Abstract
A recent study, looking at the lifetime risk of developing malignant peripheral nerve sheath tumour (MPNST) in patients with neurofibromatosis type 1 (NF1), estimated the risk to be 8–13%. Prior to this, longitudinal studies had shown that patients with NF1 had a risk of 4–5% of developing MPNST, and cross‐sectional studies had found that only 1–2% of patients with NF1 had MPNST. The aim of this study was to estimate the lifetime risk of MPNST in patients with NF1 in southern Scotland, using patient records obtained from the Edinburgh and Glasgow Genetic Units and Scottish Cancer Register. In the period 1993–2002, 14 patients with NF1 were diagnosed with MPNST in a population of 3.5 million. The lifetime risk of MPNST in the Scottish patients with NF1 was calculated to be 5.9–10.3%. This provides further evidence that patients with NF1 are at greater risk of developing MPNST than was previously estimated, and emphasises the importance of educating patients about suspicious symptoms, which may need an urgent medical opinion. The mean age at diagnosis of MPNST (p<0.05) and 5‐year survival (p<0.01) were significantly lower in patients with NF1 than in unaffected individuals. This may be due to patients with NF1 presenting later, because the tumour is mistaken for a neurofibroma, or due to MPNST having a more aggressive course in NF1.
Neurofibromatosis type 1 (NF1) is an autosomal dominant disorder with a predisposition to malignancy and a population prevalence of 1 in 4000.1 The diagnosis is made according to the criteria set out by the 1987 National Institute of Health (NIH) Consensus Development Conference Statement on Neurofibromatosis.2 In earlier studies of tumour prevalence, it was estimated that the relative risk of developing malignancy in patients with NF1 was between 2.1 and 4 times greater than that in the general population.3,4 Patients with NF1 are particularly predisposed to tumours of the nervous system,5 one of which is malignant peripheral nerve sheath tumour (MPNST). The early diagnosis of MPNST is imperative to improve survival.6
Evans et al7 attempted to identify all the coexistent diagnoses of NF1 and MPNST in the north‐west of England over a 13‐year period by collecting information from both the local cancer register and the NorthWest Regional NF1 Genetic Register. They then calculated the lifetime risk of MPNST in patients with NF1 to be 8–13%. Prior to this, the accepted lifetime risk of MPNST in patients with NF1 from cross‐sectional studies was 1–2%.1
There has been discussion in the literature about the effect on survival of a coexistent diagnosis of MPNST and NF1 compared with a diagnosis of sporadic MPNST. Ducatman et al8 studied 120 patients with MPNST and concluded that those with a coexistent diagnosis of NF1 have a poorer prognosis, and most other studies concur with this. Evans et al7 found a 5‐year survival of 42% in their patients with sporadic MPNST compared with 21% in patients with a coexistent diagnosis of MPNST and NF1 (p = 0.09).
In the present study, we have estimated the lifetime risk of developing MPNST in patients with NF1 living in southern Scotland, using data from the records of the Edinburgh and Glasgow Genetic Units and the Scottish Cancer Register (SCR) for the 10‐year period 1993–2002. Age at diagnosis and survival have been compared in patients with MPNST with and without NF1.
Methods
The Edinburgh and Glasgow Genetics Units serve a population of 3.5 million. We have used the records in the local genetics units as well as the SCR to obtain maximal ascertainment of patients in southern Scotland with coexistent diagnoses of NF1 and MPNST in the period from 1993 to 2002 inclusive, and thus to assess the lifetime risk of MPNST in patients with NF1. This study involved seeking ethical approval from a number of bodies including the Caldicott Guardian, the Information and Statistics Division of NHS Scotland, the Central Office for Research Ethics Committees, and the Research and Development departments responsible for each of the hospitals in which there were patient records which were necessary to be accessed.
Records in the genetics departments
The medical records of all patients seen from 1993 to 2002 inclusive, with a possible diagnosis of NF1, were reviewed, and all patients with a confirmed diagnosis according to the NIH diagnostic criteria2 were entered into the study. Patients were classified as unknown if they were under 5 years old9 and either had no clinical features of NF1 but not sufficient to fulfil the NIH diagnostic criteria and no family history, as in both instances signs may still develop. Subsequently, the genetics notes of patients with confirmed NF1 were reviewed for diagnoses of all neoplasia within the study period. The diagnoses and sites of the neoplasia were confirmed from pathology reports that were requested from the oncology treatment centres.
The Scottish Cancer Register
To identify all cases of MPNST within the study region, a list of all cases of MPNST (ICD‐O 9540/3,9560/3, 9561/3) in individuals resident in the population area served by the Edinburgh and Glasgow Genetics Units and diagnosed from 1993 to 2002 inclusive was obtained from the SCR. The SCR monitors cancer trends in the Scottish population using pathology records and death certificates. Information is held in confidence and is released only when approval has been given by the information services division, privacy advisory committee and a research ethics committee.
On receipt of the SCR data, medical case notes were reviewed for confirmation of the pathology and evidence of a coexistent diagnosis of NF1. Informed consent was sought from all living patients and the notes of deceased patients were reviewed after consent had been sought from the relevant hospital consultant. A diagnosis of NF1 was confirmed by evidence of the NIH diagnostic criteria2 recorded in the notes.
Statistics
The annual incidence of MPNST in NF1 was calculated assuming a population prevalence of NF1 of 1/4000,1 and secondly assuming a birth incidence of 1/2500,1 using the method described by Evans et al.7 In each case, the estimated number of individuals with NF1 in the population was calculated and the annual incidence was taken as the annual number of cases of MPNST per patient with NF1. The lifetime risk was calculated by multiplying the annual incidence by the number of years of life. We assumed a life expectancy of 60–65 years based on the findings of two studies,10,11 which showed that patients with NF1 survive approximately 15 years less than individuals in the general population.
Patients with MPNST with and without NF1 were compared in terms of age at diagnosis (t test) and survival since diagnosis (Kaplan–Meier survival curves). Vital status of patients was recorded as on 1 January 2005. Spearman's rank correlation coefficient was calculated between age at diagnosis and survival since diagnosis in each group.
All calculations were made using SPSS V.14.0.
Results
In the review of the genetics records, 628 patients met the diagnostic criteria for NF1, 249 did not meet the criteria and the diagnosis was unknown in 38 cases (table 1). There were nine patients identified from this review with a coexistent diagnosis of MPNST within the study period. On review of the pathology, three of these patients were excluded because the pathology report was not consistent with MPNST, leaving a total of six coexistent NF1 and MPNST diagnoses from 1993 to 2002 inclusive.
Table 1 Neurofibromatosis type 1 status of patients after review of their genetics notes.
| NF1 status | Affected | Unaffected | Unknown |
|---|---|---|---|
| Edinburgh | 227 | 165 | 21 |
| Glasgow | 401 | 84 | 17 |
| Total | 628 | 249 | 38 |
NF1, neurofibromatosis type 1.
The Scottish Cancer Register provided a list of 46 patients diagnosed with MPNST from 1993 to 2002 inclusive. Four of these patients were already known to the genetics departments. Consent was sought via the general practitioner from the 21 living patients to access their medical records: 10 consented, 1 refused consent, 6 did not reply, and in 4 cases general practitioners were able to exclude NF1 and/or MPNST. The medical records from the patients who had consented were reviewed to confirm MPNST and identify possible coexistent NF1. The records of the 25 deceased patients were also reviewed. We were unable to verify medical history in one case as all the notes pertaining to this patient had been destroyed. Thus, the clinical details of 38 patients were available to us.
The diagnosis of MPNST was confirmed in 25 of the patients on the SCR, and 12 of these had a coexistent diagnosis of NF1. MPNST was excluded as a diagnosis in 10 of the SCR patients. For the remaining three patients, information available was inadequate to confirm or refute a diagnosis of MPNST (table 2).
Table 2 Conclusion of the review of the Scottish Cancer Register records.
| Diagnosis | Patients (n) |
|---|---|
| Sporadic MPNST | 13 |
| MPNST + NF1 | 12 |
| Not MPNST | 10 |
| Unknown | 3 |
MPNST, malignant peripheral nerve sheath tumour; NF1, neurofibromatosis type 1.
Two patients with MPNST and NF1 were identified from the records of the genetics units only. Details of both these patients were found on the SCR. One patient was diagnosed with MPNST in the study period, but was not registered until later, while the other had another diagnosis recorded on the SCR. The SCR alone identified eight coexistent diagnoses, and four cases were identified by both sources. Therefore, the overall number of individuals identified with a coexistent diagnosis of NF1 and MPNST in the study period was 14 (table 3).
Table 3 Sources identifying coexistent cases of malignant peripheral nerve sheath tumour and neurofibromatosis type 1.
| Record source | Patients (n) |
|---|---|
| Genetics records | 2 |
| SCR | 8 |
| Genetics records + SCR | 4 |
SCR, Scottish Cancer Register.
NF1 MPNST lifetime risk
Taking the prevalence of NF1 as 1/4000,1 the estimated number of individuals with NF1 in a population of 3 546 490 is 887. The annual incidence of MPNST in the NF1 population is calculated at 0.158% per annum or 1.58 per 1000 patients (1.4/887). If patients live to 60–65 years, this corresponds to a lifetime risk of 9.5–10.3%.
The birth incidence of NF1, however, has been quoted at 1/2500,1 which would correspond to an annual incidence of MPNST of 0.99 per 1000 patients. This corresponds to a lifetime risk of 5.9–6.4%. This is the minimum lifetime risk of developing MPNST for patients with NF1, because of the likelihood that there is under‐reporting.
Age at diagnosis
The mean (SD) age at diagnosis for MPNST alone was 59.0 (21.2) years, compared with 42.1 (16.6) years in patients with coexistent diagnoses of NF1 and MPNST. This difference is significant (p<0.05).
Survival
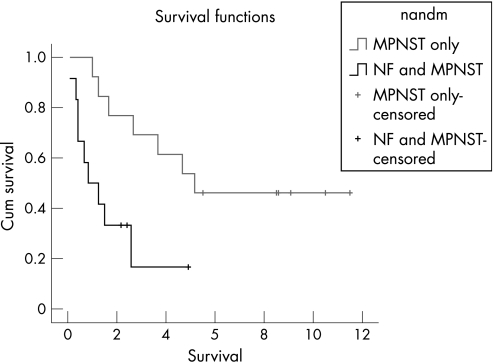
Figure 1 shows the Kaplan–Meier survival curves for the two patient groups. The Mantel–Cox log‐rank test indicated a significant difference between the groups (p<0.01). Among the patients with MPNST alone, the 5‐year survival rate was 54% (95% CI 29% to 77%). None of the 12 patients with a coexistent diagnosis of MPNST and NF1 survived for >5 years (95% CI 0% to 24%), although three were still alive 2.2–4.9 years after diagnosis. There was no significant correlation between age at diagnosis and survival time in either of the two groups of patients with and without NF1.
Figure 1 Kaplan–Meier curves comparing survival from malignant peripheral nerve sheath tumour (MPNST) alone with survival from MPNST with neurofibromatosis (NF) type 1.
Discussion
The predisposition to the development of malignant neoplasia, and particularly MPNST, in NF1 is well known. Before 2002, it was generally thought that the lifetime risk of developing MPNST in patients with NF1 was low. This was based on finding only 1–2% of patients with NF1 with MPNST in cross‐sectional studies.1 However, this might be an underestimate of the lifetime risk, because it is based on the percentage of patients with NF1 who are affected with MPNST at any one time and does not take into account loss of affected individuals from the cohort because of early death.
In 2002, Evans et al7 attempted to achieve full ascertainment of coexistent diagnoses of MPNST and NF1 over a 13‐year period and, using accepted values for the incidence and prevalence of NF1 in the population, to calculate the lifetime risk of developing MPNST in patients with NF1. In their study, they estimated a lifetime risk of 8–13%. This study has shown the lifetime risk to be around 5.9–10.3% in the Scottish cohort using the same statistical method as Evans. This estimated figure is smaller than that of Evans et al,7 but would have been very similar, had we assumed the same life span as Evans et al7 in their study.
This is the minimum lifetime risk, as there may have been further cases of coexistent diagnoses of MPNST and NF1 where the medical records were not accessible. This is illustrated by the fact that two of the cases of coexistent MPNST and NF1 were not recorded in the SCR but only in the genetics case notes. This may be because of a delay in coding and recording data, or because of alternative terminology being used in reporting histology, but the SCR estimated that at least 95% of MPNST diagnosed between 1993 and 2002 would have been registered at the time of data collection (personal communication). In addition, the genetics departments would not routinely see all individuals affected by NF1; hence, there may be further cases of coexistent diagnoses within the study period and area that have been missed.
The mean age at diagnosis of MPNST in patients with NF1 in our study was 42.1 years. In the study of Evans et al,7 the median age at diagnosis was 26 years, compared with 38 years in our study. This difference is probably due to the small number of patients involved in each study. The range of age at diagnosis in our study was 22–73 years, which was very similar to the range of 16–77 years found by Evans et al.7
The difference in survival from MPNST in patients with NF1 when compared with sporadic MPNST has been noted previously,7,8,12,13 and our study found a striking difference in the 5‐year survival. Evans et al calculated a 5‐year survival of 42% in sporadic cases of MPNST and 21% in patients with a coexistent diagnosis.7 In our study, the 5‐year survival in patients with sporadic MPNST was 54%, compared with 0% in those with a coexistent diagnosis. The Kaplan–Meier curves are striking, and a 0% 5‐year survival from MPNST in patients with NF1 is a major concern. This may be because patients with NF1 present later, as they are accustomed to developing new lumps and therefore have a higher consulting threshold than the general population, or it may be that MPNST has a more aggressive course in NF1. There may also be an element of bias towards those surviving for a shorter time, because of the necessity of obtaining consent from patients who are still alive, some of whom refused consent or did not reply. There is no reason, however, why this should have more effect on the NF1 group than on the group of sporadic cases.
A recent study identified microdeletions in the NF1 region in 23.7% of patients with a coexistent diagnosis of MPNST and NF1. This is significantly greater than the accepted microdeletion frequency of 5–10% in the NF1 population, and the authors deduced that MPNST is approximately twice as common in patients with NF1 with a microdeletion as in those with an alternative mutation on the NF gene.14 Patients who have received radiotherapy,7,8 have NF1 neuropathy15 or have internal plexiform neurofibromatosis16 are also thought to be at an increased risk of developing MPNST.
Key points
We have estimated the lifetime risk of malignant peripheral nerve sheath tumour (MPNST) in patients with neurofibromatosis type 1 (NF1) to be 5.9–10.3%, which is further evidence that it is greater than what was thought previously.
Our study gives further evidence that survival after diagnosis of MPNST is shorter in patients with NF1 compared with patients without NF1.
All patients with NF1 should seek specialist advice about suspicious symptoms, and current research indicates that there may be particular subgroups requiring regular surveillance.
Conclusions
The lifetime risk for patients with NF1 developing MPNST is significant. It is imperative that patients with NF1 are informed of this risk and educated about suspicious symptoms. They must access specialist opinion as a matter of urgency if these develop. More work is needed into the identification of an at‐risk subgroup within the NF1 population in relation to the De Raedt study.14 This is with the view to screening for this group, who would then have a high index of suspicion about new symptoms.
Acknowledgements
We thank all patients who kindly consented to this study, and the staff of the Scottish Cancer Registry and the Research and Development Offices of the hospitals who treated patients included in the study, for their help and advice.
Abbreviations
MPNST - malignant peripheral nerve sheath tumour
NF1 - neurofibromatosis type 1
NIH - National Institute of Health
SCR - Scottish Cancer Register
Footnotes
Competing interests: None.
References
- 1.Huson S M, Compston D A S, Harper P S. A genetic study of von Recklinghausen neurofibromatosis in south east Wales. I. Prevalence, fitness, mutation rate, and effect of parental transmission on severity. J Med Genet 198926704–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Diagnostic criteria for neurofibromatosis type 1 from the 1987 NIH Consensus Development Conference Statement on Neurofibromatosis
- 3.Zöller M E, Rembeck B, Odén A, Samuelsson M, Angervall L. Malignant and benign tumors in patients with neurofibromatosis type 1 in a defined Swedish population. Cancer 1997792125. [PubMed] [Google Scholar]
- 4.Sorensen S A, Mulvihill J J, Nielsen A. Long‐term follow‐up of von Recklinghausen neurofibromatosis survival and malignant neoplasms. N Engl J Med 19863141010–1015. [DOI] [PubMed] [Google Scholar]
- 5.Riccardi V M. Von Recklinghausen neurofibromatosis. N Engl J Med 19813051617–1627. [DOI] [PubMed] [Google Scholar]
- 6.Wanebo J, Malik J, VandenBerg S, Wanebo H, Driessen N, Persing J. Malignant peripheral nerve sheath tumors: a clinicopathological study of 28 cases. Cancer 1993711247–1253. [DOI] [PubMed] [Google Scholar]
- 7.Evans D G, Baser M E, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet 200239311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ducatman B S, Scheithauer B W, Piepgras D G, Reiman H M, Ilstrup D M. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer 1986572006–2021. [DOI] [PubMed] [Google Scholar]
- 9.Bundey S, Emery A E H. Phacomatoses and tumours. In: ed. Genetics and neurology. 2nd edn. Edinburgh: Churchill Livingstone, 199237–66.
- 10.Zöller M, Rembeck B, Åkesson H O, Angervall L. Life expectancy, mortality and prognostic factors in neurofibromatosis type 1: a twelve‐year follow‐up of an epidemiological study in Göteborg, Sweden. Acta Dermatol Venereol 199575136–140. [DOI] [PubMed] [Google Scholar]
- 11.Rasmussen S A, Yang Q, Friedman J M. Mortality in neurofibromatosis 1: an analysis using US death certificates. Am J Hum Genet 2001681110–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gosh B C, Gosh L, Huvos A G. Malignant schwannoma; a clinicopathological study. Cancer 197331184–190. [DOI] [PubMed] [Google Scholar]
- 13.Dasgupta T K, Brasfield R D. Solitary malignant schwannoma. Ann Surg 1970171419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Raedt T, Brems H, Wolkenstein P, Vidaud D, Pilotti S, Perrone F, Mautner V, Frahm S, Sciot R, Legius E. Elevated risk for MPNST in NF1 microdeletion patients. Am J Hum Genet 2003721288–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferner R E, Hughes R A C, Hall S M, Upadhyaya M, Johnson M R. Neurofibromatous neuropathy in neurofibromatosis 1 (NF1). J Med Genet 200441837–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tucker T, Wolkenstein P, Revuz J, Zeller J, Friedman J M. Association between benign and malignant peripheral nerve sheath tumors in NF1. Neurology 200565205–211. [DOI] [PubMed] [Google Scholar]