Abstract
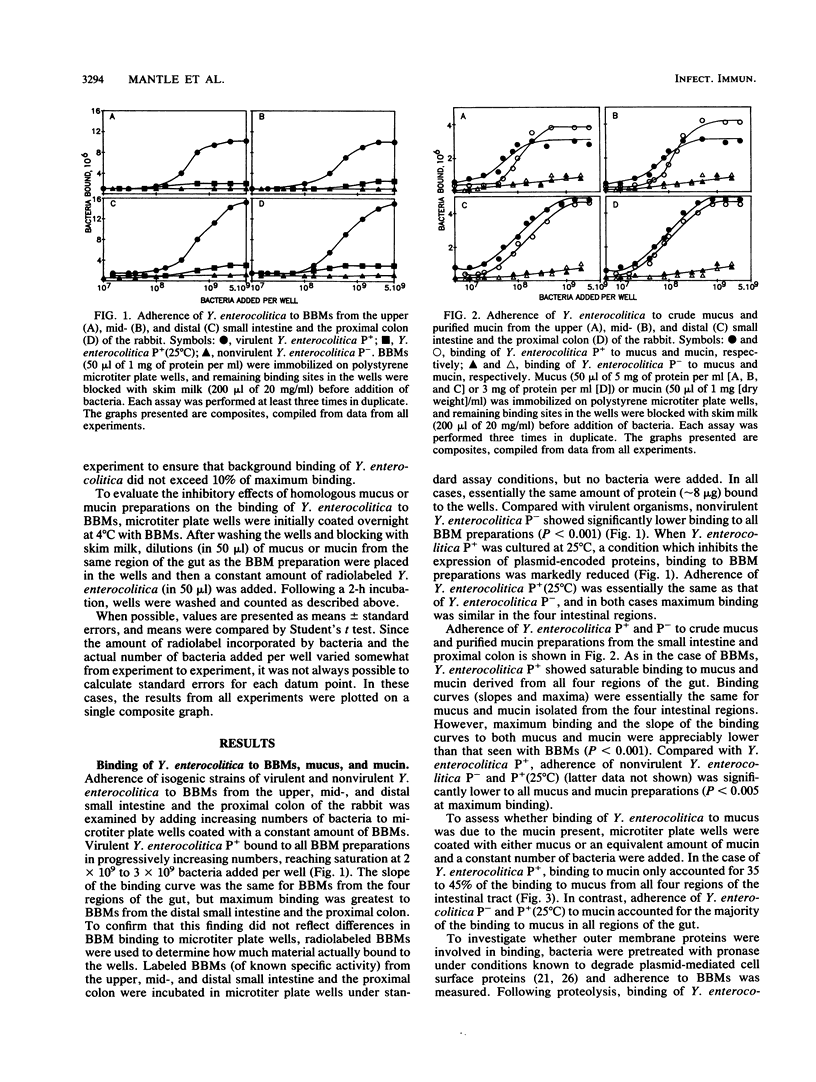
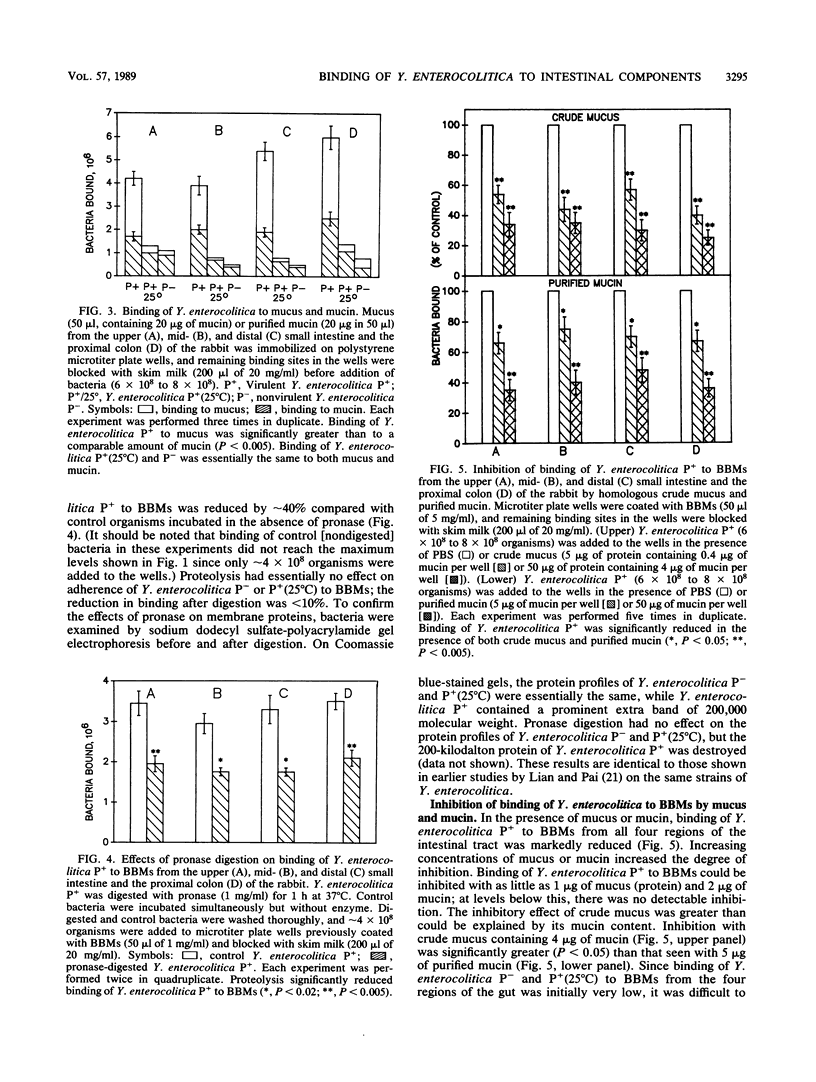
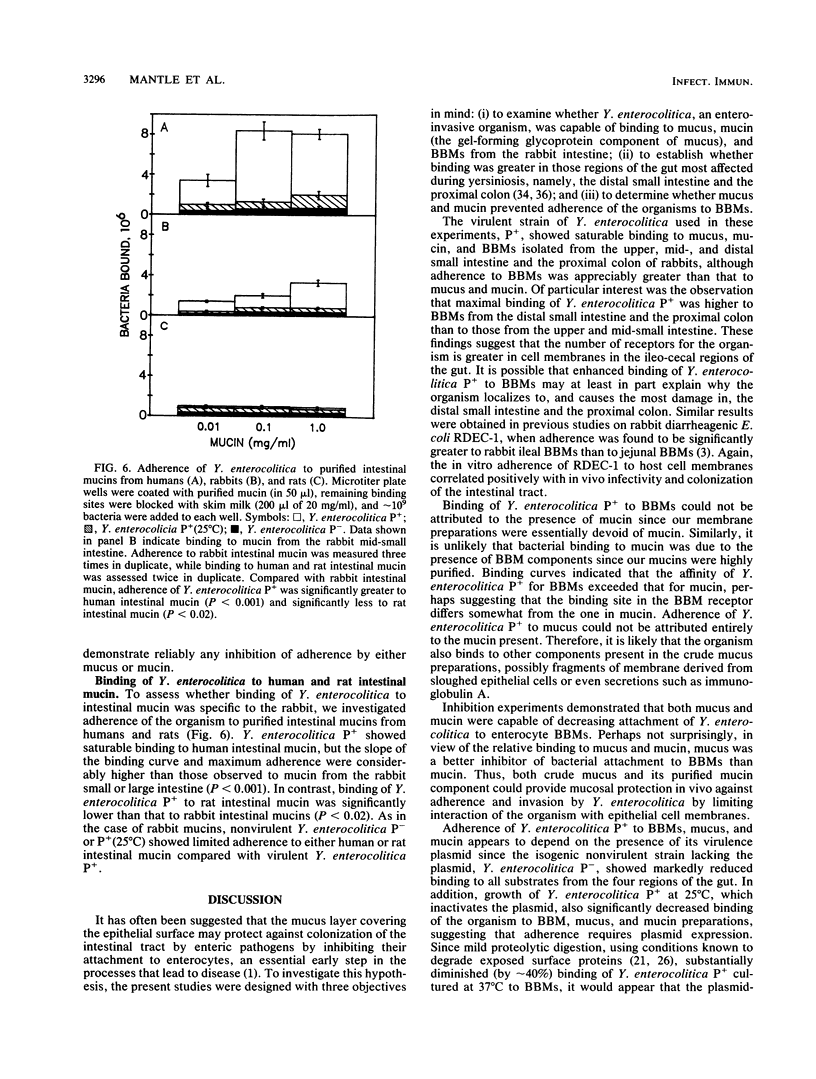
Mucus and its gel-forming glycoprotein component, mucin, are thought to protect the gastrointestinal tract from enteric pathogens by inhibiting their attachment to enterocytes. In this study, we investigated interactions between Yersinia enterocolitica (isogenic strains of virulent and nonvirulent organisms) and crude mucus, highly purified mucin, and brush border membranes (BBMs) isolated from the upper mid-, and distal small intestine and the proximal colon of the rabbit. Adherence of radiolabeled bacteria was assessed to BBMs, mucus, and mucin immobilized in polystyrene microtiter plate wells. Virulent Y. enterocolitica showed saturable binding to mucus, mucin, and BBMs from all four regions of the intestinal tract, although adherence to BBMs was appreciably greater than that to mucus or mucin. Maximal binding of bacteria was higher to BBMs from the distal small intestine and the proximal colon than to those from the upper and mid-small intestine, which may in part explain why the organism localizes to the ileo-caecal regions of the gut. Adherence of virulent Y. enterocolitica to BBMs was significantly reduced in the presence of homologous mucus or mucin preparations. Binding of virulent bacteria appears to depend on plasmid-encoded proteins located on the outer surface membrane, since (i) the isogenic strain lacking the virulence plasmid showed markedly less binding to all BBM, mucus, and mucin preparations; (ii) growth of the virulent strain at 25 degrees C, which inactivates its plasmid, significantly diminished binding to BBMs, mucus, and mucin; and (iii) mild proteolysis substantially decreased adherence of virulent bacteria to BBMs. Compared with rabbit intestinal and colonic mucins, binding of virulent Y. enterocolitica was significantly greater to purified human intestinal mucin and significantly less to rat intestinal mucin. These findings provide support for the role of mucus and mucin in host defense by preventing adherence of virulent Y. enterocolitica to epithelial cell membranes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Chadee K., Petri W. A., Jr, Innes D. J., Ravdin J. I. Rat and human colonic mucins bind to and inhibit adherence lectin of Entamoeba histolytica. J Clin Invest. 1987 Nov;80(5):1245–1254. doi: 10.1172/JCI113199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney C. P., Schad P. A., Formal S. B., Boedeker E. C. Species specificity of in vitro Escherichia coli adherence to host intestinal cell membranes and its correlation with in vivo colonization and infectivity. Infect Immun. 1980 Jun;28(3):1019–1027. doi: 10.1128/iai.28.3.1019-1027.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. S., Arruda J. C., Williams T. J., Laux D. C. Adhesion of a human fecal Escherichia coli strain to mouse colonic mucus. Infect Immun. 1985 Apr;48(1):139–145. doi: 10.1128/iai.48.1.139-145.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumm B., Roberton A. M., Sherman P. M. Inhibition of attachment of Escherichia coli RDEC-1 to intestinal microvillus membranes by rabbit ileal mucus and mucin in vitro. Infect Immun. 1988 Sep;56(9):2437–2442. doi: 10.1128/iai.56.9.2437-2442.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner G. G. (1-14C)glucosamine incorporation by subcellular fractions of small intestinal mucosa. Identification by precursor labeling of three functionally distinct glycoprotein classes. J Biol Chem. 1970 Jul 25;245(14):3584–3592. [PubMed] [Google Scholar]
- Forstner G. G., Sabesin S. M., Isselbacher K. J. Rat intestinal microvillus membranes. Purification and biochemical characterization. Biochem J. 1968 Jan;106(2):381–390. doi: 10.1042/bj1060381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemski P., Lazere J. R., Casey T. Plasmid associated with pathogenicity and calcium dependency of Yersinia enterocolitica. Infect Immun. 1980 Feb;27(2):682–685. doi: 10.1128/iai.27.2.682-685.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesemann J., Algermissen B., Laufs R. Genetically manipulated virulence of Yersinia enterocolitica. Infect Immun. 1984 Oct;46(1):105–110. doi: 10.1128/iai.46.1.105-110.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapperud G., Namork E., Skarpeid H. J. Temperature-inducible surface fibrillae associated with the virulence plasmid of Yersinia enterocolitica and Yersinia pseudotuberculosis. Infect Immun. 1985 Feb;47(2):561–566. doi: 10.1128/iai.47.2.561-566.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapperud G., Namork E., Skurnik M., Nesbakken T. Plasmid-mediated surface fibrillae of Yersinia pseudotuberculosis and Yersinia enterocolitica: relationship to the outer membrane protein YOP1 and possible importance for pathogenesis. Infect Immun. 1987 Sep;55(9):2247–2254. doi: 10.1128/iai.55.9.2247-2254.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. J., Volz P. A., Edwards C. A., Yancey R. J. Mechanisms of association of Candida albicans with intestinal mucosa. J Med Microbiol. 1987 Dec;24(4):333–341. doi: 10.1099/00222615-24-4-333. [DOI] [PubMed] [Google Scholar]
- Khavkin T. N., Kudryavtseva M. V., Dragunskaya E. M., Polotsky Y. E., Kudryavtsev B. N. Fluorescent PAS-reaction study of the epithelium of normal rabbit ileum and after challenge with enterotoxigenic Escherichia coli. Gastroenterology. 1980 Apr;78(4):782–790. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lachica R. V., Zink D. L., Ferris W. R. Association of fibril structure formation with cell surface properties of Yersinia enterocolitica. Infect Immun. 1984 Oct;46(1):272–275. doi: 10.1128/iai.46.1.272-275.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachica R. V., Zink D. L. Plasmid-associated cell surface charge and hydrophobicity of Yersinia enterocolitica. Infect Immun. 1984 May;44(2):540–543. doi: 10.1128/iai.44.2.540-543.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian C. J., Pai C. H. Inhibition of human neutrophil chemiluminescence by plasmid-mediated outer membrane proteins of Yersinia enterocolitica. Infect Immun. 1985 Jul;49(1):145–151. doi: 10.1128/iai.49.1.145-151.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Stewart G. Intestinal mucins from normal subjects and patients with cystic fibrosis. Variable contents of the disulphide-bound 118 kDa glycoprotein and different reactivities with an anti-(118 kDa glycoprotein) antibody. Biochem J. 1989 Apr 1;259(1):243–253. doi: 10.1042/bj2590243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Thakore E., Hardin J., Gall D. G. Effect of Yersinia enterocolitica on intestinal mucin secretion. Am J Physiol. 1989 Feb;256(2 Pt 1):G319–G327. doi: 10.1152/ajpgi.1989.256.2.G319. [DOI] [PubMed] [Google Scholar]
- Mantle M., Thakore E. Rabbit intestinal and colonic mucins: isolation, partial characterization, and measurement of secretion using an enzyme-linked immunoassay. Biochem Cell Biol. 1988 Oct;66(10):1045–1054. doi: 10.1139/o88-121. [DOI] [PubMed] [Google Scholar]
- Martinez R. J. Plasmid-mediated and temperature-regulated surface properties of Yersinia enterocolitica. Infect Immun. 1983 Sep;41(3):921–930. doi: 10.1128/iai.41.3.921-930.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSweegan E., Walker R. I. Identification and characterization of two Campylobacter jejuni adhesins for cellular and mucous substrates. Infect Immun. 1986 Jul;53(1):141–148. doi: 10.1128/iai.53.1.141-148.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Falkow S. Evidence for two genetic loci in Yersinia enterocolitica that can promote invasion of epithelial cells. Infect Immun. 1988 May;56(5):1242–1248. doi: 10.1128/iai.56.5.1242-1248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Farmer J. J., 3rd, Hill W. E., Falkow S. The ail locus is found uniquely in Yersinia enterocolitica serotypes commonly associated with disease. Infect Immun. 1989 Jan;57(1):121–131. doi: 10.1128/iai.57.1.121-131.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H. W., Whipp S. C., Baetz A. L. Comparative effects of enterotoxins from Escherichia coli and Vibrio cholerae on rabbit and swine small intestine. Lab Invest. 1971 Aug;25(2):133–140. [PubMed] [Google Scholar]
- Mouricout M. A., Julien R. A. Pilus-mediated binding of bovine enterotoxigenic Escherichia coli to calf small intestinal mucins. Infect Immun. 1987 May;55(5):1216–1223. doi: 10.1128/iai.55.5.1216-1223.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Loughlin E. V., Humphreys G., Dunn I., Kelly J., Lian C. J., Pai C., Gall D. G. Clinical, morphological, and biochemical alterations in acute intestinal Yersiniosis. Pediatr Res. 1986 Jul;20(7):602–608. doi: 10.1203/00006450-198607000-00005. [DOI] [PubMed] [Google Scholar]
- Pai C. H., DeStephano L. Serum resistance associated with virulence in Yersinia enterocolitica. Infect Immun. 1982 Feb;35(2):605–611. doi: 10.1128/iai.35.2.605-611.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai C. H., Mors V., Seemayer T. A. Experimental Yersinia enterocolitica enteritis in rabbits. Infect Immun. 1980 Apr;28(1):238–244. doi: 10.1128/iai.28.1.238-244.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Moseley S. L., Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981 Feb;31(2):775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Wolf-Watz H., Bolin I., Beeder A. B., Falkow S. Characterization of common virulence plasmids in Yersinia species and their role in the expression of outer membrane proteins. Infect Immun. 1984 Jan;43(1):108–114. doi: 10.1128/iai.43.1.108-114.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemann D. A., Crane M. R., Swanz P. J. Surface properties of Yersinia species and epithelial cell interactions in vitro by a method measuring total associated, attached and intracellular bacteria. J Med Microbiol. 1987 Nov;24(3):205–218. doi: 10.1099/00222615-24-3-205. [DOI] [PubMed] [Google Scholar]
- Skurnik M. Expression of antigens encoded by the virulence plasmid of Yersinia enterocolitica under different growth conditions. Infect Immun. 1985 Jan;47(1):183–190. doi: 10.1128/iai.47.1.183-190.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesikari T., Nurmi T., Mäki M., Skurnik M., Sundqvist C., Granfors K., Grönroos P. Plasmids in Yersinia enterocolitica serotypes O:3 and O:9: correlation with epithelial cell adherence in vitro. Infect Immun. 1981 Sep;33(3):870–876. doi: 10.1128/iai.33.3.870-876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesikari T., Sundqvist C., Mäki M. Adherence and toxicity of Yersinia enterocolitica 0:3 and 0:9 containing virulence-associated plasmids for various cultured cells. Acta Pathol Microbiol Immunol Scand B. 1983 Apr;91(2):121–127. doi: 10.1111/j.1699-0463.1983.tb00020.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Yokota T. Vibrio cholerae non-O1: production of cell-associated hemagglutinins and in vitro adherence to mucus coat and epithelial surfaces of the villi and lymphoid follicles of human small intestines treated with formalin. J Clin Microbiol. 1988 Oct;26(10):2018–2024. doi: 10.1128/jcm.26.10.2018-2024.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



