Abstract
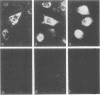
The principal efferent role of the macrophage in acquired resistance to intracellular pathogens depends on activation by T-cell lymphokines, primarily gamma interferon (IFN-gamma). However, mouse macrophages that are heavily burdened with Mycobacterium leprae are refractory to activation by IFN-gamma and are thus severely compromised in their capacity for both enhanced microbicidal and tumoricidal activities. We report here that lipoarabinomannan (LAM), a highly immunogenic lipopolysaccharide that is a prominent component of the cell walls of M. leprae and M. tuberculosis, was a potent inhibitor of IFN-gamma-mediated activation of mouse macrophages in vitro. Inhibition of macrophage activation by LAM required preincubation for approximately 24 h, resulting in uptake of LAM into cytoplasmic vacuoles of macrophages. Intact LAM was necessary to inhibit IFN-gamma-mediated activation, as this property was lost when the acyl side chains were removed from LAM by mild alkaline hydrolysis. In addition, LAM was an abundant constituent of macrophages isolated from lepromatous granulomas of M. leprae-infected nude mice and likely contributed to the defective activation of granuloma macrophages by IFN-gamma.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brennan P. J. The phthiocerol-containing surface lipids of Mycobacterium leprae--a perspective of past and present work. Int J Lepr Other Mycobact Dis. 1983 Sep;51(3):387–396. [PubMed] [Google Scholar]
- Celada A., Allen R., Esparza I., Gray P. W., Schreiber R. D. Demonstration and partial characterization of the interferon-gamma receptor on human mononuclear phagocytes. J Clin Invest. 1985 Dec;76(6):2196–2205. doi: 10.1172/JCI112228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada A., Schreiber R. D. Internalization and degradation of receptor-bound interferon-gamma by murine macrophages. Demonstration of receptor recycling. J Immunol. 1987 Jul 1;139(1):147–153. [PubMed] [Google Scholar]
- Collins F. M. Mycobacterium avium-complex infections and development of the acquired immunodeficiency syndrome: casual opportunist or causal cofactor? Int J Lepr Other Mycobact Dis. 1986 Sep;54(3):458–474. [PubMed] [Google Scholar]
- Draper P., Rees R. J. Electron-transparent zone of mycobacteria may be a defence mechanism. Nature. 1970 Nov 28;228(5274):860–861. doi: 10.1038/228860a0. [DOI] [PubMed] [Google Scholar]
- Drutz D. J., Cline M. J., Levy L. Leukocyte antimicrobial function in patients with leprosy. J Clin Invest. 1974 Feb;53(2):380–386. doi: 10.1172/JCI107570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellner J. J., Daniel T. M. Immunosuppression by mycobacterial arabinomannan. Clin Exp Immunol. 1979 Feb;35(2):250–257. [PMC free article] [PubMed] [Google Scholar]
- Gaylord H., Brennan P. J., Young D. B., Buchanan T. M. Most Mycobacterium leprae carbohydrate-reactive monoclonal antibodies are directed to lipoarabinomannan. Infect Immun. 1987 Nov;55(11):2860–2863. doi: 10.1128/iai.55.11.2860-2863.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godal T., Myklestad B., Samuel D. R., Myrvang B. Characterization of the cellular immune defect in lepromatous leprosy: a specific lack of circulating Mycobacterium leprae-reactive lymphocytes. Clin Exp Immunol. 1971 Dec;9(6):821–831. [PMC free article] [PubMed] [Google Scholar]
- Hard G. C. Comparative toxic effect of the surface lipid of Corynebacterium ovis on peritoneal macrophages. Infect Immun. 1975 Dec;12(6):1439–1449. doi: 10.1128/iai.12.6.1439-1449.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Levis W. R., Cohn Z. A. Defective production of monocyte-activating cytokines in lepromatous leprosy. J Exp Med. 1984 Mar 1;159(3):666–678. doi: 10.1084/jem.159.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. W., Gaylord H., Brennan P. J. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J Biol Chem. 1986 Sep 15;261(26):12345–12351. [PubMed] [Google Scholar]
- Kaplan G., Gandhi R. R., Weinstein D. E., Levis W. R., Patarroyo M. E., Brennan P. J., Cohn Z. A. Mycobacterium leprae antigen-induced suppression of T cell proliferation in vitro. J Immunol. 1987 May 1;138(9):3028–3034. [PubMed] [Google Scholar]
- Kaplan G., Nathan C. F., Gandhi R., Horwitz M. A., Levis W. R., Cohn Z. A. Effect of recombinant interferon-gamma on hydrogen peroxide-releasing capacity of monocyte-derived macrophages from patients with lepromatous leprosy. J Immunol. 1986 Aug 1;137(3):983–987. [PubMed] [Google Scholar]
- Krahenbuhl J. L., Remington J. S. Cytostatic effects of activated macrophages on tumor target cells: inhibition of cytotoxic action of ARA-C. J Immunopharmacol. 1980;2(3):325–348. doi: 10.3109/08923978009046465. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B. Resistance to intracellular infection. J Infect Dis. 1971 Apr;123(4):439–445. doi: 10.1093/infdis/123.4.439. [DOI] [PubMed] [Google Scholar]
- Mehra V., Mason L. H., Rothman W., Reinherz E., Schlossman S. F., Bloom B. R. Delineation of a human T cell subset responsible for lepromin-induced suppression in leprosy patients. J Immunol. 1980 Sep;125(3):1183–1188. [PubMed] [Google Scholar]
- Misaki A., Azuma I., Yamamura Y. Structural and immunochemical studies on D-arabino-D-mannans and D-mannans of Mycobacterium tuberculosis and other Mycobacterium species. J Biochem. 1977 Dec;82(6):1759–1770. doi: 10.1093/oxfordjournals.jbchem.a131874. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Kaplan G., Levis W. R., Nusrat A., Witmer M. D., Sherwin S. A., Job C. K., Horowitz C. R., Steinman R. M., Cohn Z. A. Local and systemic effects of intradermal recombinant interferon-gamma in patients with lepromatous leprosy. N Engl J Med. 1986 Jul 3;315(1):6–15. doi: 10.1056/NEJM198607033150102. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks D. R., Bryan V. M., Oi V. T., Herzenberg L. A. Antigen-specific identification and cloning of hybridomas with a fluorescence-activated cell sorter. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1962–1966. doi: 10.1073/pnas.76.4.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley D. S. Histological classification and the immunological spectrum of leprosy. Bull World Health Organ. 1974;51(5):451–465. [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., Steele J., Fraher L., Barker S., Karmali R., O'Riordan J., Stanford J. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986 Jan;57(1):159–163. [PMC free article] [PubMed] [Google Scholar]
- Ryan J. L., Gobran L., Morrison D. C. Modulation of murine macrophage metabolism by glycolipids: inhibition of LPS-induced metabolism by specific gangliosides. J Leukoc Biol. 1986 Oct;40(4):367–379. doi: 10.1002/jlb.40.4.367. [DOI] [PubMed] [Google Scholar]
- Sibley L. D., Franzblau S. G., Krahenbuhl J. L. Intracellular fate of Mycobacterium leprae in normal and activated mouse macrophages. Infect Immun. 1987 Mar;55(3):680–685. doi: 10.1128/iai.55.3.680-685.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley L. D., Krahenbuhl J. L. Defective activation of granuloma macrophages from Mycobacterium leprae-infected nude mice. J Leukoc Biol. 1988 Jan;43(1):60–66. doi: 10.1002/jlb.43.1.60. [DOI] [PubMed] [Google Scholar]
- Sibley L. D., Krahenbuhl J. L. Mycobacterium leprae-burdened macrophages are refractory to activation by gamma interferon. Infect Immun. 1987 Feb;55(2):446–450. doi: 10.1128/iai.55.2.446-450.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]