Abstract
Background
Mutations in the PTEN gene cause two disorders that predispose to cancer, Bannayan–Riley–Ruvalcaba and Cowden syndromes. Some patients with a PTEN mutation have only macrocephaly and autism, but they may still be at risk for neoplasms. Vascular anomalies occur in patients with a PTEN mutation, but they have not been systematically studied or precisely defined.
Method
We analysed the clinical and radiological features of the vascular anomalies in 26 patients with PTEN mutations who were either seen or had their medical records reviewed at Children's Hospital Boston.
Results
All 23 patients who had their head circumference measured were macrocephalic, and all 13 male patients who were fully examined had penile freckling. Vascular anomalies were found in 14/26 (54%) of patients: 8/14 (57%) had multiple lesions and 11/13 (85%) who had cross‐sectional imaging had intramuscular vascular lesions. Radiographic studies showed that 12/14 (86%) were fast‐flow vascular anomalies, and angiography typically showed focal segmental dilatation of draining veins. Excessive ectopic fat in the vascular anomalies was present in 11/12 (92%) of patients on CT or MRI. Intracranial developmental venous anomalies (DVAs) were found in 8/9 (89%) of patients who had brain MRI with contrast.
Conclusions
Vascular anomalies in patients with a PTEN mutation are typically multifocal intramuscular combinations of fast‐flow channels and ectopic fat. Cerebral DVAs are very common. PTEN mutational analysis should be considered for all macrocephalic patients with fast‐flow vascular anomalies or multiple intracranial DVAs.
Keywords: PTEN hamartoma syndrome, Bannayan–Riley–Ruvalcaba syndrome, Cowden syndrome, vascular malformations, arteriovenous malformations
The PTEN (phosphatase and tensin homologue) gene on chromosome 10q23.31 encodes a tumour suppressor protein that regulates the phosphoinositide‐3 kinase (PI3K) pathway, which is involved in cell‐cycle regulation, angiogenesis, and cellular growth and proliferation.1PTEN was isolated and cloned in 1997,2,3,4 and germline heterozygous mutations have been found to be responsible for both Bannayan–Riley–Ruvalcaba syndrome (BRRS) and Cowden syndrome (CS).
BRRS is characterised by macrocephaly, macrosomia at birth, lipomas, hamartomatous intestinal polyposis, proximal myopathy, variable degrees of developmental delay, and pigmented macules on the glans penis.5,6,7 Pathognomonic criteria for the diagnosis of CS include mucocutaneous lesions, such as facial trichilemmomas, cobblestone‐like papules on the oral mucosa, acral keratoses and various papillomatous lesions. Major criteria include adult‐onset Lhermitte–Duclos disease, macrocephaly, thyroid cancer (especially follicular carcinoma), breast cancer and endometrial carcinoma. Lipomas and hamartomatous intestinal polyps are considered minor criteria for the diagnosis of Cowden syndrome.8
Following the identification of the PTEN gene, Marsh et al.9,10 found that 81% of patients with Cowden syndrome, 60% of patients with BRRS, and 91% of patients in families with features of both BRRS and Cowden syndrome had germline mutations in the PTEN gene. These findings confirmed that BRRS and Cowden syndrome are allelic disorders, now designated “PTEN hamartoma–tumour syndrome” (PHTS).10 Some patients with PTEN mutations have macrocephaly and autism, without any of the other clinical features associated with PHTS.11 Although the long‐term follow‐up of these patients has not been reported, the risk of cancer is likely to be similar to those of patients with PHTS.
A variety of vascular anomalies, termed “arteriovenous malformation” (AVM), “vascular hamartoma” or “haemangioma”, have been reported in some patients with BRRS12,13 or Cowden syndrome.14,15,16 Nevertheless, there have been no systematic studies of the frequency or the precise types of vascular anomalies that occur in these patients. Monitoring for vascular anomalies is not currently part of the “standard of care” for these patients.5,8 Moreover, it is unclear which patients with vascular anomalies should be tested for a PTEN mutation.
The purpose of this study was to characterise the cerebral and peripheral vascular anomalies in PHTS that may merit close follow‐up or possible intervention. Furthermore, we attempted to determine the clinical and radiological features that should prompt clinicians to test for a PTEN mutation.
Methods
This study was approved by the Committee on Clinical Investigation at Children's Hospital Boston.
Subjects
This study included all patients with a PTEN mutation who had been seen or had their clinical findings reviewed at Children's Hospital Boston (including the Vascular Anomalies Center and the Division of Genetics) between 1997 and 2005, regardless of their phenotype. All these patients were tested for PTEN mutations between July 1999 and October 2005. Before November 2002, testing was performed at a research laboratory directed by Dr C Eng (Dana‐Farber Cancer Institute, Boston, Massachusetts, USA, and Ohio State University, Columbus, Ohio, USA), except for one patient who was tested in North Adelaide, Australia. Subsequently, most samples were sent to a commercial diagnostic laboratory (GeneDx, Gaithersburg, Maryland, USA).
Classification of vascular anomalies
We classified all anomalies based on the binary system accepted by the International Society for the Study of Vascular Anomalies: vascular tumours, which are endothelial neoplasms with increased cell proliferation and include haemangiomas, haemangioendotheliomas and angiosarcomas; and vascular malformations, which are composed of abnormally formed vascular channels and subclassified according to the type of vessels involved (ie, arterial, capillary, lymphatic or venous, or a combination of the different types of channels, such as arteriovenous and capillary‐lymphatico‐venous malformations).17,18
All available neuroradiological images were reviewed by a neuroradiologist (CDR), and the angiograms, MRI, CT and sonographic images of the torso and limbs were reviewed by two vascular/interventional radiologists (PEB and AA). Vascular anomalies were characterised according to published imaging criteria.19,20,21 The angioarchitecture of the vascular anomalies was categorised based on the structure of the arterial and venous components. We defined three types of fast‐flow vascular anomalies: (1) arteriovenous fistula, in which no more than three arteries feed into a single draining vein; (2) arteriolovenous fistula, in which several small arteries shunt into a single draining vein; and (3) arteriolovenulous fistula, in which the afferent arterioles branching from an artery are connected to their corresponding venules, all of which coalesce to form a draining vein.22 Ectopic fat was noted when MRI or CT studies showed abnormal collections of tissue with imaging characteristics typical of adipose tissue.
Results
In total, 26 patients (15 male, 11 female) were included in the study. We excluded 1 patient who had an intronic mutation of unknown significance (IVS1+35C→T) and another patient who had a mutation in the PTEN promoter, which has been reported to be a normal variant (−903G→A).23 The major clinical features and PTEN mutations are listed in table 1. Table 2 shows the presence of features that constitute the diagnostic criteria for Cowden syndrome and BRRS and the age at which each patient was last evaluated. Most of the patients presented with macrocephaly, developmental delay, or a “mass” lesion. One patient (patient 7) had been previously misdiagnosed as having Cohen syndrome, but was found to have a PTEN mutation following the development of an ovarian granulosa cell tumour. Two patients (patients 8 and 13) were initially labelled as having Sotos syndrome. Patient 8 was tested for PTEN mutation when it was discovered that her father had penile freckling, and patient 13 was tested because of macrocephaly. In addition to a germline mutation in PTEN, patient 25 had a different somatic PTEN mutation in trans in the tissues from the muscles, epidermal naevus and vessels of the affected limb, as previously reported.24
Table 1 Summary of clinical and neuroradiological findings in patients with PTEN mutations.
| Patient | Sex | Age at diagnosis* (years)† | Symptoms and signs at first visit leading to diagnosis | Age at first PHTS symptom/sign | Height (centile) | Weight (centile) | OFC (centile) | PF | MR/ GDD | PDD | VA | Brain MRI | Indication for brain MRI | PTEN mutation | Exon/ IVS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 5½ | Hypotonia; developmental delay; “haemangioma” and lipoma (both excised); penile freckling | 2 years; lipoma in groin | >97 | >97 | >97 | Yes | No | No | Yes | Small bilateral frontal and left cerebellar DVAs | Macrocephaly; abnormal EEG without clinical seizures | A34D | Exon 2 |
| 2 | M | <25 months | Macrocephaly; lipoma; “haemangioma”; penile freckling | <25 months | <3 | 10–25 | >97 | Yes | No | No | Yes | Not available | N/A | M35T | Exon 2 |
| 3 | F | 11½ | Macrocephaly; lipoma; “capillary haemangioma” on scalp; Graves disease | Prenatal; macrocephaly | 90 | 50–75 | >97 | N/A | No | No | Yes | Not available | N/A | IVS3+1G→A | Intron 3 |
| 4 | M | 5 | Macrocephaly; hypotonia; lipoma; penile freckling | Possibly at birth; cervical lipoma | 90–97 | 50–75 | >97 | Yes | No | Yes | No | Small left parieto‐occipital and bilateral cerebellar DVAs | Macrocephaly; speech delay; cervical mass | IVS3+5G→A | Intron 3 |
| 5 | F | 22½ | Haematoma of arm with minor injury; gastrointestinal mucosal ganglioneuromas | 15 years ; vascular malformation presenting as haematoma after a minor injury | 25–50 | >97 | ? | N/A | No | No | Yes | (NC) AC I; punctate T2 prolongation (bright spots) in white matter, possibly migraine‐related | Headache; worsening diplopia | c.220delA | Exon 4 |
| 6 | F | 54½ | AVM in right foot and pelvis | 16 years; AVM in sole of right foot | ? | ? | ? | N/A | No | No | Yes | Not available | N/A | c.302_304 delTCAinsCC | Exon 5 |
| 7 | F | 17½ | Macrocephaly; learning disability; ovarian granulosa cell tumour | 8 years; macrocephaly | 75 | 90 | >97 | N/A | Yes | No | Yes | (NC) Normal | Macrocephaly; motor difficulties; failure to thrive | L108P | Exon 5 |
| 8 | F | 3 | Macrocephaly; hypotonia; gross motor delay; vascular staining on right flank; paternal penile freckles | Birth; vascular stain | 90–97 | 90 | >97 | N/A | No | Yes | Yes | DVAs; slightly prominent CSF spaces,spontaneously resolved with time | Macrocephaly; Hypotonia | c.346_358dup | Exon 5 |
| 9 | M | 38 | Penile freckling; suspicion in daughter (patient 8) | Asymptomatic (except penile freckles) | 90–97 | ? | >97 | Yes | No | No | No | Not available | N/A | c.346_358dup | Exon 5 |
| 10 | M | 20 | Macrocephaly; GI polyps; mucosal neuroma, ganglioneuroma | 8 years; bloody diarrhoea (probably from GI polyps) | 75–90 | 90–97 | >97 | Yes | No | No | No | Small right cerebellar DVAs | Unclear | R130Q | Exon 5 |
| 11 | M | 15 | Macrocephaly; paraspinal mass/AVM | Birth; pinkish‐blue cutaneous stain | 25–50 | 75 | >97 | ? | No | No | Yes | [AC I on cervical spine MRI; Brain MRI not available] | Vascular anomaly in cervical/thoracic region (spine MRI) | R130X | Exon 5 |
| 12 | M | 5 | Macrocephaly; lipoma/lipoblastoma; penile freckling | 4.5 months; lipoma | 95 | >95 | >97 | Yes | No | No | No | (NC) Normal | BRRS with lipomatous growth on left tonsil | R130X | Exon 5 |
| 13 | F | 15 | Macrocephaly; language delay | Prenatal; macrocephaly | 25–50 | 75–90 | >97 | N/A | No | No | No | DVAs associated with left frontal cavernous malformation | Subcortical lesion on prior head CT | G132A | Exon 5 |
| 14 | F | 3½ | Frontal bossing; recurrent GI polyps; occult blood in stool; hypoalbuminaemia | 2 years; occult blood in stool and eosinophilia | 10–25 | 3–10 | >97 | N/A | No | No | No | Not available | N/A | IVS5‐2A→C | Intron 5 |
| 15 | M | 12 | Penile freckling; right lower limb AVM | 2 years; AVM right thigh | 25–50 | 25 | >97 | Yes | No | No | Yes | Left frontal lobe and right basal ganglia DVAs | Thigh AVM, to rule out brain AVM | c.499_505del7 | Exon 6 |
| 16 | F | 8½ | GI polyps; maternal uncle with PTEN mutation | 7 years; multiple GI polyps | <3 | 25 | >97 | N/A | No | No | No | (NC) Normal | Macrocephaly; large anterior fontanelle at 23 months | c.513 InsA | Exon 6 |
| 17 | M | 1 | Penile freckling | 1 year; penile freckling | 90–97 | 50–75 | >97 | Yes | No | No | No | (NC) Normal | Macrocephaly; gross motor delay | R173C | Exon 6 |
| 18 | M | 7½ | Lipoma; penile freckling | 2.5 years; lipoma | 25–50 | 75 | >97 | Yes | No | No | No | Not available | N/A | Y180X | Exon 6 |
| 19 | F | 37 | Son's diagnosis (patient 18); facial skin tags | 29 years; lipoma | 50–75 | >97 | >97 | N/A | No | No | No | Not available | N/A | Y180X | Exon 6 |
| 20 | F | 6½ | Macrocephaly; vascular lesion on leg | 4.5 years; macrocephaly, vascular lesion | 10–25 | 50 | >97 | N/A | No | No | Yes | Not available | N/A | L181P | Exon 6 |
| 21 | F | 5 | Left thigh mass; adipose tissue and lymphoid follicle on biopsy | 3.5 years; left thigh mass | 50–75 | 75–90 | >97 | N/A | Yes | Yes | Yes | Normal | Macrocephaly; GDD | R233X | Exon 7 |
| 22 | M | 23 | Swelling on left lower face and neck | 15 years; left cheek AVM | ? | ? | ? | ? | No | No | Yes | Not available | N/A | IVS7+1G→T | Intron 7 |
| 23 | M | 6½ | Macrocephaly; developmental delay; penile freckling | 17 months; motor and language delay | 90–97 | >97 | >97 | Yes | No | No | Yes | DVAs; Dural arteriovenous fistula; Sinus pericranii | BRRS and headache; prominent veins on face, intracranial vascular anomaly? | c.955insA | Exon 8 |
| 24 | M | 14 | Macrocephaly; thyroid nodules; lingual papules; penile freckling | 13 years; thyroid nodules | 25–50 | 50–75 | >97 | Yes | No | No | No | Not available | N/A | c.968dupA | Exon 8 |
| 25 | M | 9½ | Macrocephaly; epidermal naevus on right leg | Birth; macrocephaly, epidermal naevus on right leg | <3 | <3 | >97 | Yes | No | No | Yes | Not available | N/A | R335X (germline), R130X (somatic;‐ in vascular malformation) in trans | Exon 8 |
| 26 | M | 3 | Macrocephaly; PDD; penile freckles | Prenatal; “large head size” on prenatal ultrasound | 75–90 | 97 | >97 | Yes | Yes | Yes | No | Right temporal and parietal DVAs | Macrocephaly; GDD; hypotonia; PDD | R335X | Exon 8 |
?, Unknown; AC I, Arnold–Chiari malformation type I; AVM, arteriovenous malformation; DVAs, developmental venous anomalies; CSF, cerebrospinal fluid; CNS, central nervous system; GDD, global developmental delay; GI, gastrointestinal; IVS, intervening sequence (ie intron); MR, mental retardation; OFC, occipitofrontal circumference; N/A, not applicable; NC, non‐contrast brain MRI; PDD, pervasive developmental disorder; PF, penile freckles; PHTS, PTEN hamartoma tumour syndrome; VA, vascular anomaly.
*Refers to the age when the patient was first diagnosed with PHTS, either clinically or by molecular testing; †unless otherwise stated.
Table 2 Diagnostic features present in patients with PTEN mutations.
| Patient | Sex | Age at last evaluation (years) | Consensus diagnostic criteria for Cowden syndrome | PTEN mutation | Exon/IVS | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pathognomonic criteria | Major criteria | Minor criteria | ||||||||||||||||||||
| FP | FT | OMC | AK | OFC >97th centile | TC (nm) | BC | EC | MR | TA/MN | FDB | HIP | GT | GM | UF | F | L | BRRS PMGP | |||||
| 1 | M | 10 | No | No | No | No | Yes | ?No | No | N/A | No | ?No | No | ?No | No | ? | N/A | No | Yes | Yes | A34D | Exon 2 |
| 2 | M | 7 | No | No | No | No | Yes | ?No | No | N/A | No | ?No | No | Yes* | No | Yes† | N/A | No | Yes | Yes | M35T | Exon 2 |
| 3 | F | 11 | No | No | No | No | Yes | No | No | No | No | No‡ | No | ?No | No | ? | ?No | No | Yes | N/A | IVS3+1G>A | Intron 3 |
| 4 | M | 6 | No | No | No | No | Yes | No | No | N/A | No | No | No | ?No | No | ? | N/A | No | Yes | Yes | IVS3+5G>A | Intron 3 |
| 5 | F | 24 | No | No | No | No | ? | Yes§ | No | No | No | Yes | No | Yes | No | No | No | No | Yes | N/A | c.220delA | Exon 4 |
| 6 | F | 58 | ? | ? | ? | ? | ? | ?No | Yes | No | No | Yes | Yes | ?No | No | ? | ?No | ? | ? | N/A | c.302_304 delTCAinsCC | Exon 5 |
| 7 | F | 18 | No | No | No | No | Yes | ?No | No | No | Yes | ?No | Yes | ?No | No¶ | No | No | No | Yes | N/A | L108P | Exon 5 |
| 8 | F | 6 | No | No | No | No | Yes | No | No | No | No | No | No | ?No | No | No | No | No | Yes | N/A | c.346_358dup | Exon 5 |
| 9 | M | 40** | No | No | No | No | Yes | ?No | No | N/A | No | No†† | No | No | No | ? | N/A | No | Yes | Yes | c.346_358dup | Exon 5 |
| 10 | M | 22 | No | No | No | No | Yes | ?No | No | N/A | No | Yes | No | Yes | No | No | N/A | No | No | Yes | R130Q | Exon 5 |
| 11 | M | 15 | ? | ? | ? | ? | Yes | ?No | No | N/A | No | ? | No | ?No | No | ? | N/A | ? | Yes | ? | R130X | Exon 5 |
| 12 | M | 9 | No | No | No | No | Yes | No | No | N/A | No | No | No | ?No | No | No | N/A | No | Yes | Yes | R130X | Exon 5 |
| 13 | F | 16 | No | No | No | No | Yes | No | No | No | No | No | No | ?No | No | No | ?No | No | No | N/A | G132A | Exon 5 |
| 14 | F | 5 | No | No | No | No | Yes | ?No | No | No | No | ?No | No | Yes* | No | ? | ?No | No | No | N/A | IVS5‐2A>C | Intron 5 |
| 15 | M | 14 | No | No | No | No | Yes | Yes | No | N/A | No | No†† | No | ?No | No | ? | N/A | No | No | Yes | c.499_505del7 | Exon 6 |
| 16 | F | 15 | No | No | No | No | Yes | Yes | No | No | No | No†† | No | Yes | No | Yes | No | No | Yes | N/A | c.513 InsA | Exon 6 |
| 17 | M | 13 | No | No | No | No | Yes | No | No | N/A | No | No | No | No | No | No | N/A | No | No | Yes | R173C | Exon 6 |
| 18 | M | 13 | No | No | No | No | Yes | No | No | N/A | No | Yes | No | Yes | No | No | N/A | No | Yes | Yes | Y180X | Exon 6 |
| 19 | F | 39 | No | Yes‡‡ | No | No | Yes | Yes | No | No | No | Yes | No | Yes* | No | ? | ?No | No | Yes | N/A | Y180X | Exon 6 |
| 20 | F | 9 | No | No | No | No | Yes | ?No | No | No | No | ?No | No | ?No | No | ? | ?No | No | No | N/A | L181P | Exon 6 |
| 21 | F | 7 | No | No | No | No | Yes | No | No | No | Yes | No†† | No | ?No | No | No | No | No | No | N/A | R233X | Exon 7 |
| 22 | M | 23 | No | No | No | No | ? | ?No | No | N/A | No | ? | No | ?No | No | ? | N/A | Yes | No | ? | IVS7+1G>T | Intron 7 |
| 23 | M | 10 | No | No | No | No | Yes | No | No | N/A | No | No | No | ?No | No | ? | N/A | No | No | Yes | c.955insA | Exon 8 |
| 24 | M | 14 | No | No | Yes | No | Yes | Yes | No | N/A | No | Yes | No | ?No | No | ? | N/A | ? | ? | Yes | c.968dupA | Exon 8 |
| 25 | M | 10 | No | No | No | Yes | Yes | ?No | No | N/A | No | ?No | No | ?Yes§§ | No | No¶¶ | N/A | No | Yes | Yes | R335X (germline), R130X (somatic ‐ in vascular malformation; in trans) | Exon 8 |
| 26 | M | 5 | No | No | No | No | Yes | No | No | N/A | Yes | No | No | ?No | No | ? | N/A | No | No | Yes | R335X | Exon 8 |
?, Unknown; ?No, presumed negative (no formal investigation); AK, acral keratoses; BC, breast cancer; EC, endometrial carcinoma; F, fibromas; FDB, fibrocystic disease of the breast; FP, facial papules; FT, facial trichilemmomas; GM, genitourinary malformations; GT, genitourinary tumours; HIP, hamartomatous intestinal polyps; IVS, intervening sequence (i.e., intron); L, lipomas; MR, mental retardation; N/A, Not applicable, OFC, Occipitofrontal circumference; OMC, oral mucosal papillomatosis; PMGP, pigmented macules on glans penis; TA/MN, thyroid adenoma/multiple nodules; TC (nm), thyroid cancer (non‐medullary); UF, Uterine fibroids.
*Intestinal polyps but unclear if hamartomatous; †Renal hamartoma, nephrolithiasis, hydronephrosis; ‡Graves disease leading to partial thyroidectomy; §Probable papillary carcinoma on cytology; ¶Granulosa cell tumour of left ovary; **Father of patient 8, not fully evaluated; ††Solitary thyroid nodule; ‡‡Facial skin tags but not biopsied; §§Peri‐rectal & anal polyps, but no faecal occult blood; ¶¶ Duplex right kidney only.
We found nonsense mutations in seven patients, missense mutations in seven, insertions, duplications or deletions in eight and splice‐site mutations in four patients (table 1). Of the four splice‐site mutations, three (in patients 3, 4 and 22) have been previously reported to be associated with Cowden Syndrome (Human Gene Mutation Database, Institute of Medical Genetics, Cardiff; http://www.hgmd.cf.ac.uk/); the other splice‐site mutation (patient 14, IVS5‐2A→C) has not been previously reported. However, using two different splice‐site prediction software programs (the Neural Network Splice Site Prediction Tool (NNSPLICE version 0.9) at the Berkeley Drosophila Genome Project (http://www.fruitfly.org/seq_tools/splice.html) and the NetGene2 Server (http://www.cbs.dtu.dk/services/NetGene2/)), we found that this splice‐site mutation resulted in the loss of the splice acceptor site. Moreover, the −2 position of splice acceptor sites is highly conserved, hence this A→C mutation is likely to adversely affect splicing of the PTEN gene in this patient. There were no specific mutational hotspots, although 8 of 26 (31%) patients had a mutation in exon 5, and another 6 of 26 (23%) patients had mutations in exon 6. To our knowledge, mutational analysis of the PTEN promoter had never been performed until Dr Eng's laboratory started this on a research basis in 2004; GeneDx still does not offer PTEN promoter analysis. Hence, most of the patients in our institution suspected of having a PTEN mutation were not tested for mutations in the promoter region of the gene.
All 23 patients whose head circumference was measured had absolute macrocephaly (>97th centile). In at least three patients (patients 3, 13 and 25), absolute macrocephaly was noted at birth. Only one patient had height >97th centile, and three patients were below the third centile for height. Each of the 13 males whose penis was examined had hyperpigmented macules on the glans. The youngest age at which penile freckling was observed was approximately 1 year. Thyroid carcinoma was documented in at least 5 of 26 (19%) patients, including a child who was diagnosed with thyroid carcinoma at 11 years old (patient 16) and two children who were diagnosed with thyroid carcinoma at 13 years old (patients 15 and 24). Mental retardation or global developmental delay was noted in 3 of 26 (12%) patients, and autistic spectrum disorder was identified in 4 of 26 (15%) patients, 2 of whom also had mental retardation.
Results of imaging studies
The following imaging studies were reviewed: brain MRI (n = 14, with intravenous gadolinium contrast in 9), limb or body MRI (n = 11), limb or body magnetic resonance angiography (n = 8), ultrasonography (n = 5) and angiography (n = 8). None of the patients had haemangiomas or other endothelial tumours (table 3). Vascular anomalies were documented in 14 of 26 (54%) patients. All of these anomalies presented clinically as cutaneous discoloration, swelling or pain. One patient had a dural arteriovenous anomaly only (patient 23; fig 2A). Of these 14 patients, 8 (57%) had a vascular anomaly at >1 non‐contiguous anatomical site. No vascular anomalies were found in intrathoracic or intra‐abdominal viscera.
Table 3 Summary of vascular findings in patients with PTEN mutations.
| Patient | Description of vascular anomaly | Sites involved | IM | Ectopic fat | Age at first vascular symptom* (years)† | Vascular anomaly symptomatic |
|---|---|---|---|---|---|---|
| 1 | Unclear: disorganised vessels within fat of L hand; abnormal veins in subcutaneous fat in R shin | L hand; R shin | No | Intramuscular | 3.5 | Yes: lump on L hand changed colour at 5 years |
| 2 | Arteriolovenous | L pelvis; L buttock; R thigh | Yes | Intramuscular; subcutaneous; intraperitoneal | <25 months | Yes: skin discoloration over vascular anomaly; pain in leg (over vascular anomaly) at 8 years |
| 3 | Arteriovenous | L gastrocnemius muscle | Yes | Yes | 11 | Yes: swollen and painful L calf at 11 years; visible veins |
| 5 | Arteriolovenous with focal venous varicosities | R upper arm and elbow | Yes | Intramuscular; Intermuscular | 15 | Yes: swollen and tender R arm at 15 years (after onset of haematoma) |
| 6 | Arteriolovenular | Pelvis; R foot | ? | ? | 16 | Yes: pain in pelvis occasionally |
| 7 | Unclear: fast‐flow | R knee | Yes | ? | 15 | Yes: lump on knee at 15 years |
| 8 | Unclear | Paraspinal; thoracic; abdominal wall; retroperitoneal | Yes | Retroperitoneal; paraspinal | Birth | Yes: skin discoloration on R flank at birth; palpable mass over R abdomen at 16 months |
| 11 | Arteriolovenous; arteriovenous fistula; arteries draining into focally dilated veins | R paraspinal thoracic region; R ankle | Yes | Paraspinal mass and ankle lesion | Birth | Yes: skin discoloration in paraspinal region at birth and palpable mass in same area from infancy |
| 15 | Arteriolovenous | Pelvis; scrotum; penile shaft; R thigh | Yes | Intramuscular; intermuscular; subcutaneous | 2 | Yes: swollen and painful R thigh mass at 2 years |
| 20 | Fast‐flow | R soleus and peroneal muscle | Yes | Intramuscular | 4.5 | Yes: swollen R calf at 4.5 years, painful from 5.5 years |
| 21 | Arterio‐capillary‐venular; tortuous small arteries and capillary blush | L thigh, quadriceps, gluteus | Yes | Subcutaneous | 3.5 | Yes: painful L thigh with ambulatory difficulties at 3.5 years |
| 22 | Arteriolovenular | L cheek, L arm, R thigh, L calf, L ankle and foot | Yes | Intramuscular | 15 | Yes: swollen L cheek at 15; Pain and stiffness in L elbow at 19 years |
| 23 | Arteriovenous | Intracranial (dural) | No | No | 7 | Yes: headaches and prominent facial veins at 7 years |
| 25 | Arteriolovenular | Both lower limbs; penis | Yes | Intramuscular; intra‐abdominal; retroperitoneal | Birth | Yes: swollen right leg at birth; ambulatory difficulties from 3 years; high output cardiac failure at 6.5 years |
L, Left; IM, intramuscular; R, Right.
*Refers to the age when the vascular anomaly was first noticed by either the patient or doctor; †unless otherwise stated.
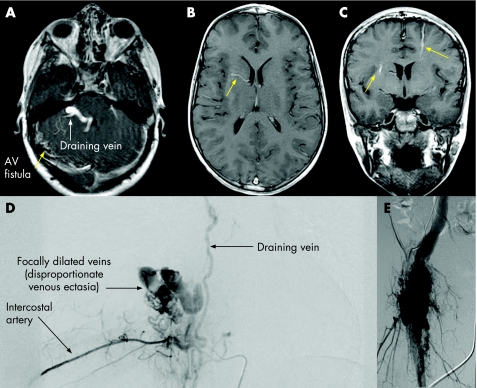
Figure 2 (A) Dural arteriovenous (AV) fistula (patient 23); (B, C) gadolinium‐enhanced T1‐weighted MRI of the brain showing developmental venous anomalies (arrows). (D, E) Aangiography of (D) patient 11 with paraspinal and (E) patient 15 with right lower limb arteriolovenous malformations, showing disproportionate venous ectasia, typical of PTEN lesions.
Fast‐flow anomalies were noted in 12 of 14 (86%) patients, and flow characteristics could not be determined for 2 patients. Of the 12 patients with fast‐flow anomalies, angiography showed arteriolovenous shunts in 4, arteriolovenular anomalies in 3, arteriovenous fistulae in 3 and unclassifiable fast‐flow anomalies with arterial, capillary and venular components without direct shunting in 1; 1 patient had arteriovenous and arteriolovenous anomalies at different sites. The type of fast‐flow lesion and the vessels involved could not be determined in two patients who had abnormal venous channels within fatty tissue because flow‐sensitive imaging was not available. Unlike patients without PTEN mutations, the vascular anomalies in many of our patients had draining veins that exhibited focal segmental dilatation that we describe as “disproportionate venous ectasia” (fig 2D,E).
The vascular anomalies were associated with variable amounts of ectopic fat in 11/12 (92%) of the patients; ectopic fat was not seen in the patient with an intracranial vascular anomaly (patient 23). We were unable to determine the presence of ectopic fat in two patients with extracranial vascular anomalies, either because they had fat‐suppressed sequences (n = 1) or had never had an MRI (n = 1). The ectopic fat was usually at the same site as the vascular anomaly (fig 1A,B). However, one patient (patient 12) had excessive intraperitoneal and extraperitoneal fat in the absence of any vascular anomalies, and another patient (patient 2) had excessive intraperitoneal fat in addition to vascular anomalies in the lower limbs.
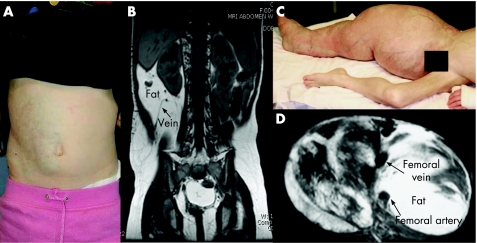
Figure 1 (A, B) Patient 8, a 5‐year‐old girl, presented with (A) right flank/abdominal mass with pinkish‐blue skin discoloration; (B) T1‐weighted MRI of this patient's abdomen showed a fast‐flow lesion, dilated draining vein (arrowed) and excessive fat. (C, D) Patient 25 had (C) an arteriolovenular anomaly and epidermoid naevus involving right lower limb, scrotum and penis; (D) T1‐weighted MRI of this patient's right thigh. Parental/guardian informed consent was obtained for publication of these figures.
Infiltrative intramuscular involvement was another imaging characteristic of vascular anomalies in patients with PTEN mutation, often resulting in severe and progressive enlargement of the affected muscles (fig 1C,D). This abnormality was seen in 85% (11/13) of the patients. We were unable to determine whether there was intramuscular involvement in one patient because appropriate imaging was unavailable.
The histology of the vascular anomalies in nine patients was reviewed by a pathologist. Major findings were skeletal muscle infiltrated with adipose tissue, fibrous bands, and mucinous matrix, often containing lymphoid aggregates and plasma cells. There were nodular aggregates of tortuous arteries with concentric transmural muscular hyperplasia and clusters of abnormal veins, which had a variable smooth muscle coat that was irregular, diminished or excessive.
Developmental venous anomalies (DVAs) of the cerebral hemispheres and/or cerebellum were noted in eight of nine (89%) of patients who underwent brain MRI with contrast. DVAs are dilated veins considered to be normal anatomical variants in the venous drainage pathways of the brain; they are found in approximately 2% of the normal population.25 Characteristic DVAs in patients with PTEN mutations are shown in Figure 2(B,C). These lesions, which are usually asymptomatic, were often not visible on MRI without intravenous contrast medium. Four of the patients with DVAs did not have any extracranial vascular anomalies. One patient (patient 23) had a dural arteriovenous fistula and sinus pericranii, requiring multiple embolisation treatments; the details of his clinical course and treatment have recently been reported.26 A cavernous vascular malformation was found in another patient who had multiple DVAs (patient 13). This patient was asymptomatic and the lesion was considered to be an incidental finding on brain MRI with contrast. Patient 5 had an Arnold–Chiari I malformation and small areas of bright signals (T2 prolongation) in the white matter, which was probably also an unrelated finding. Furthermore, the patient had chronic daily headaches and a history of probable migraine, which could account for the T2 prolongation in the white matter.
Discussion
This retrospective study was undertaken to characterise the types of vascular anomalies that occur in patients with a PTEN mutation, thereby expanding the phenotype of PHTS. Macrocephaly and penile freckling are well‐known features.5 All of our patients who had their head circumference measured were macrocephalic, and all male patients who were fully examined had penile freckling. Previous studies of PTEN‐positive BRRS patients had documented macrocephaly in all patients, but penile freckling had been noted in only 67–85% of patients.6,27 This study was not designed to examine the prevalence of the various clinical features seen in patients with a PTEN mutation. Nevertheless, it is of note that at least 8 of 26 (31%) patients had thyroid involvement in the form of multinodular goitre, thyroid adenoma or thyroid cancer. Similarly, 8 of 26 patients had gastrointestinal polyps, none of which was found to be malignant. Some authors have reported an association between PTEN mutation and autism,11,28 and our findings support these observations.
The terminology for the extracranial vascular anomalies in these patients is controversial. Although radiologically these lesions have features of fast‐flow vascular malformations, histopathologically, they appear to be disordered growths of blood vessels, adipose and fibrous tissue, with a low level of proliferation. Therefore, we elected to use the generic term “anomaly” to describe these lesions, recognising that further radiological, histopathological and molecular studies should provide a more precise nosology.
We identified vascular anomalies in 54% of our patients with a PTEN mutation, although none of them had haemangiomas or other “pure” vascular tumours. This relatively high frequency could be due to selection bias, as the Vascular Anomalies Center, Children's Hospital Boston, is a major referral centre. Moreover, it is likely that asymptomatic patients will not be tested for the mutation. For example, two of our patients were found to have the mutation only after their children had been diagnosed with PHTS.
In the patients who underwent angiography, the deep vascular anomalies were all fast‐flow with some degree of arteriovenous shunting. There was a spectrum of findings, ranging from infiltration of the affected tissue with fine, tortuous arterial and venous channels and a tissue blush, to direct arteriovenous fistulae with massive enlargement of the proximal draining veins (termed arteriovenous or arteriolovenous). We believe that the angioarchitecture in these vascular anomalies is relatively unique, characterised by the unusual segmental dilatation of the draining veins. In contrast, arteriovenous anomalies in patients without a PTEN mutation exhibit diffuse, smooth dilatation of the draining veins. Moreover, cross‐sectional imaging (MRI, CT and ultrasonography) showed that the intramuscular vascular anomalies in our patients disrupted the muscular architecture and had excessive disorganised ectopic fat. Non‐syndromic intramuscular AVMs are usually associated with symmetrical overgrowth of the affected muscle, without an eccentric mass effect, and are not accompanied by excess adipose tissue. Involvement of multiple non‐contiguous sites, seen in 57% of the PTEN positive patients, is also very uncommon in patients with non‐syndromic AVM.
DVAs are usually considered normal anatomical variants of no clinical significance. Nonetheless, the fact that 8 of 9 of our patients who had brain MRI with contrast were found to have DVAs is striking. Further brain imaging studies are needed to determine the true prevalence and significance of DVAs in these patients. Cavernous vascular malformations are known to occur in association with DVAs, as noted in one of our patients,29 but the pathogenesis of these two entities appears to be different.30
This study was not designed to examine the neuroanatomical characteristics of patients with PTEN mutations. Nonetheless, it is noteworthy that, other than Arnold–Chiari I malformation in 2 patients (one of whom only had a cervical spine MRI), no other structural brain abnormalities were seen in the 14 patients who underwent a brain MRI for a variety of indications, including global developmental delay or mental retardation.
Heterozygous deletion of PTEN in mouse endothelial cells increases the sensitivity of the cells to various vascular growth factors, resulting in enhanced angiogenesis and growth of tumours.31 These murine studies could explain the predisposition to vascular anomalies in our patients. Other experiments in mice have also shown that anti‐angiogenic therapy can diminish adipose tissue,32 suggesting that the ectopic fat in vascular anomalies may be due to the increased angiogenesis caused by a PTEN mutation.
The PTEN gene encodes a lipid phosphatase that mediates cell‐cycle arrest and apoptosis. PTEN has two key domains, an N‐terminal phosphatase domain encoded by exons 1–6, and a C‐terminal domain encoded by exons 6–9, which is involved in protein–protein interactions.33 Marsh et al.9,10 reported that mutations in exons 5, 7 and 8 of the PTEN gene together accounted for 63–75% of the pathogenic mutations, with 31–50% of all mutations occurring in exon 5. In contrast, only 50% of our patients had mutations in exons 5, 7 and 8. Moreover, 23% of our patients had mutations in exon 6, compared with only 6–13% in previous reports (table 4).9,10 Exon 6 lies at the interface between the phosphatase domain and the N‐terminal domain involved in the binding of phospholipids. It is a highly conserved region, and mutations in that region result in reduction in the activity of the phophatase against phosphatidylinositol‐(3,4,5)‐triphosphate (PI(3,4,5)P3). This suggests an important role for exon 6 in the binding of PI(3,4,5)P3 by PTEN.33
Table 4 Distribution and types of mutations in PTEN identified by previous studies and in our study.
| Studies by Marsh et al. | Our study (PTEN‐positive patients) | |||||||
|---|---|---|---|---|---|---|---|---|
| Cowden syndrome and BRRS combined10 | BRRS*9 | Cowden syndrome/ BRRS overlap9 | ||||||
| Proportion | % | Proportion | % | Proportion | % | Proportion | % | |
| Exon | ||||||||
| 5 | 10/30 | 33 | 5/16 | 31 | 4/8 | 50 | 8/26 | 31 |
| 5; core motif | 4/10 | 40 | 1/5 | 20 | 2/4 | 50 | 4/8 | 50 |
| 6 | 4/30 | 13 | 1/16 | 6 | 0/8 | 0 | 6/26 | 23 |
| 7 | 6/30 | 20 | 3/16 | 19 | 0/8 | 0 | 1/26 | 4 |
| 8 | 4/30 | 13 | 2/16 | 13 | 2/8 | 25 | 4/26 | 15 |
| 5, 6 | 14/30 | 47 | 6/16 | 38 | 4/8 | 50 | 14/26 | 54 |
| 7, 8 | 10/30 | 33 | 5/16 | 31 | 2/8 | 25 | 5/26 | 19 |
| 5, 7, 8 | 20/30 | 67 | 10/16 | 63 | 6/8 | 75 | 13/26 | 50 |
| Types of mutation | ||||||||
| Nonsense | 9/30 | 30 | 3/16 | 19 | 4/8 | 50 | 7/26 | 27 |
| Missense | 6/30 | 20 | 6/16 | 38 | 0/8 | 0 | 7/26 | 27 |
| Ins/del | 12/30 | 40 | 4/16 | 25 | 2/8 | 25 | 8/26 | 31 |
| Splice site | 3/30 | 10 | 3/16 | 19 | 2/8 | 25 | 4/26 | 15 |
Ins/del, insertion/deletion.
*Excluding one patient with a cytogenetically visible deletion on chromosome 10q23.2q24.1 [46,XY,del(10)(q23.2q24.1)] and one patient with an apparently balanced translocation [46,XY,t(10;13)(q23.2;q33)].
R173 in exon 6 is one of the eight most commonly mutated residues in patients with PTEN‐related tumours, suggesting that the integrity of the interface is important for the function of PTEN. It is therefore interesting that of our six patients with mutations in exon 6, two had both thyroid carcinoma and intestinal polyps, one had both multiple thyroid nodules and intestinal polyps, and another had thyroid carcinoma with only a solitary thyroid nodule. In contrast, the only patient in our series with a mutation in R173 had neither thyroid carcinoma nor thyroid nodules and did not have intestinal polyps.
This study has documented a high frequency of deep vascular anomalies in patients with a PTEN mutation. These extracranial vascular anomalies are almost all fast‐flow and have consistent, unusual features, including multifocality (involvement of multiple non‐contiguous sites), musculoskeletal location, ectopic adipose tissue and drainage into disproportionately dilated veins. Multifocality is not a feature of non‐syndromic AVM. Clinicians who care for these patients should consider regular monitoring for vascular anomalies. MRI, with MRA, is appropriate for the initial evaluation of a suspicious lesion.
In conclusion, our study suggests that all patients who present with fast‐flow vascular anomalies or who have multiple DVAs on brain MRI should have their head circumference measured, and male patients should also be examined for penile freckling. Those who are macrocephalic or have penile freckling should be considered candidates for PTEN testing. MRI should be used to evaluate symptomatic mass lesions. Multiple fast‐flow vascular anomalies, the presence on MRI of ectopic fat with disruption of the architecture of the affected muscles, and angiographic finding of disproportionate dilation of the immediate draining veins appear to be the typical features of these lesions. These radiological features should also prompt consideration for PTEN testing. A molecular diagnosis of PTEN mutation is critical because these patients have an increased risk of malignancy, even when they are relatively young, as suggested by our three patients who developed thyroid carcinoma in their early teenage years. Timely identification of these patients also facilitates the diagnosis of asymptomatic carriers and enables physicians to institute appropriate surveillance for thyroid, breast and endometrial cancers for all people found to have a PTEN mutation.
Acknowledgments
We are indebted to Robert Pilarski, MS, CGC, Ohio State University; Charis Eng, MD, PhD, Cleveland Clinic; Gabriele Richard, MD and John Compton, PhD, GeneDx Inc; and Renee Hickey, Tricia Lane, PNP and Carole Roberts, PNP, Vascular Anomalies Center, Children's Hospital Boston, for their invaluable assistance with this study. We are also grateful to Harry Kozakewich, MD, Children's Hospital Boston, for helping us with the pathological assessment of the anomalies.
Abbreviations
AVM - arteriovenous malformation
BRRS - Bannayan–Riley–Ruvalcaba syndrome
CS - Cowden syndrome
DVA - developmental venous anomaly
PI3Kl - phosphoinositide‐3 kinase
PI(3,4,5)P3 - phosphatidylinositol‐(3,4,5)‐triphosphate
PHTS - PTEN hamartoma–tumour syndrome
Footnotes
Competing interests: None declared.
Parental/guardian informed consent was obtained for publication of figure 1.
References
- 1.Sansal I, Sellers W R. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol 2004222954–2963. [DOI] [PubMed] [Google Scholar]
- 2.Li D M, Sun H. TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta. Cancer Res 1997572124–2129. [PubMed] [Google Scholar]
- 3.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang S I, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner S H, Giovanella B C, Ittmann M, Tycko B, Hibshoosh H, Wigler M H, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast and prostate cancer. Science 19972751943–2017. [DOI] [PubMed] [Google Scholar]
- 4.Steck P A, Pershouse M A, Jasser S A, Yung W K, Lin H, Ligon A H, Langford L A, Baumgard M L, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng D H, Tavtigian S V. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet 199715356–362. [DOI] [PubMed] [Google Scholar]
- 5.Zbuk K M, Stein J L, Eng C.PTEN hamartoma tumor syndrome (PHTS). In: GeneReviews at GeneTests: Medical Genetics Information Resource (online database) Copyright, University of Washington, Seattle, 1993–2007 (updated 10 Jan 2006)]. http://www.genetests.org/ Accessed 11 Mar 2007
- 6.Parisi M A, Dinulos M B, Leppig K A, Sybert V P, Eng C, Hudgins L. The spectrum and evolution of phenotypic findings in PTEN mutation positive cases of Bannayan‐Riley‐Ruvalcaba syndrome. J Med Genet 20013852–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorlin R J, Cohen M M, Jr, Condon L M, Burke B A. Bannayan‐Riley‐Ruvalcaba syndrome. Am J Med Genet 199244307–314. [DOI] [PubMed] [Google Scholar]
- 8.Pilarski R, Eng C. Will the real Cowden syndrome please stand up (again)? Expanding mutational and clinical spectra of the PTEN hamartoma tumour syndrome. J Med Genet 200441323–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marsh D J, Kum J B, Lunetta K L, Bennett M J, Gorlin R J, Ahmed S F, Bodurtha J, Crowe C, Curtis M A, Dasouki M, Dunn T, Feit H, Geraghty M T, Graham J M, Jr, Hodgson S V, Hunter A, Korf B R, Manchester D, Miesfeldt S, Murday V A, Nathanson K L, Parisi M, Pober B, Romano C, Tolmie J L, Trembath R, Winter R M, Zackai E H, Zori R T, Weng L P, Dahia P L, Eng C. PTEN mutation spectrum and genotype‐phenotype correlations in Bannayan‐Riley‐Ruvalcaba syndrome suggest a single entity with Cowden syndrome. Hum Mol Genet 199981461–1472. [DOI] [PubMed] [Google Scholar]
- 10.Marsh D J, Coulon V, Lunetta K L, Rocca‐Serra P, Dahia P L, Zheng Z, Liaw D, Caron S, Duboue B, Lin A Y, Richardson A L, Bonnetblanc J M, Bressieux J M, Cabarrot‐Moreau A, Chompret A, Demange L, Eeles R A, Yahanda A M, Fearon E R, Fricker J P, Gorlin R J, Hodgson S V, Huson S, Lacombe D, LePrat F, Odent S, Toulouse C, Olopade O I, Sobol H, Tishler S, Woods C G, Robinson B G, Weber H C, Parsons R, Peacocke M, Longy M, Eng C. Mutation spectrum and genotype‐phenotype analyses in Cowden disease and Bannayan‐Zonana syndrome, two hamartoma syndromes with germline PTEN mutation. Hum Mol Genet 19987507–515. [DOI] [PubMed] [Google Scholar]
- 11.Butler M G, Dasouki M J, Zhou X P, Talebizadeh Z, Brown M, Takahashi T N, Miles J H, Wang C H, Stratton R, Pilarski R, Eng C. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J Med Genet 200542318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naidich J J, Rofsky N M, Rosen R, Karp N. Arteriovenous malformation in a patient with Bannayan‐Zonana syndrome. Clin Imaging 200125130–132. [DOI] [PubMed] [Google Scholar]
- 13.Miles J H, Zonana J, McFarlane J, Aleck K A, Bawle E. Macrocephaly with hamartomas: Bannayan‐Zonana syndrome. Am J Med Genet 198419225–234. [DOI] [PubMed] [Google Scholar]
- 14.Lok C, Viseux V, Avril M F, Richard M A, Gondry‐Jouet C, Deramond H, Desfossez‐Tribout C, Courtade S, Delaunay M, Piette F, Legars D, Dreno B, Saiag P, Longy M, Lorette G, Laroche L, Caux F. Brain magnetic resonance imaging in patients with Cowden syndrome. Medicine (Baltimore) 200584129–136. [DOI] [PubMed] [Google Scholar]
- 15.Turnbull M M, Humeniuk V, Stein B, Suthers G K. Arteriovenous malformations in Cowden syndrome. J Med Genet 200542e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weary P E, Gorlin R J, Gentry W C, Jr, Comer J E, Greer K E. Multiple hamartoma syndrome (Cowden's disease). Arch Dermatol 1972106682–690. [PubMed] [Google Scholar]
- 17.Mulliken J B, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg 198269412–422. [DOI] [PubMed] [Google Scholar]
- 18.Enjolras O, Mulliken J B. Vascular tumors and vascular malformations (new issues). Adv Dermatol 199713375–423. [PubMed] [Google Scholar]
- 19.Konez O, Burrows P E. Magnetic resonance of vascular anomalies. Magn Reson Imaging Clin N Am 200210363–88, vii. [DOI] [PubMed] [Google Scholar]
- 20.Paltiel H J, Burrows P E, Kozakewich H P, Zurakowski D, Mulliken J B. Soft‐tissue vascular anomalies: utility of US for diagnosis. Radiology 2000214747–754. [DOI] [PubMed] [Google Scholar]
- 21.Burrows P E, Laor T, Paltiel H, Robertson R L. Diagnostic imaging in the evaluation of vascular birthmarks. Dermatol Clin 199816455–488. [DOI] [PubMed] [Google Scholar]
- 22.Houdart E, Gobin Y P, Casasco A, Aymard A, Herbreteau D, Merland J J. A proposed angiographic classification of intracranial arteriovenous fistulae and malformations. Neuroradiology 199335381–385. [DOI] [PubMed] [Google Scholar]
- 23.Zhou X P, Waite K A, Pilarski R, Hampel H, Fernandez M J, Bos C, Dasouki M, Feldman G L, Greenberg L A, Ivanovich J, Matloff E, Patterson A, Pierpont M E, Russo D, Nassif N T, Eng C. Germline PTEN promoter mutations and deletions in Cowden/Bannayan‐Riley‐Ruvalcaba syndrome result in aberrant PTEN protein and dysregulation of the phosphoinositol‐3‐kinase/Akt pathway. Am J Hum Genet 200373404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou X P, Marsh D J, Hampel H, Mulliken J B, Gimm O, Eng C. Germline and germline mosaic PTEN mutations associated with a Proteus‐like syndrome of hemihypertrophy, lower limb asymmetry, arteriovenous malformations and lipomatosis. Hum Mol Genet 20009765–768. [DOI] [PubMed] [Google Scholar]
- 25.Lasjaunias P, Burrows P, Planet C. Developmental venous anomalies (DVA): the so‐called venous angioma. Neurosurg Rev 19869233–242. [DOI] [PubMed] [Google Scholar]
- 26.Srinivasa R N, Burrows P E. Dural arteriovenous malformation in a child with Bannayan‐Riley‐Ruvalcaba Syndrome. AJNR Am J Neuroradiol 2006271927–1929. [PMC free article] [PubMed] [Google Scholar]
- 27.Hendriks Y M, Verhallen J T, van der Smagt J J, Kant S G, Hilhorst Y, Hoefsloot L, Hansson K B, van der Straaten P J, Boutkan H, Breuning M H, Vasen H F, Brocker‐Vriends A H. Bannayan‐Riley‐Ruvalcaba syndrome: further delineation of the phenotype and management of PTEN mutation‐positive cases. Fam Cancer 2003279–85. [DOI] [PubMed] [Google Scholar]
- 28.Goffin A, Hoefsloot L H, Bosgoed E, Swillen A, Fryns J P. PTEN mutation in a family with Cowden syndrome and autism. Am J Med Genet 2001105521–524. [DOI] [PubMed] [Google Scholar]
- 29.Wilms G, Bleus E, Demaerel P, Marchal G, Plets C, Goffin J, Baert A L. Simultaneous occurrence of developmental venous anomalies and cavernous angiomas. AJNR Am J Neuroradiol 1994151247–54 discussion 1255–7. [PMC free article] [PubMed] [Google Scholar]
- 30.Guclu B, Ozturk A K, Pricola K L, Seker A, Ozek M, Gunel M. Cerebral venous malformations have distinct genetic origin from cerebral cavernous malformations. Stroke 2005362479–2480. [DOI] [PubMed] [Google Scholar]
- 31.Hamada K, Sasaki T, Koni P A, Natsui M, Kishimoto H, Sasaki J, Yajima N, Horie Y, Hasegawa G, Naito M, Miyazaki J, Suda T, Itoh H, Nakao K, Mak T W, Nakano T, Suzuki A. The PTEN/PI3K pathway governs normal vascular development and tumor angiogenesis. Genes Dev 2005192054–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rupnick M A, Panigrahy D, Zhang C Y, Dallabrida S M, Lowell B B, Langer R, Folkman M J. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci U S A 20029910730–10735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J O, Yang H, Georgescu M M, Di Cristofano A, Maehama T, Shi Y, Dixon J E, Pandolfi P, Pavletich N P. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell 199999323–334. [DOI] [PubMed] [Google Scholar]