Abstract
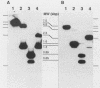
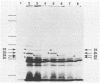
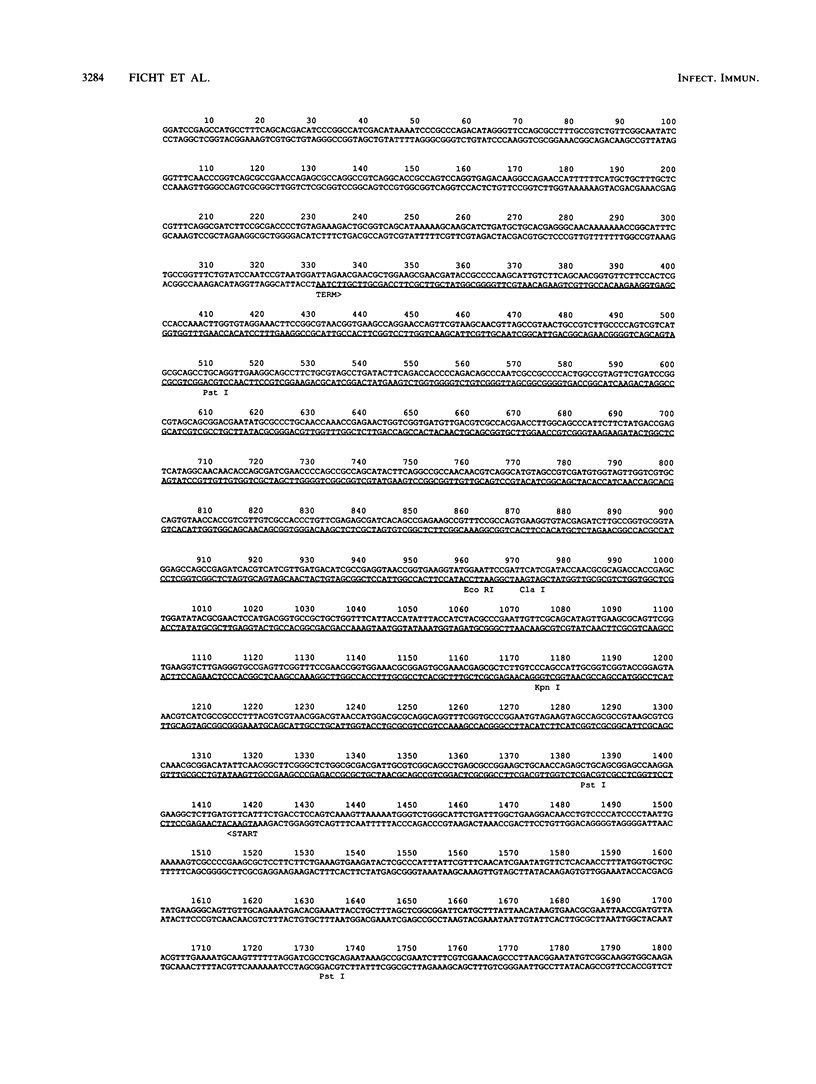
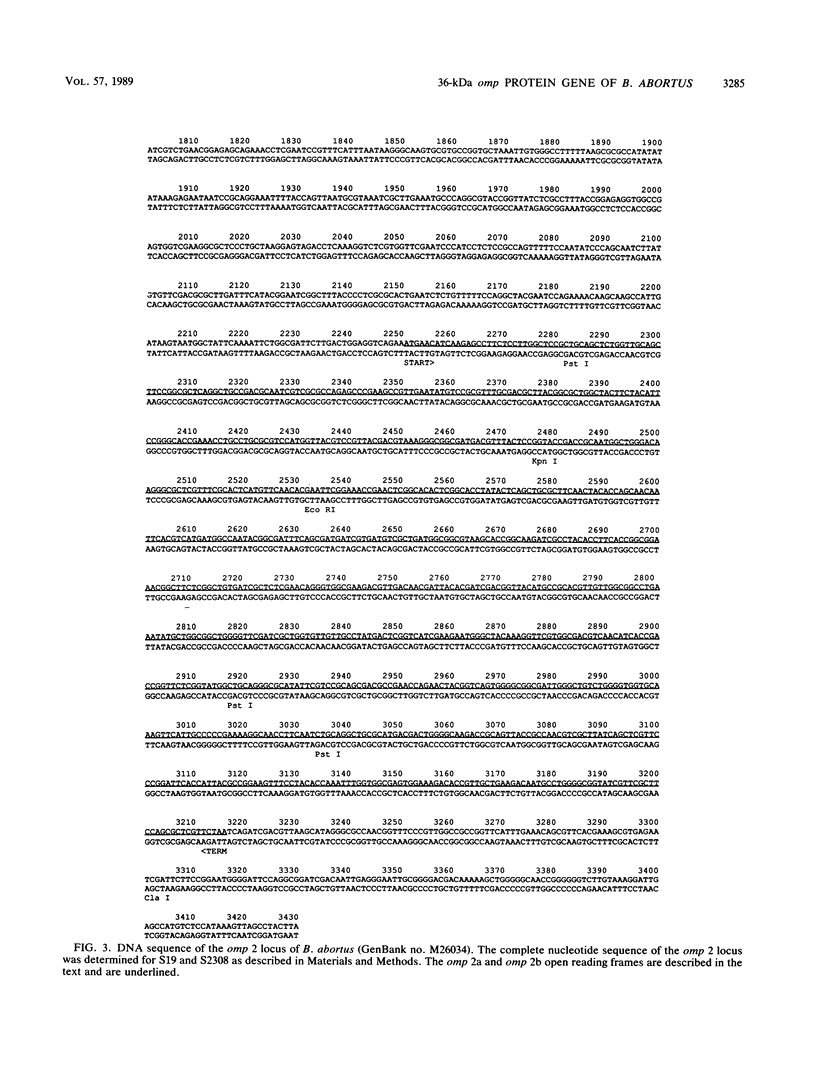
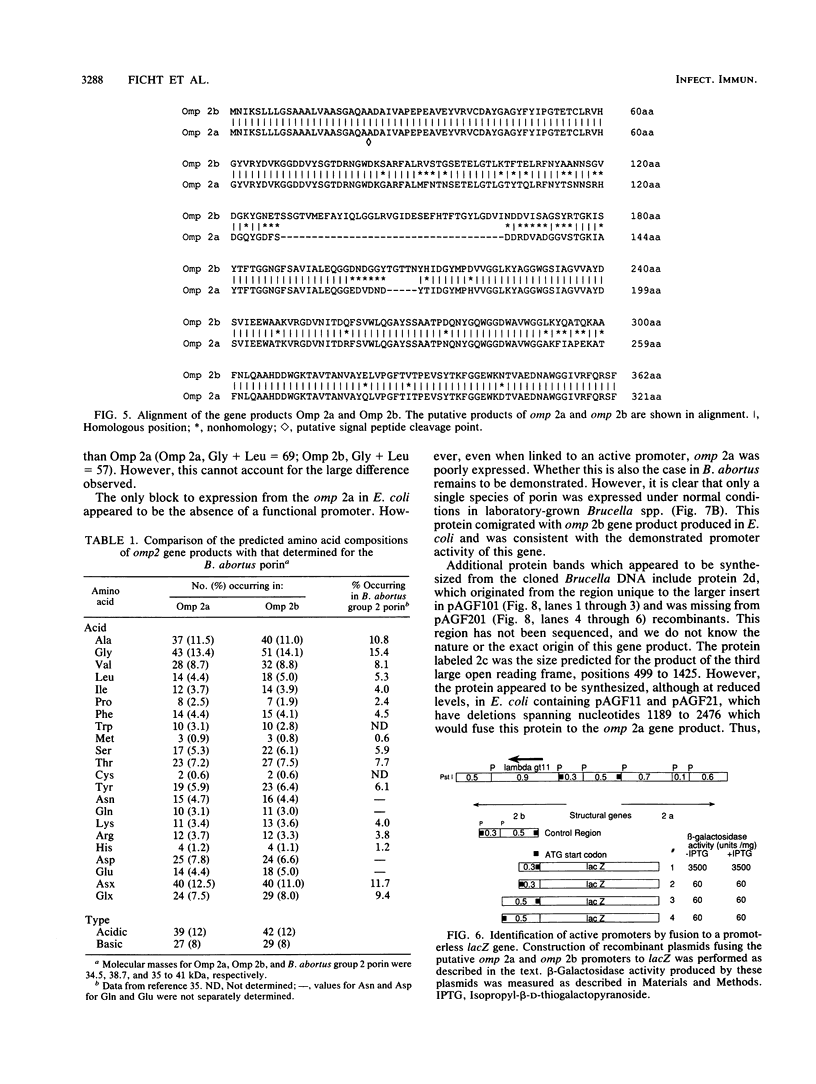
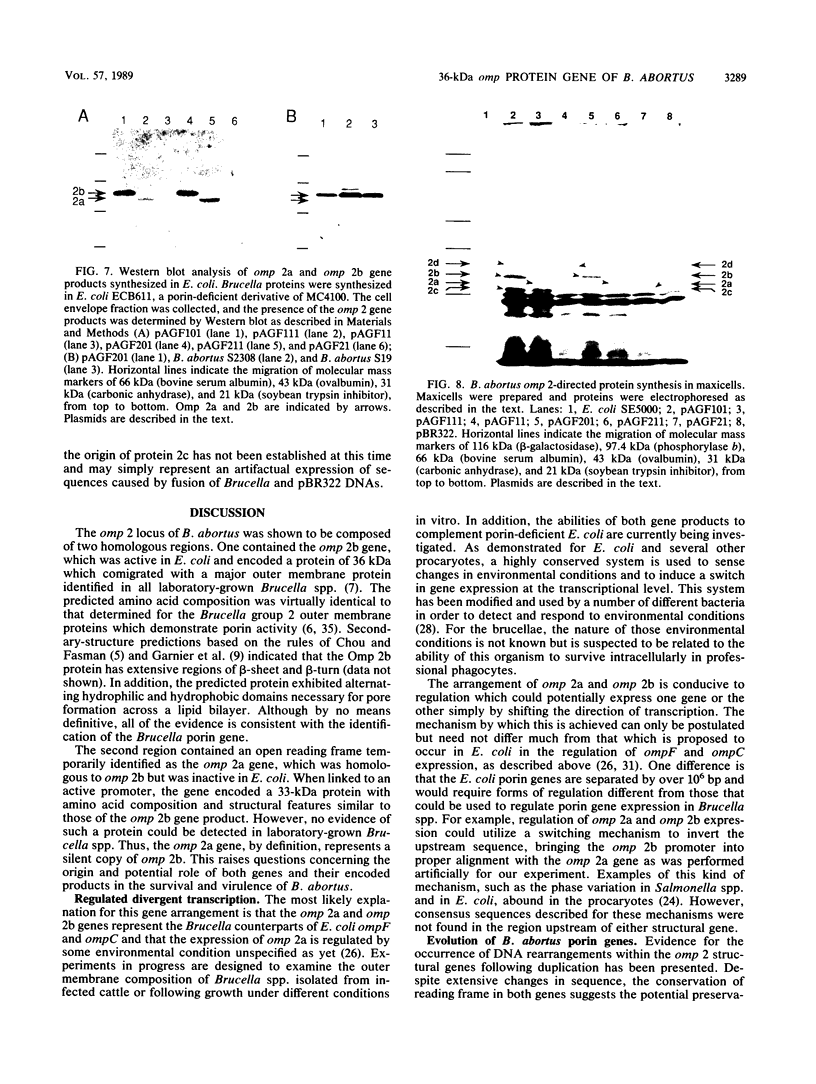
The cloning of the gene(s) encoding a 36-kilodalton (kDa) cell envelope protein of Brucella abortus has been previously described (T. A. Ficht, S. W. Bearden, B. A. Sowa, and L. G. Adams, Infect, Immun. 56:2036-2046, 1988). In an attempt to define the nature of the previously described duplication at this locus we have sequenced 3,500 base pairs of genomic DNA encompassing this region. The duplication represented two similar open reading frames which shared more than 85% homology at the nucleotide level but differed primarily because of the absence of 108 nucleotides from one of the two gene copies. These two genes were read from opposite strands and potentially encoded proteins which are 96% homologous. The predicted gene products were identical over the first 100 amino acids, including 22-amino-acid-long signal sequences. The amino acid composition of the predicted proteins was similar to that obtained for the Brucella porin isolated by Verstreate et al. (D. R. Verstreate, M. T. Creasy, N. T. Caveney, C. L. Baldwin, M. W. Blab, and A. J. Winter, Infect. Immun. 35:979-989, 1982) and presumably represented two copies of the porin gene, tentatively identified as omp 2a (silent) and omp 2b (expressed). The homology between the two genes extended to and included Shine-Dalgarno sequences 7 base pairs upstream from the ATG start codons. Homology at the 3' ends extended only as far as the termination codon, but both genes had putative rho-independent transcription termination sites. Localization of the promoters proved more difficult, since the canonical procaryotic sequences could not be identified in the region upstream of either gene. Promoter activity was demonstrated by ligation to a promoterless lacZ gene in pMC1871. However, only one active promoter could be identified by using this system. A 36-kDa protein was synthesized in E. coli with the promoter in the native orientation and was identical in size to the protein produced in laboratory-grown B. abortus. When the promoter-containing fragment was inverted, a 33-kDa protein was expressed. These results were consistent with the predicted sizes based on the nucleotide sequences of the open reading frames in omp 2b and omp 2a. Whether this locus contains one active and one silent or cryptic porin gene, or two active Brucella porin genes expressed under different environmental conditions, is discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Canning P. C., Roth J. A., Deyoe B. L. Release of 5'-guanosine monophosphate and adenine by Brucella abortus and their role in the intracellular survival of the bacteria. J Infect Dis. 1986 Sep;154(3):464–470. doi: 10.1093/infdis/154.3.464. [DOI] [PubMed] [Google Scholar]
- Canning P. C., Roth J. A., Tabatabai L. B., Deyoe B. L. Isolation of components of Brucella abortus responsible for inhibition of function in bovine neutrophils. J Infect Dis. 1985 Nov;152(5):913–921. doi: 10.1093/infdis/152.5.913. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Douglas J. T., Rosenberg E. Y., Nikaido H., Verstreate D. R., Winter A. J. Porins of Brucella species. Infect Immun. 1984 Apr;44(1):16–21. doi: 10.1128/iai.44.1.16-21.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficht T. A., Bearden S. W., Sowa B. A., Adams L. G. A 36-kilodalton Brucella abortus cell envelope protein is encoded by repeated sequences closely linked in the genomic DNA. Infect Immun. 1988 Aug;56(8):2036–2046. doi: 10.1128/iai.56.8.2036-2046.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost A. J., Smith H., Witt K., Keppie J. The chemical basis of the virulence of Brucella abortus. X. A surface virulence factor which facilitates intracellular growth of Brucella abortus in bovine phagocytes. Br J Exp Pathol. 1972 Dec;53(6):587–596. [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Hall B. G., Yokoyama S., Calhoun D. H. Role of cryptic genes in microbial evolution. Mol Biol Evol. 1983 Dec;1(1):109–124. doi: 10.1093/oxfordjournals.molbev.a040300. [DOI] [PubMed] [Google Scholar]
- Hu N., Messing J. The making of strand-specific M13 probes. Gene. 1982 Mar;17(3):271–277. doi: 10.1016/0378-1119(82)90143-3. [DOI] [PubMed] [Google Scholar]
- Kreutzer D. L., Robertson D. C. Surface macromolecules and virulence in intracellular parasitism: comparison of cell envelope components of smooth and rough strains of Brucella abortus. Infect Immun. 1979 Mar;23(3):819–828. doi: 10.1128/iai.23.3.819-828.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lutkenhaus J. F. Role of a major outer membrane protein in Escherichia coli. J Bacteriol. 1977 Aug;131(2):631–637. doi: 10.1128/jb.131.2.631-637.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield J. E., Bricker B. J., Godfrey H., Crosby R. M., Knight D. J., Halling S. M., Balinsky D., Tabatabai L. B. The cloning, expression, and nucleotide sequence of a gene coding for an immunogenic Brucella abortus protein. Gene. 1988;63(1):1–9. doi: 10.1016/0378-1119(88)90540-9. [DOI] [PubMed] [Google Scholar]
- McClure W. R. Mechanism and control of transcription initiation in prokaryotes. Annu Rev Biochem. 1985;54:171–204. doi: 10.1146/annurev.bi.54.070185.001131. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Meyer M. E. Metabolic characterization of the genus Brucella. VI. Growth stimulation by i-erythritol compared with strain virulence for guinea pigs. J Bacteriol. 1967 Mar;93(3):996–1000. doi: 10.1128/jb.93.3.996-1000.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A comparative study on the genes for three porins of the Escherichia coli outer membrane. DNA sequence of the osmoregulated ompC gene. J Biol Chem. 1983 Jun 10;258(11):6932–6940. [PubMed] [Google Scholar]
- Mutoh N., Inokuchi K., Mizushima S. Amino acid sequence of the signal peptide of OmpF, a major outer membrane protein of Escherichia coli. FEBS Lett. 1982 Jan 25;137(2):171–174. doi: 10.1016/0014-5793(82)80341-4. [DOI] [PubMed] [Google Scholar]
- Parker L. L., Hall B. G. A fourth Escherichia coli gene system with the potential to evolve beta-glucoside utilization. Genetics. 1988 Jul;119(3):485–490. doi: 10.1093/genetics/119.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasterk R. H., Brinkman A., van de Putte P. DNA inversions in the chromosome of Escherichia coli and in bacteriophage Mu: relationship to other site-specific recombination systems. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5355–5358. doi: 10.1073/pnas.80.17.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T. Transcription termination and the regulation of gene expression. Annu Rev Biochem. 1986;55:339–372. doi: 10.1146/annurev.bi.55.070186.002011. [DOI] [PubMed] [Google Scholar]
- Riley L. K., Robertson D. C. Ingestion and intracellular survival of Brucella abortus in human and bovine polymorphonuclear leukocytes. Infect Immun. 1984 Oct;46(1):224–230. doi: 10.1128/iai.46.1.224-230.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronson C. W., Nixon B. T., Ausubel F. M. Conserved domains in bacterial regulatory proteins that respond to environmental stimuli. Cell. 1987 Jun 5;49(5):579–581. doi: 10.1016/0092-8674(87)90530-7. [DOI] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siehnel R. J., Worobec E. A., Hancock R. E. Cloning of the Pseudomonas aeruginosa outer membrane porin protein P gene: evidence for a linked region of DNA homology. J Bacteriol. 1988 May;170(5):2312–2318. doi: 10.1128/jb.170.5.2312-2318.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slauch J. M., Garrett S., Jackson D. E., Silhavy T. J. EnvZ functions through OmpR to control porin gene expression in Escherichia coli K-12. J Bacteriol. 1988 Jan;170(1):439–441. doi: 10.1128/jb.170.1.439-441.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreate D. R., Creasy M. T., Caveney N. T., Baldwin C. L., Blab M. W., Winter A. J. Outer membrane proteins of Brucella abortus: isolation and characterization. Infect Immun. 1982 Mar;35(3):979–989. doi: 10.1128/iai.35.3.979-989.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]