increases sample throughput. This setup of pre-reduction can be easily adopted for an automated system for the oxidation state specific speciation of inorganic and methylated arsenicals by selective hydride generation-cryotrapping-AAS [13,14]. Another prospective application of presented on-line pre-reduction by TGA would be in the As speciation analysis methods employing post-separation HG approach, where high and uniform generation efficiency for all forms would be highly desirable.
Acknowledgments
This work was supported by NIH-FIRCA project No. 1 R03 TW007057-01; Academy of Sciences of the Czech Republic, Institutional research plan No. AV0Z 40310501 and GA ASCR grant No. A400310507; the Grant Agency of Charles University (Project GA UK No. 133008) and by the Czech Ministry of Education, Youths and Sports (project MSM 0021620857). The authors are much obliged to Dr. Jiøí Dědina for valuable comments to the manuscript and to Professor William R. Cullen, Department of Chemistry, University of British Columbia, who provided TMAs(V)O for this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hughes MF. Biomarkers of Exposure: A Case Study with Inorganic Arsenic. Environ Health Persp. 2006;114:1790–1796. doi: 10.1289/ehp.9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hughes MF. Arsenic toxicity and potential mechanisms of action. Toxicol Lett. 2002;133:1–16. doi: 10.1016/s0378-4274(02)00084-x. [DOI] [PubMed] [Google Scholar]
- 3.Tsalev DL, Zaprianov ZK. Atomic Absorption Spectrometry in Occupational and Environmental Health Practice. Vol. 1. CRC Press; Florida: 1983. [Google Scholar]
- 4.Valenzuela OL, Borja-Aburto VH, Garcia-Vargas GG, Cruz-Gonzales MB, Garcia-Montalvo EA, Calderon-Aranda ES, Del Razo LM. Urinary Trivalent Methylated Arsenic Species in a Population Chronically Exposed to Inorganic Arsenic. Environ Health Persp. 2005;113:250–254. doi: 10.1289/ehp.7519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cullen WR, McBride BC, Reglinski J. The reaction of methylarsenicals with thiols - some biological implications. J Inorg Biochem. 1984;21:179–194. [Google Scholar]
- 6.Devesa V, Del Razo LM, Adair B, Drobná Z, Waters SB, Hughes MF, Stýblo M, Thomas DJ. Comprehensive analysis of arsenic metabolites by pH-specific hydride generation atomic absorption spectrometry. J Anal At Spectrom. 2004;19:1460–1467. [Google Scholar]
- 7.Thomas DJ, Li JX, Waters SB, Xing WB, Adair BM, Drobná Z, Devesa V, Stýblo M. Arsenic (+3 oxidation state) methyltransferase and the methylation of arsenicals. Exp Biol Med. 2007;232:3–13. [PMC free article] [PubMed] [Google Scholar]
- 8.Liu ZJ, Stýblo M, Rosen BP. Methylarsonous acid transport by aquaglyceroporins. Environ Health Persp. 2006;114:527–531. doi: 10.1289/ehp.8600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drobná Z, Waters SB, Devesa V, Harmon AW, Thomas DJ, Stýblo M. Metabolism and toxicity of arsenic in human urothelial cells expressing rat arsenic (+3 oxidation state)-methyltransferase. Toxicol Appl Pharmacol. 2005;207:147–159. doi: 10.1016/j.taap.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braman RS, Foreback CC. Methylated Forms of Arsenic in the Enviroment. Science. 1973;182:1247–1249. doi: 10.1126/science.182.4118.1247. [DOI] [PubMed] [Google Scholar]
- 11.Razo LM, Stýblo M, Cullen WR, Thomas DJ. Determination of Trivalent Methylated Arsenicals in Biological Matrices. Toxicol Appl Pharmacol. 2001;174:282–293. doi: 10.1006/taap.2001.9226. [DOI] [PubMed] [Google Scholar]
- 12.Gong ZL, Lu XF, Cullen WR, Le XC. Unstable trivalent arsenic metabolites, monomethylarsonous acid and dimethylarsinous acid. J Anal At Spectrom. 2001;16:1409–1413. [Google Scholar]
- 13.Matoušek T, Hernández-Zavala A, Svoboda M, Langerová L, Adair BM, Drobná Z, Thomas DJ, Stýblo M, Dědina J. Oxidation state specific generation of arsines from methylated arsenicals based on L-cysteine treatment in buffered media for speciation analysis by hydride generation - automated cryotrapping - gas chromatography - atomic absorption spectrometry with the multiatomizer. Spectrochim. Acta, Part B. 2008;63:396–406. doi: 10.1016/j.sab.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernández-Zavala A, Matoušek T, Drobná Z, Paul DS, Walton FS, Adair BM, Dědina J, Thomas DJ, Stýblo M. Speciation analysis of arsenic in biological matrices by automated hydride generation-cryotrapping-atomic absorption spectrometry with multiple microflame quartz tube atomizer (multiatomizer) J Anal At Spectrom. 2008;23:342–351. doi: 10.1039/b706144g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howard AG. (Boro)Hydride Techniques in Trace Element Speciation. J Anal At Spectrom. 1997;12:267–272. [Google Scholar]
- 16.Frank J, Krachler M, Shotyk W. Direct determination of arsenic in acid digests of plant and peat samples using HG-AAS and ICP-SF-MS. Anal Chim Acta. 2005;530:307–316. [Google Scholar]
- 17.González JC, Lavilla I, Bendicho C. Evaluation of non-chromatograpfic approaches for speciation of extractable As(III) and As(V) in environmental solid samples by FI-HGAAS. Talanta. 2003;59:525–534. [PubMed] [Google Scholar]
- 18.Sigrist ME, Beldoménico HR. Determination of inorganic arsenic species by flow injection hydride generation atomic absorption spectrometry with variable sodium tetrahydroborate concentrations. Spectrochim Acta, Part B. 2004;59:1041–1045. [Google Scholar]
- 19.Abrankó L, Stefánka Z, Fodor P. Possibilities and limits of the simultaneous determaination of As, Bi, Ge, Sb, Se, and Sn by flow injection-hydride generation-inductively coupled plasma-time-of-flight mass spectrometry (FI-HG-ICP-TOFMS) Anal Chim Acta. 2003;493:13–21. [Google Scholar]
- 20.Maity S, Chakravarty S, Thakur P, Gupta KK, Bhattacharjee S, Roy BC. Evaluation and standardisation of a simple HG-AAS method for rapid speciation of As(III) and As(V) in some contamined groundwater samples of West Bengal, India. Chemosphere. 2004;54:1199–1206. doi: 10.1016/j.chemosphere.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 21.Bortoleto GG, Cadore S. Determination of total inorganic arsenic in water using on-line pre-concentration and hydride-generation atomic absorption spectrometry. Talanta. 2005;67:169–174. doi: 10.1016/j.talanta.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Chen H, Brindle ID, Le XC. Prereduction of Arsenic(V) to Arsenic(III), Enhancement of the Signal, and Reduction of Interferences by L-Cysteine in the Determination of Arsenic by Hydride Generation. Anal Chem. 1992;64:667–672. [Google Scholar]
- 23.Cordos EA, Frentiu T, Ponta M, Abraham B, Marginean J. Optimisation of analytical parametres in inorganic arsenic (III and V) speciation by hydride generation using L-cysteine as prereducing agent in diluted HCl medium. Chem Speciation and Bioavailability. 2006;18:1–9. [Google Scholar]
- 24.Welz B, Šucmanová M. L-cysteine as a Reducing and Releasing Agent for the Determination of Antimony and Arsenic Using Flow Injection Hydride Generation Atomic Absorption Spectrometry, Part 1. Optimization of the Analytical Parameters. Analyst. 1993;118:1417–1423. [Google Scholar]
- 25.Pitzalis E, Ajala D, Onor M, Zamboni R, Ulivo AD’. Chemical Vapour Generation of Arsane in the Presence of L-Cysteine. Mechanistic Studies and Their Analytical Feedback. Anal Chem. 2007;79:6324–6333. doi: 10.1021/ac070513p. [DOI] [PubMed] [Google Scholar]
- 26.Howard AG, Salou C. Cysteine enhancement of the cryogenic trap hydride AAS determination of dissolved arsenic species. Anal Chim Acta. 1996;333:89–96. [Google Scholar]
- 27.Carrero P, Malave A, Burguera JL, Burguera M, Rondon C. Determination of various arsenic species by flow injection hydride generation atomic absorption spectrometry: investigation of the effects of the acid concentration of different reaction media on the generation of arsines. Anal Chim Acta. 2001;438:195–204. [Google Scholar]
- 28.Howard AG, Salou C. Arsenic speciation by cryogenic trap hydride generation atomic absorption spectroscopy: performance enhancement by pre-derivatization. J Anal At Spectrom. 1998;13:683–686. [Google Scholar]
- 29.Tsalev DL, Sperling M, Welz B. Flow-injection hydride generation atomic absorption spectrometric study of the automated on-line pre-reduction of arsenate, methylarsonate and dimethylarsinate and high-performance liquid chromatographic separation of their L-cysteine complexes. Talanta. 2000;51:1059–1068. doi: 10.1016/s0039-9140(00)00297-6. [DOI] [PubMed] [Google Scholar]
- 30.Brindle ID, Alarabi H, Karshman S, Le XC, Zheng SG, Chen HW. Combined Generator/Separator for Continuous Hydride Generation - Application to Online Pre-Reduction of Arsenic(V) and Determination of Arsenic in Water by Atomic Emission-Spectrometry. Analyst. 1992;117:407–411. [Google Scholar]
- 31.Nielsen S, Hansen EH. Determination of As(III) and As(V) by flow injection-hydride generation-atomic absorption spectrometry via on-line reduction of As(V) by KI. Anal Chim Acta. 1997;343:5–17. [Google Scholar]
- 32.Cullen WR, McBride BC, Reglinski J. The reduction of trimethylarsine oxide to trimethylarsine by thiols: a mechanistic model for the biological reduction of arsenicals. J Inorg Biochem. 1984;21:45–60. [Google Scholar]
- 33.Matoušek T, Dědina J, Selecká A. Multiple microflame quartz tube atomizer --further development towards the ideal hydride atomizer for atomic absorption spectrometry. Spectrochim Acta, Part B. 2002;57:451–462. [Google Scholar]