Abstract
The nutritionally variant streptococci (NVS) are a fastidious group of bacteria first recognized in the early 1960s in the blood cultures of patients with subacute bacterial endocarditis. Since that time, the NVS have been implicated in 5 to 6% of all cases of human bacterial endocarditis. The NVS possess membrane-associated amphipathic molecules different from those described for other streptococci. Unitl recently, chemical characterization of these new amphipathic polymers was hampered by unsuccessful attempts at isolating large quantities of these molecules in a form free from other bacterial components. Presently, stationary-phase-culture supernatants provide an optimum source of crude material for amphiphile purification procedures. Hydrophobic-interaction chromatography in conjunction with immunoaffinity chromatography yields an NVS serotype I amphiphile preparation free of contaminants, as determined by immunoelectrophoretic and chemical analyses. Tandem crossed immunoelectrophoresis of the purified extracellular NVS amphiphile demonstrated that it is immunologically similar to the intracellular amphiphile. Finally, this amphiphile serves as the NVS serotype I antigen.
Full text
PDF

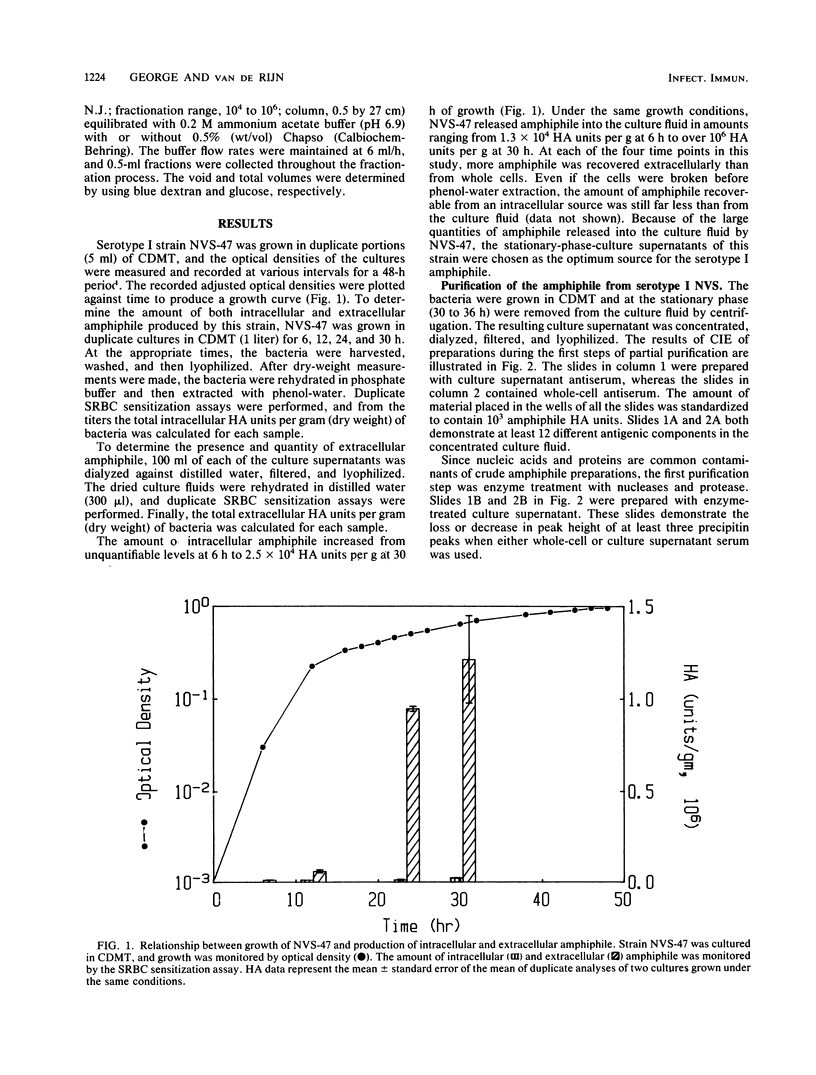

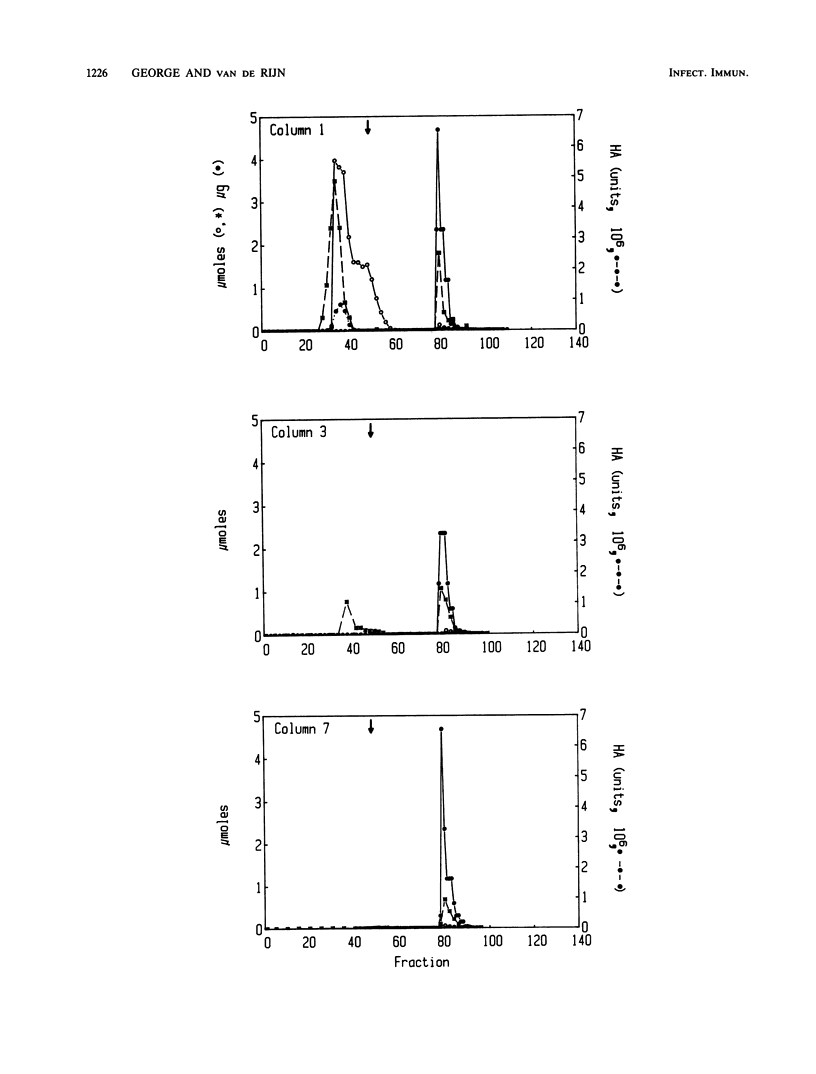

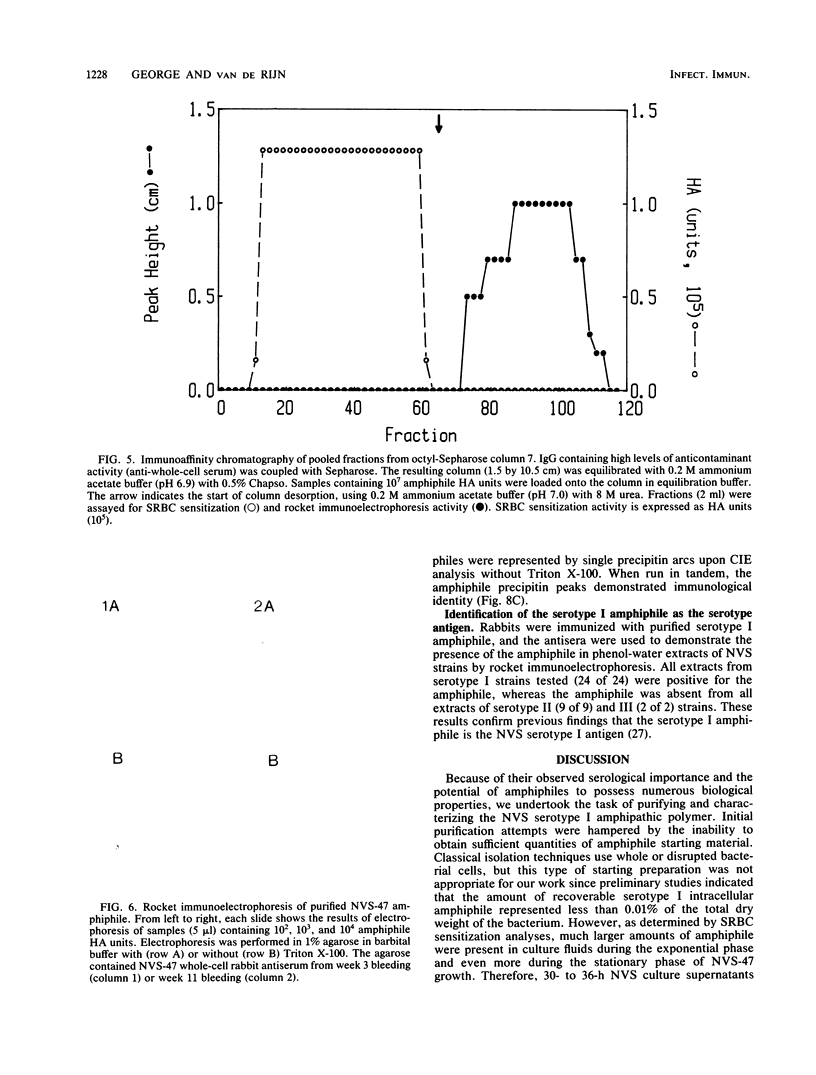


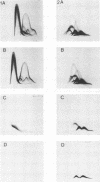
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouvet A., Ryter A., Fréhel C., Acar J. F. Nutritionally deficient streptococci: electron microscopic study of 14 strains isolated in bacterial endocarditis. Ann Microbiol (Paris) 1980 Sep-Oct;131B(2):101–120. [PubMed] [Google Scholar]
- Bouvet A., van de Rijn I., McCarty M. Nutritionally variant streptococci from patients with endocarditis: growth parameters in a semisynthetic medium and demonstration of a chromophore. J Bacteriol. 1981 Jun;146(3):1075–1082. doi: 10.1128/jb.146.3.1075-1082.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey R. B., Gross K. C., Roberts R. B. Vitamin B6-dependent Streptococcus mitior (mitis) isolated from patients with systemic infections. J Infect Dis. 1975 Jun;131(6):722–726. doi: 10.1093/infdis/131.6.722. [DOI] [PubMed] [Google Scholar]
- Cayeux P., Acar J. F., Chabbert Y. A. Bacterial persistence in streptococcal endocarditis due to thiol-requiring mutants. J Infect Dis. 1971 Sep;124(3):247–254. doi: 10.1093/infdis/124.3.247. [DOI] [PubMed] [Google Scholar]
- ELLIOTT S. D. TEICHOIC ACID AND THE GROUP ANTIGEN OF LACTIC STREPTOCOCCI (GROUP N). Nature. 1963 Dec 21;200:1184–1185. doi: 10.1038/2001184a0. [DOI] [PubMed] [Google Scholar]
- FRENKEL A., HIRSCH W. Spontaneous development of L forms of streptococci requiring secretions of other bacteria or sulphydryl compounds for normal growth. Nature. 1961 Aug 12;191:728–730. doi: 10.1038/191728a0. [DOI] [PubMed] [Google Scholar]
- Fischer W., Koch H. U., Haas R. Improved preparation of lipoteichoic acids. Eur J Biochem. 1983 Jul 1;133(3):523–530. doi: 10.1111/j.1432-1033.1983.tb07495.x. [DOI] [PubMed] [Google Scholar]
- GOODMAN J. W., KABAT E. A. Immunochemical studies on cross-reactions of antipneumococcal sera. III. The effect of variation in molecular weight on the cross-reactivity of dextran with type II antipneumococcal serum. J Immunol. 1960 Oct;85:342–346. [PubMed] [Google Scholar]
- George R. H. The isolation of symbiotic streptococci. J Med Microbiol. 1974 Feb;7(1):77–83. doi: 10.1099/00222615-7-1-77. [DOI] [PubMed] [Google Scholar]
- Hewett M. J., Knox K. W., Wicken A. J. Studies on the group F antigen of lactobacilli: detection of antibodies by haemagglutination. J Gen Microbiol. 1970 Mar;60(3):315–322. doi: 10.1099/00221287-60-3-315. [DOI] [PubMed] [Google Scholar]
- Kessler R. E., Shockman G. D. Precursor-product relationship of intracellular and extracellular lipoteichoic acids of Streptococcus faecium. J Bacteriol. 1979 Feb;137(2):869–877. doi: 10.1128/jb.137.2.869-877.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R. E., van de Rijn I. Quantitative immunoelectrophoretic analysis of Streptococcus pyogenes membrane. Infect Immun. 1979 Dec;26(3):892–902. doi: 10.1128/iai.26.3.892-902.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Hewett M. J., Wicken A. J. Studies on the group F antigen of lactobacilli: antigenicity and serological specificity of teichoic acid preparations. J Gen Microbiol. 1970 Mar;60(3):303–313. doi: 10.1099/00221287-60-3-303. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Markham J. L., Knox K. W., Wicken A. J., Hewett M. J. Formation of extracellular lipoteichoic acid by oral streptococci and lactobacilli. Infect Immun. 1975 Aug;12(2):378–386. doi: 10.1128/iai.12.2.378-386.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy L. R., Bottone E. J. Bacteremia and endocarditis caused by satelliting streptococci. Am J Clin Pathol. 1974 May;61(5):585–591. doi: 10.1093/ajcp/61.5.585. [DOI] [PubMed] [Google Scholar]
- Rothfield L., Pearlman-Kothencz M. Synthesis and assembly of bacterial membrane components. A lipopolysaccharide-phospholipid-protein complex excreted by living bacteria. J Mol Biol. 1969 Sep 28;44(3):477–492. doi: 10.1016/0022-2836(69)90374-x. [DOI] [PubMed] [Google Scholar]
- Rotta J., Krause R. M., Lancefield R. C., Everly W., Lackland H. New approaches for the laboratory recognition of M types of group A streptococci. J Exp Med. 1971 Nov 1;134(5):1298–1315. doi: 10.1084/jem.134.5.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri L. J., Craig R. A., Ingram L. O., Hoffmann E. M., Bleiweis A. S. Purification of lipoteichoic acids by using phosphatidyl choline vesicles. Infect Immun. 1978 Oct;22(1):107–118. doi: 10.1128/iai.22.1.107-118.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WICKEN A. J., ELLIOTT S. D., BADDILEY J. The identity of streptococcal group D antigen with teichoic acid. J Gen Microbiol. 1963 May;31:231–239. doi: 10.1099/00221287-31-2-231. [DOI] [PubMed] [Google Scholar]
- van de Rijn I., Bouvet A. Characterization of a pH-dependent chromophore from nutritionally variant streptococci. Infect Immun. 1984 Jan;43(1):28–31. doi: 10.1128/iai.43.1.28-31.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rijn I., George M., Bouvet A., Roberts R. B. Enzyme-linked immunosorbent assay for the detection of antibodies to nutritionally variant streptococci in patients with endocarditis. J Infect Dis. 1986 Jan;153(1):116–121. doi: 10.1093/infdis/153.1.116. [DOI] [PubMed] [Google Scholar]
- van de Rijn I., George M. Immunochemical study of nutritionally variant streptococci. J Immunol. 1984 Oct;133(4):2220–2225. [PubMed] [Google Scholar]