Abstract
Purpose
Familial partial lipodystrophy caused by mutations in the PPARG gene is characterised by altered distribution of subcutaneous fat, muscular hypertrophy and symptoms of metabolic syndrome. PPARG encodes peroxisome proliferator‐activated receptor (PPAR)γ, a nuclear hormone receptor playing a crucial role in lipid and glucose metabolism and in several other cellular regulatory processes.
Methods
PPARG was screened for mutations by direct sequencing in two patients with lipodystrophy, one unaffected family member and 124 controls. Body composition was examined in affected patients, and they were investigated for abnormalities in laboratory results. Functional analysis of the mutant protein was assessed by determining transcriptional activity and possible interference with the wild‐type protein.
Results
In two patients with familial partial lipodystrophy, we identified a nucleotide substitution in the PPARG gene. This mutation results in the substitution of aspartate by asparagine at residue 424 (D424N) in the ligand‐binding domain of PPARγ. The unaffected family member and all 124 controls did not carry this mutation. D424N PPARγ had a significantly lower ability than wild‐type PPARγ to activate a PPARγ‐stimulated reporter gene, but did not exert a negative effect on the wild‐type protein. Partial activation of D424N PPARγ was achieved in the presence of the agonist rosiglitazone.
Conclusion
We report a new PPARG mutation, D424N, which is located in the ligand‐binding domain of the protein and leads to familial partial lipodystrophy. D424N PPARγ exhibited a loss of function, which was partially restored by adding the PPARγ agonist rosiglitazone, suggesting possible treatment potential of this agent.
Keywords: lipodystrophy, lamin, PPARγ, insulin resistance, metabolic syndrome
Autosomal dominant familial partial lipodystrophy is a rare disease, characterised by loss of subcutaneous fat, mainly from the extremities, and accumulation of subcutaneous fat in the face and neck area. Affected people also have insulin resistance, diabetes mellitus, hypertriglyceridaemia, hepatic steatosis, and arterial hypertension.1,2,3 Mutations in two different genes cause familial partial lipodystrophy. Mutations in the LMNA gene, encoding A‐type nuclear lamins, cause Dunnigan familial partial lipodystrophy (OMIM 151660).4,5,6 The other gene is PPARG, encoding the peroxisome proliferator‐activated receptor (PPAR)γ. Familial partial lipodystrophy (OMIM 604367) caused by PPARG mutations is phenotypically similar to Dunnigan familial partial lipodystrophy.7,8,9,10,11,12,13,14,15
PPARγ belongs to the superfamily of nuclear hormone receptors and is involved in glucose metabolism, adipocyte differentiation, inflammation, and carcinogenesis.16,17 By forming heterodimers with retinoid X receptor α and binding to PPAR‐responsive elements, PPARγ regulates transcription of numerous PPAR‐responsive genes.18 PPARγ contains a ligand‐binding domain (LBD), a DNA‐binding domain (DBD) and an A/B domain.19 Alternative PPARG promoters and differential RNA splicing generate four different PPARγ isoforms. PPARγ1 and PPARγ3 are widely expressed in most differentiated cells,20,21 whereas little is known about PPARγ4 except for its presence in adipose tissue.22 PPARγ2 is expressed in adipose tissue only, emphasising its important role in adipocyte metabolism.23 Several mutations in PPARG cause lipodystrophic phenotypes or metabolic symptoms.7,8,9,10,11,12,13,14,15
We report a novel PPARG mutation, leading to partial lipodystrophy and loss of PPARγ transcriptional activity. The functionally abnormal protein can be stimulated by rosiglitazone.
Methods
Patients and clinical evaluations
Informed consent was obtained from all participants or their legal guardian. The study was approved by the ethics committee of the Charité Hospital, Berlin, Germany.
The index patient was referred to the Charité Hospital by her general practitioner. Her mother and half‐sister were also available for examination. In all affected patients, body weight and height were obtained to calculate body mass index. Body composition was evaluated by measuring skinfold thickness with a Lange caliper (Cambridge Scientific Industries, Cambridge, MD). Laboratory results were obtained from the clinical laboratory facility at the Charité Hospital.
Mutational analysis
DNA was isolated from blood samples containing ethylenediaminetetraacetic acid. LMNA was directly sequenced as previously described.24 For amplification of PPARG, primers flanking each exon were designed and the products were amplified by PCR, purified and sequenced by cycle sequencing with fluorescent dye terminators on an automatic sequencer (ABI 310; Applied Biosystems, Darmstadt, Germany). The mutation within PPARG was confirmed by restriction fragment length polymorphism analysis using MnII.
Plasmid constructions
Constructs expressing wild‐type PPARγ and D424N PPARγ were cloned into the plasmid pSVK3 (GE Healthcare Bio‐Sciences Corp., Piscataway, New Jersey, USA) as described previously.15 To generate constructs encoding the protein fused to an epitope tag, wild‐type and D424N PPARγ cDNAs were cloned into the vector pSVF, which is identical to pSVK3 except for a FLAG tag coding sequence inserted in the multiple cloning site.
Reporter gene assays
293T cells were cultured in Dulbecco modified Eagle medium containing 10% fetal bovine serum (both Invitrogen, Carlsbad, California, USA) and transfected in 6‐well plates (Lipofectamine 2000; Invitrogen). Luciferase assay and normalisation after cotransfection with pSV‐β‐galactosidase plasmid (Promega, Madison, Wisconsin, USA) were performed as previously described.15 Assays were performed in the presence and absence of the PPARγ agonist rosiglitazone (Cayman Chemical, Ann Arbor, Michigan, USA), and interference of the mutant with the wild‐type receptor activity and the dose response to rosiglitazone were assessed. Results were compared using Student t test with significance set at p<0.05.
Immunoblot analysis
Cos7 cells were cultured in Dulbecco modified Eagle medium containing 10% fetal bovine serum. Cells were transiently transfected with pSVF or pSVF containing wild‐type or D424N PPARγ cDNA in frame with a FLAG epitope coding sequence. Transfection with Lipofectamine 2000 and immunoblot analysis were performed as described previously.15
Results
Patients
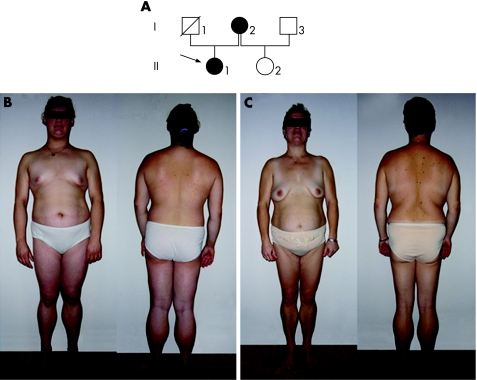
The family's pedigree is shown in figure 1, and demographic and laboratory data of the affected family members are given in table 1. The index patient (II.1, fig 1B), a 14 year‐old Caucasian girl, was referred to the Charité Hospital for hypertriglyceridaemia. Laboratory analysis showed raised serum triglyceride and cholesterol levels and raised aminotransferase and γ‐glutamyl transpeptidase activities. Abdominal ultrasound showed increased echogenecity, consistent with hepatic steatosis. Fasting glucose was normal, but a Homeostasis Model Assessment (HOMA) Index of 2.7, determined using the HOMA2 calculator (http://www.dtu.ox.ac.uk/), indicated insulin resistance. Physical examination found loss of subcutaneous fat and muscular hypertrophy, predominantly on the legs. Triceps skinfold thickness was <5th centile compared with a normal population,25 indicating loss of subcutaneous fat from the arms. Accumulation of subcutaneous fat was present in face, chin, trunk and abdomen. Skinfold thickness at the mid‐abdomen was 3.4 cm, indicating the presence of subcutaneous fat. Pronounced acanthosis nigricans was observed at the neck, axillae and inguinal region. Arterial hypertension had been diagnosed at 12 years of age and menstrual cycles were irregular.
Figure 1 Pedigree and photographs of members of a family with partial lipodystrophy. (A) Pedigree of the family. The index patient is marked with an arrow. Filled symbols, affected members; unfilled symbols, unaffected patients; symbol with a diagonal line, deceased member. (B) Index patient (II.1), showing loss of fat and muscular hypertrophy especially of the legs, and accumulation of subcutaneous fat on the trunk and in the face. (C) The index patient's mother (I.1) shows similar clinical features. Parental/guardian informed consent was obtained for publication of this figure.
Table 1 Demographic and laboratory data of the family members with the PPARG D424N mutation.
| Subject II.1 | Subject I.2 | |
|---|---|---|
| Age | 14 | 36 |
| BMI (kg/m2) | 28.7 | 29.7 |
| HbA1c (%) | 5.2 | 5.4 |
| Plasma glucose (mmol/l) | 4.4 | 4.4 |
| Insulin (pmol/l) | 154.9 | 84.7 |
| HOMA | 2.7 | 1.5 |
| Total cholesterol (mmol/l) | 9.73 | 4.05 |
| HDL cholesterol (mmol/l) | 0.62 | 0.57 |
| Triglycerides (mmol/l) | 28.91 | 8.45 |
| ALT (U/l) | 82 | 19 |
| GGT (U/l) | 150 | 43 |
ALT, alanine aminotransferase; BMI, body mass index; GGT, γ‐glutamyl transpeptidase; HDL, high‐density lipoprotein; HOMA, Homeostasis Model Assessment.
Blood samples were obtained after a 10‐hour fasting period.
Reference ranges: ALT (females) <34 U/l; GGT (females) <38 U/l; haemoglobin A1c <6.2%; HDL cholesterol >1.2 mmol/l; HOMA age <25 years: <2.0, age 25–35 years: <3.0, age >35 years: <4.0; insulin <173 pmol/l; plasma glucose <5.5 mmol/l; total cholesterol <5.2 mmol/l; triglycerides <2.0 mmol/l.
The index subject's mother (I.2, fig 1C) was 36 years old and presented with an abnormal distribution of subcutaneous fat similar to her daughter, showing loss from the legs and accumulation in face, chin, trunk, and abdomen, with a mid‐abdominal skinfold thickness of 4.1 cm. Triceps skinfold thickness was <5th centile. Muscular hypertrophy was observed on the legs. Slight acanthosis nigricans was present on the neck, axillae and inguinal folds. Laboratory results revealed raised serum triglyceride concentration, normal total serum cholesterol concentration, low serum high‐density lipoprotein cholesterol concentration, and raised γ‐glutamyl transpeptidase activity. HOMA index and fasting glucose were normal. Arterial hypertension was diagnosed at 21 years of age. A left ovarian cyst had been found during a routine gynaecological examination. This subject had experienced one episode of acute pancreatitis in the past.
The index patient's father (I.1) had died at the age of 31 years from unknown cause. Her 4‐year‐old half‐sister (II.2), who has a different biological father (I.3) was healthy and did not show clinical signs of partial lipodystrophy.
Identification of the PPARG D424N mutation
Because the index patient presented with signs and symptoms of partial lipodystrophy, we sequenced the candidate genes LMNA and PPARG. Patient II.1 had a heterozygous guanine to adenine transversion at nucleotide 1270 in exon 5 of PPARG, leading to the replacement of aspartate by asparagine at residue 424 (D424N) in the encoded protein (amino acid nomenclature refers to PPARγ isoform 2). This patient's affected mother had the same heterozygous mutation, whereas the half‐sister did not. The mutation created an MnII endonuclease site. The absence of the PPARG D424N mutation from 248 control chromosomes of the same ethnic background and from 25 people with partial lipodystrophy due to LMNA mutations was confirmed using restriction fragment length polymorphism analysis (data not shown).
D424N PPARγ has decreased transcriptional activity
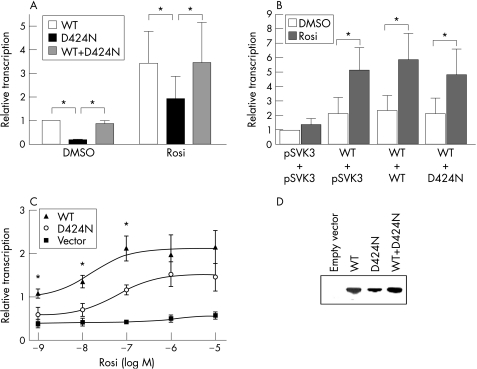
The ability of D424N PPARγ to activate transcription was investigated by measurement of reporter gene activity in transfected 293T cells. In the absence of rosiglitazone, mean (SD) reporter gene activity in cells expressing D424N PPARγ was 18.4 (1.9)% of that in cells expressing wild‐type PPARγ. Rosiglitazone enhanced the transcriptional activity of wild‐type PPARγ and of D424N PPARγ, so that the difference between wild‐type and mutant PPARγ was no longer statistically significant (fig 2A). This indicates a partial loss of function of D424N PPARγ and shows that stimulation of the mutant receptor with a synthetic agonist is possible. Mixing experiments with equal concentrations of plasmids encoding wild‐type and mutant PPARγ in the absence and presence of rosiglitazone led to a reporter gene activity that was similar to the activity of wild‐type PPARγ alone (fig 2A).
Figure 2 Transcriptional activity of wild‐type and D424N PPARγ in transfected 293T cells. (A) Cells were transfected with 1 μg of empty vector or constructs containing wild‐type and D424N PPARγ cDNA, either individually or in combination and were treated with either vehicle (dimethyl sulphoxide; DMSO) or 10 μM rosiglitazone (Rosi) for 24 h. Relative luciferase activity was measured in extracts and normalised to β‐galactosidase activities. Background activity of cells transfected with empty vector was subtracted from results and relative transcriptional activity was calculated as a percentage of the maximum activity achieved by wild‐type PPARγ in the absence of rosiglitazone. Values are means (SD) for three independent experiments. Asterisks indicate statistically significant differences (p<0.05). (B) 293T cells were transfected with 2.4 μg empty vector (pSVK3) or 1.2 μg wild‐type PPARγ cDNA construct plus 1.2 μg empty vector, or wild‐type or D424N PPARγ cDNA construct. Cells were treated with either DMSO or 10 μmol/l rosiglitazone. Transcriptional activity was measured and results are shown as a percentage of the maximum activity measured in cells transfected with empty vector and treated with DMSO. Values are mean (SD) for three independent experiments. Asterisks indicate statistically significant differences (p<0.05). Whereas transcriptional activity was significantly increased in each group after rosiglitazone was added, the comparison of the different groups with each other did not reveal statistically significant differences, indicating that D424N PPARγ does not exert a negative effect on wild‐type PPARγ. (C) Rosiglitazone dose–response curves for cells transfected with 1 μg plasmid encoding wild‐type and D424N PPARγ cDNA construct as well as empty vector. Transfected 293T cells were treated with increasing concentrations of rosiglitazone. Results were normalised to β‐galactosidase activity and calculated as a percentage of the wild‐type form treated with vehicle. Data are presented as mean (SD) (n = 3). Asterisks indicate statistically significant differences (p<0.05) between the transcriptional activity of wild‐type and D424N PPARγ. (D) Immunoblot analysis of FLAG‐tagged wild‐type PPARγ, D424N PPARγ and the combination of wild‐type and D424N PPARγ. Cells were transfected with either empty vector, plasmids encoding wild‐type PPARγ, D424N PPARγ or equal amounts of both plasmids. Proteins were fused to a FLAG epitope, which was detected with an anti‐FLAG antibody. One representative blot out of three experiments is shown.
To confirm this observation, cells were transfected with plasmid encoding wild‐type PPARγ and equal amounts of empty vector, or wild‐type or D424N PPARγ cDNA constructs. Compared with the activity of cells transfected with wild‐type PPARγ cDNA, transcriptional activity did not decrease when D424N PPARγ cDNA was added. Thus, the mutant protein did not interfere with the wild‐type receptor activity (fig 2B).
The rosiglitazone dose‐response curve showed that transcriptional activity of D424N PPARγ could be stimulated by rosiglitazone. With concentrations of 1–100 nmol/l rosiglitazone, the maximum transcriptional activity of D424N PPARγ was significantly reduced compared with wild‐type PPARγ. When 1 μmol/l or 10 μmol/l of the agonist were added, the difference between wild‐type and mutant PPARγ was no longer significant (fig 2C).
To show that D424N PPARγ was expressed in the reporter gene assays, cells were transfected with plasmids encoding D424N and wild‐type PPARγ fused to a FLAG epitope. Immunoblot analysis verified that D424N and wild‐type PPARγ were expressed (fig 2D). Reporter gene assays were also performed with constructs encoding the protein fused to the FLAG tag; the results did not differ from those performed with constructs encoding proteins without FLAG tag (data not shown).
Discussion
We have identified an amino acid substitution in the LBD of PPARγ in two related people with familial partial lipodystrophy. Both had maldistribution of subcutaneous fat with a prominent accumulation of fat in the region of the abdomen, which was confirmed by caliper measurement of the mid‐abdominal skinfold thickness. The abdominal accumulation of subcutaneous fat has previously been reported to be different between patients with familial partial lipodystrophy due to PPARG and LMNA mutations, with patients with LMNA mutations having less accumulation of subcutaneous fat in the abdomen.26 Our results further support this observation, although intra‐abdominal fat content was not measured and additional patients have to be carefully evaluated to confirm this hypothesis. Other symptoms of familial partial lipodystrophy in our two patients were muscular hypertrophy, arterial hypertension and metabolic symptoms. The index patient was more severely affected than her mother, who presented with hypertriglyceridaemia but not insulin resistance. The different severity of symptoms might be due to modifier genes present in II.1 but not in I.2, which could enhance the development of metabolic dysfunction.
Functional analyses of D424N PPARγ showed a significantly reduced transcriptional activity compared with wild‐type PPARγ. We did not detect any negative effect of D424N PPARγ on the activity of the wild‐type protein. This confirms previous observations that haploinsufficiency with a reduction of the abnormal protein's transcriptional activity ⩾50% is sufficient to cause partial lipodystrophy.9,12,14 Treatment with 1 μmol/l or 10 μmol/l of the PPARγ agonist rosiglitazone led to an almost normal transcriptional activation of D424N PPARγ compared with wild‐type PPARγ. This suggests that loss of function of the abnormal protein can be corrected. Treatment with PPARγ agonists could therefore be promising in patients with familial partial lipodystrophy due to this or similar mutations.
Since the first description of a PPARG mutation in 1999,7 several other mutations in the LBD and the DBD of PPARγ have been reported to cause familial partial lipodystrophy. The pathophysiological mechanism of these mutations is either haploinsufficiency or interference with the wild‐type protein. Of the seven mutations in the LBD, four have been shown to exert a negative effect on the activity of the wild‐type protein7,11 and one, R425C, has not been functionally evaluated.8 In the DBD, three of six mutations were shown to interfere with the wild‐type protein. In cases without negative interference with wild‐type PPARγ, loss of function of only one allele seems to be sufficient to cause the disease.11,14,15 A frameshift mutation, leading to a truncation of both DBD and LBD, was not functionally assessed but is suspected to cause partial lipodystrophy due to haploinsufficiency.13 Mutations in the DBD and the LBD affect all PPARγ isoforms, whereas a mutation in the promoter region of PPARG encoding PPARγ4 leads to a selective deficiency of isoform 4 but also to partial lipodystrophy.10 Another mutation in PPARG has been reported to cause a lipodystrophic phenotype only if patients are heterozygous for a second mutation in another gene.27 Hence, mutations or polymorphisms in PPARG in combination with certain genetic backgrounds might predispose individuals to the development of the metabolic syndrome and could also explain different forms and degrees of obesity in the broad population. This hypothesis remains to be confirmed.
Acknowledgements
AL was supported by a fellowship from the Deutsche Forschungsgemeinschaft (DFG LU 1206/1‐1).
Abbreviations
DBD - DNA‐binding domain
HOMA - Homeostasis Model Assessment
LBD - ligand‐binding domain
OMIM - Online Mendelian Inheritance in Man
PPAR - peroxisome proliferator‐activated receptor
Footnotes
Competing interests: None declared.
Parental/guardian informed consent was obtained for publication of figure 1.
References
- 1.Schmidt H H, Genschel J, Baier P, Schmidt M, Ockenga J, Tietge U J, Propsting M, Buttner C, Manns M P, Lochs H, Brabant G. Dyslipemia in familial partial lipodystrophy caused by an R482W mutation in the LMNA gene. J Clin Endocrinol Metab 2001862289–2295. [DOI] [PubMed] [Google Scholar]
- 2.Garg A. Acquired and inherited lipodystrophies. N Engl J Med 20043501220–1234. [DOI] [PubMed] [Google Scholar]
- 3.Ludtke A, Genschel J, Brabant G, Bauditz J, Taupitz M, Koch M, Wermke W, Worman H J, Schmidt H H. Hepatic steatosis in Dunnigan‐type familial partial lipodystrophy. Am J Gastroenterol 20051002218–2224. [DOI] [PubMed] [Google Scholar]
- 4.Cao H, Hegele R A. Nuclear lamin A/C R482Q mutation in Canadian kindreds with Dunnigan‐type familial partial lipodystrophy. Hum Mol Genet 20009109–112. [DOI] [PubMed] [Google Scholar]
- 5.Shackleton S, Lloyd D J, Jackson S N, Evans R, Niermeijer M F, Singh B M, Schmidt H, Brabant G, Kumar S, Durrington P N, Gregory S, O'Rahilly S, Trembath R C. LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nat Genet 200024153–156. [DOI] [PubMed] [Google Scholar]
- 6.Speckman R A, Garg A, Du F, Bennett L, Veile R, Arioglu E, Taylor S I, Lovett M, Bowcock A M. Mutational and haplotype analyses of families with familial partial lipodystrophy (Dunnigan variety) reveal recurrent missense mutations in the globular C‐terminal domain of lamin A/C. Am J Hum Genet 2000661192–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barroso I, Gurnell M, Crowley V E, Agostini M, Schwabe J W, Soos M A, Maslen G L, Williams T D, Lewis H, Schafer A J, Chatterjee V K, O'Rahilly S. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature 1999402880–883. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal A K, Garg A. A novel heterozygous mutation in peroxisome proliferator‐activated receptor‐gamma gene in a patient with familial partial lipodystrophy. J Clin Endocrinol Metab 200287408–411. [DOI] [PubMed] [Google Scholar]
- 9.Hegele R A, Cao H, Frankowski C, Mathews S T, Leff T. PPARG F388L, a transactivation‐deficient mutant, in familial partial lipodystrophy. Diabetes 2002513586–3590. [DOI] [PubMed] [Google Scholar]
- 10.Al‐Shali K, Cao H, Knoers N, Hermus A R, Tack C J, Hegele R A. A single‐base mutation in the peroxisome proliferator‐activated receptor gamma4 promoter associated with altered in vitro expression and partial lipodystrophy. J Clin Endocrinol Metab 2004895655–5660. [DOI] [PubMed] [Google Scholar]
- 11.Agostini M, Schoenmakers E, Mitchell C, Szatmari I, Savage D, Smith A, Rajanayagam O, Semple R, Luan J, Bath L, Zalin A, Labib M, Kumar S, Simpson H, Blom D, Marais D, Schwabe J, Barroso I, Trembath R, Wareham N, Nagy L, Gurnell M, O'Rahilly S, Chatterjee K. Non‐DNA binding, dominant‐negative, human PPARgamma mutations cause lipodystrophic insulin resistance. Cell Metab 20064303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francis G A, Li G, Casey R, Wang J, Cao H, Leff T, Hegele R A. Peroxisomal proliferator activated receptor‐gamma deficiency in a Canadian kindred with familial partial lipodystrophy type 3 (FPLD3). BMC Med Genet 200673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hegele R A, Ur E, Ransom T P, Cao H. A frameshift mutation in peroxisome‐ proliferator‐activated receptor‐gamma in familial partial lipodystrophy subtype 3 (FPLD3; MIM 604367). Clin Genet 200670360–362. [DOI] [PubMed] [Google Scholar]
- 14.Monajemi H, Zhang L, Li G, Jeninga E H, Cao H, Maas M, Brouwer C B, Kalkhoven E, Stroes E, Hegele R A, Leff T. Familial partial lipodystrophy phenotype resulting from a single‐base mutation in DNA binding domain of peroxisome proliferator‐activated receptor gamma. J Clin Endocrinol Metab 2007921606–1612. [DOI] [PubMed] [Google Scholar]
- 15.Ludtke A, Buettner J, Wu W, Muchir A, Schroeter A, Zinn‐Justin S, Spuler S, Schmidt H H, Worman H J. Peroxisome proliferator‐activated receptor gamma C190S mutation causes partial lipodystrophy. J Clin Endocrinol Metab 2007922248–2255. [DOI] [PubMed] [Google Scholar]
- 16.Rosen E D, Sarraf P, Troy A E, Bradwin G, Moore K, Milstone D S, Spiegelman B M, Mortensen R M. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell 19994611–617. [DOI] [PubMed] [Google Scholar]
- 17.Kota B P, Huang T H, Roufogalis B D. An overview on biological mechanisms of PPARs. Pharmacol Res 20055185–94. [DOI] [PubMed] [Google Scholar]
- 18.Kliewer S A, Umesono K, Noonan D J, Heyman R A, Evans R M. Convergence of 9‐cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature 1992358771–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freedman L P, Luisi B F, Korszun Z R, Basavappa R, Sigler P B, Yamamoto K R. The function and structure of the metal coordination sites within the glucocorticoid receptor DNA binding domain. Nature 1988334543–546. [DOI] [PubMed] [Google Scholar]
- 20.Elbrecht A, Chen Y, Cullinan C A, Hayes N, Leibowitz M, Moller D E, Berger J. Molecular cloning, expression and characterization of human peroxisome proliferator activated receptors gamma 1 and gamma 2. Biochem Biophys Res Commun 1996224431–437. [DOI] [PubMed] [Google Scholar]
- 21.Fajas L, Fruchart J C, Auwerx J. PPARgamma3 mRNA: a distinct PPARgamma mRNA subtype transcribed from an independent promoter. FEBS Lett 199843855–60. [DOI] [PubMed] [Google Scholar]
- 22.Sundvold H, Lien S. Identification of a novel peroxisome proliferator‐activated receptor (PPAR) gamma promoter in man and transactivation by the nuclear receptor RORalpha1. Biochem Biophys Res Commun 2001287383–390. [DOI] [PubMed] [Google Scholar]
- 23.Fajas L, Auboeuf D, Raspe E, Schoonjans K, Lefebvre A M, Saladin R, Najib J, Laville M, Fruchart J C, Deeb S, Vidal‐Puig A, Flier J, Briggs M R, Staels B, Vidal H, Auwerx J. The organization, promoter analysis, and expression of the human PPARgamma gene. J Biol Chem 199727218779–18789. [DOI] [PubMed] [Google Scholar]
- 24.Fatkin D, MacRae C, Sasaki T, Wolff M R, Porcu M, Frenneaux M, Atherton J, Vidaillet H J, Jr, Spudich S, De Girolami U, Seidman J G, Seidman C, Muntoni F, Muehle G, Johnson W, McDonough B. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction‐system disease. N Engl J Med 19993411715–1724. [DOI] [PubMed] [Google Scholar]
- 25.Frisancho A R. New norms of upper limb fat and muscle areas for assessment of nutritional status. Am J Clin Nutr 1981342540–2545. [DOI] [PubMed] [Google Scholar]
- 26.Al‐Attar S A, Pollex R L, Robinson J F, Miskie B A, Walcarius R, Harper Little C, Rutt B K, Hegele R A. Quantitative and qualitative differences in subcutaneous adipose tissue stores across lipodystrophy types shown by magnetic resonance imaging. BMC Med Imaging 2007123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savage D B, Agostini M, Barroso I, Gurnell M, Luan J, Meirhaeghe A, Harding A H, Ihrke G, Rajanayagam O, Soos M A, George S, Berger D, Thomas E L, Bell J D, Meeran K, Ross R J, Vidal‐Puig A, Wareham N J, O'Rahilly S, Chatterjee V K, Schafer A J. Digenic inheritance of severe insulin resistance in a human pedigree. Nat Genet 200231379–384. [DOI] [PubMed] [Google Scholar]