Abstract
Background
GJA8 encodes connexin‐50, a gap junction protein in the eye lens. Mutations in GJA8 have been reported in families with autosomal dominant cataract.
Objective
To identify the disease gene in a family with congenital cataract of autosomal recessive inheritance.
Methods
Eight candidate genes were screened for pathogenic alterations in affected and unaffected family members and in normal unrelated controls.
Results
A single base insertion leading to frameshift at codon 203 of connexin 50 was found to co‐segregate with disease in the family.
Conclusions
These results confirm involvement of GJA8 in autosomal recessive cataract.
Keywords: GJA8, connexin, hereditary cataract, recessive cataract, mutations
Cataract is defined as any opacity of the lens resulting in partial or total loss of transparency. Hereditary cataracts are clinically and genetically heterogeneous, often presenting as congenital or developmental cataracts that arise at birth or during the first decade of life, respectively. As these opacities can cause blurring of the vision during form vision development, they are clinically very important. Cataracts may account for about one‐tenth of total childhood blindness in Southern India1 and hereditary cataracts account for about one‐fifth of childhood cataracts in this region.2 The majority of hereditary cataracts that have been genetically characterised to date are of autosomal dominant inheritance.3 Mutations in six genes (CRYAA,4LIM2,5GCNT2,6HSF4,7CRYBB3,8 and BFSP19) have been associated with the autosomal recessive cataracts.
MATERIALS AND METHODS
The study protocol was approved by the institutional review board of the L. V. Prasad Eye Institute and followed the tenets of the Declaration of Helsinki. A family of southern Indian origin was recruited for the study. The proband and five available family members underwent a complete ophthalmic evaluation and blood samples were obtained after informed consent. Diagnosis of hereditary cataract was based on the presence of a bilateral familial lenticular opacity of any size of congenital or developmental type (based on history/examination and age of onset <16 years) as evaluated independently by two examiners. Patients with a history of trauma, or having unilateral (nonfamilial) cataract, co‐existing ocular disease, mental retardation, microcephaly, cerebral palsy, systemic syndromes, or a maternal history of intrauterine infections or antenatal steroid use were excluded.
Genomic DNA was isolated from peripheral blood leukocytes using standard protocols. The gene sequence for GJA8 was retrieved from the Ensembl database (ENSG00000121634). Primers were designed using the Primer 3 software (http://frodo.wi.mit.edu) for PCR amplification of the coding region of GJA8, which is present in exon 2. Seven pairs of overlapping primers were used to obtain fragments of <300 bp in length for single‐strand conformation polymorphism (SSCP) analysis (listed in table 1). PCR products were mixed with two volumes of formamide, denatured by heating at 90°C, snap‐chilled and loaded onto 8% non‐denaturing polyacrylamide gels with 5% glycerol. All samples were subjected to electrophoresis at 4°C and at room temperature. Gels were fixed and subsequently stained with silver nitrate, and DNA visualised under visible light. Variants on the SSCP were subjected to bidirectional sequencing by automated methods. Screening for the observed mutations was performed on 75 ethnically matched unrelated normal controls by SSCP.
Table 1 Primers used for amplification of GJA8.
| Name of primer | Primer sequence (5′ to 3′) |
|---|---|
| GJA8‐2A(F) | CGCGTTAGCAAAAACAGA |
| GJA8‐2A(R) | TCGTAGCAGACGTTCTCG |
| GJA8‐2B(F) | GGATGAGCAATCCGACTT |
| GJA8‐2B(R) | CCAGCCGGAACTTCTTAG |
| GJA8‐2C(F) | ACCAGGGCAGCGTCAA |
| GJA8‐2C(R) | CAGAGGCCACAGACAA |
| GJA8‐2D(F) | CCACGGAGAAAACCATCT |
| GJA8‐2D(R) | TCGGTCAAGGGGAAATAG |
| GJA8‐2E(F) | CTGTCTCCTCCATCCAGAA |
| GJA8‐2E(R) | CGTAGGAAGGCAGTGTCTC |
| GJA8‐2F(F) | TCAGGTCGAGGAGAAGATCA |
| GJA8‐2F(R) | TTTCACCCTCCTTATCCACT |
| GJA8‐2G(F) | GGAGCAGGAGAAGGTG |
| GJA‐2G(R) | TTCCTTTCATCTTGCC |
Primers used for amplification of the coding regions of GJA8 within the second exon are listed above. F and R in parentheses refer to forward and reverse primers respectively.
RESULTS AND DISCUSSION
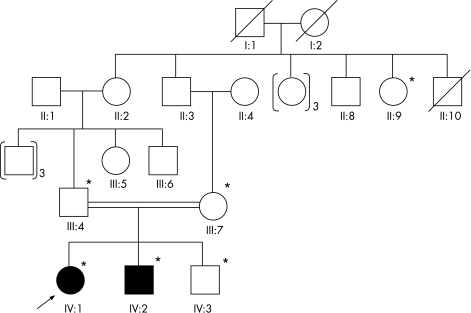
The proband (IV: 1 in fig 1) presented at our institution at 12 years of age. She had a history of poor vision, white opacities in both eyes and nystagmus since birth. She had undergone cataract surgery elsewhere, and had an unaided visual acuity in both eyes of counting fingers at about 1 metre. Her brother (IV: 2 in fig 1), who was similarly affected, had a history of decreased vision since birth and on examination, had total cataracts, nystagmus and a visual acuity in both eyes of counting fingers. The pedigree obtained upon examination of all available members of the family was suggestive of autosomal recessive inheritance (fig 1).
Figure 1 Pedigree of family with autosomal recessive cataract. The dark symbols indicate affected individuals and open symbols indicate unaffected individuals. Symbols marked with asterisks represent individuals who were analysed.
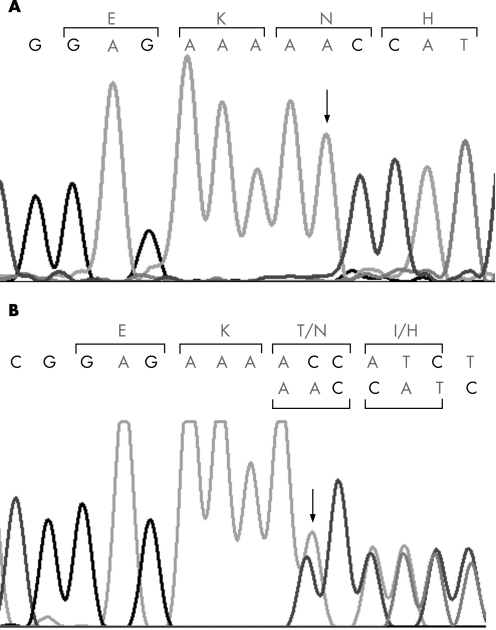
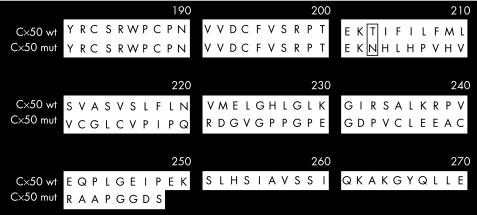
We employed a candidate gene approach consisting of SSCP‐based screening and sequencing. We screened eight genes including six crystallin genes and two connexin genes to identify mutations. Screening of the coding regions of GJA8 revealed a single base insertion causing a frameshift at codon 203 (c.670insA; p.Thr203AsnfsX47; shown in fig 2) that was homozygous in the two affected members IV:1 and IV:2 (fig 1), and heterozygous in the parents (III:4 and III:7 in fig 1), sibling (IV:3 in fig 1) and a second‐degree relative of the proband (II:9 in fig 1), all of whom were unaffected. This change was not found in 75 unrelated controls. The mutation is predicted to result in a frameshift at codon 203 with a stop codon after 46 amino acids of altered reading frame, producing a truncated protein consisting of 248 amino acid residues (fig 3).
Figure 2 Sequence of the GJA8 coding region. The sequence of GJA8 showing the insertion (arrow) of an A at position 670 of the cDNA (c.670insA) (A) homozygous in the proband and (B) heterozygous in unaffected parent. Codons are marked by brackets and amino acids indicated above. Codons and amino acids for both wild type and mutant alleles are shown in (B).
Figure 3 Sequences of wild type and mutant GJA8 proteins. Partial protein sequences of the wild type GJA8/Cx50 (wt) and predicted sequence of the insertion mutant (mut) (c.670insA, p.Thr203AsnfsX47) are shown. The residue (position 203) at the start of the frameshift is boxed. The mutant protein terminates at 248 amino acids. Residues are numbered with respect to the wild‐type Cx50 sequence.
GJA8 encodes the gap junction protein connexin 50 (Cx50), which is one of the major lens connexins along with connexins 43 (locus GJA1) and 46 (locus GJA3). Connexins 50 and 46 are expressed in differentiating lens fibres and persist in mature fibres, and connexin 43 is expressed in lens epithelial cells.10,11,12,13,14 Connexins form intercellular channels consisting of two halves or hemichannels, the connexons, each made up of six connexin monomers. Mutations in GJA3 and GJA8 are known to result in autosomal dominant cataract. Eight different mutations have been reported in the GJA8 gene (table 2), all of which are missense changes.
Table 2 Cx50 (GJA8) mutations reported in human cataracts.
| Mutation | Phenotype | Reference | ||
|---|---|---|---|---|
| Arg23Thr | Progressive congenital nuclear | 28 | ||
| Val44Glu | Congenital or developmental cataract with microcornea | 29 | ||
| Glu48Lys | Zonular nuclear pulverulent | 30 | ||
| Pro88Ser | Zonular pulverulent | 19 | ||
| Pro88Gln | Lamellar pulverulent | 21 | ||
| Val79Leu | “Full moon” with Y‐sutural opacity | 31 | ||
| Arg198Glu | Congenital or developmental cataract with microcornea | 29 | ||
| Ile247Met | Zonular pulverulent | 32 |
The insertion described here is located in codon 203, which is predicted to be in the second extracellular domain of connexin 50; a frameshift at this position would be expected to lead to the disruption of the C‐terminal half of the protein (amino acids 203–433) and thereby produce a functionally null allele. Possible consequences could be instability or non‐functionality of the mutant protein, or degradation of the mRNA through the nonsense‐mediated decay pathway. A mechanism of disease involving loss of function at connexin loci has also been suggested in mouse models of recessive cataract. GJA3 or GJA8 homozygous knockout mice are reported to have a cataractous phenotype, whereas heterozygous knockout mice (GJA3+/−, GJA8+/−) have normal lenses.15,16
DeRosa et al.17 studied the properties of Cx50 proteins with C‐terminal truncations at residue 290 that correspond to physiological truncations occurring during lens maturation. Such truncated Cx50 proteins were found to be expressed and localised to the cell membrane effectively when transiently expressed in HeLa cells.17 Interestingly, they also retained the ability to form channels, but had significantly impaired conductance compared with wild‐type connexin 50.17 Truncation at residue 290 would be expected to result in loss of the C‐terminal cytoplasmic domain of the protein (residues 228–433),18 with all putative transmembrane domains intact.
In comparison, the mutation identified in the present study would be predicted to result in the loss of the second extracellular domain and the subsequent transmembrane and cytoplasmic domains. Heterologous expression in cell lines and in Xenopus oocytes would be required to determine the level of inactivation of the protein.
Studies on various mutant connexin proteins causing dominant cataract in humans and mice, have suggested varied mechanisms of action. Dominant negative effects have been proposed for the GJA8 mutant proteins Pro88Ser19,20 and Pro88Gln21 based on studies in Xenopus oocytes. Studies on the effect of the GJA8 mutation Gly22Arg (found in Lop10 mice), in mouse lenses also revealed dominant negative effects.22 In that study, the mutant proteins were found to interfere with the formation of gap‐junction channels. In contrast, other mutants of both GJA3 and GJA8, when tested in Xenopus oocytes, have been observed to result in loss of function without any dominant negative effects. These are the GJA8 mutant Asp47Ala (D47A) in the No2 mice23 and two GJA3 mutants Asn63Ser and fs380, causing cataract in humans.24 Yet another mechanism of action suggested for the GJA3 (Cx46) fs380 mutant,25 upon expression in mammalian cells, is a gain of function, resulting in mislocalisation, caused by the frameshifted protein.26 Overall, these observations do not point to any unifying mechanisms that may explain how specific connexin mutations could cause dominant versus recessive phenotypes. As has been suggested, interactions between connexin isoforms and the effects of connexins on other lens proteins may determine the phenotype.16,27
In the present study, the homozygous GJA8 insertion mutation was associated with a severe phenotype in both affected siblings as indicated by the presence of opacities evident at birth, as well as nystagmus and amblyopia due to severe visual deprivation. One affected family member also had microcornea and microphthalmia, whereas her affected sibling was normal with respect to these parameters. Although it is not possible to conclude here as to whether the occurrence of microphthalmia is causally linked to deficiency of GJA8/Cx50, it is worth noting that microphthalmia was a feature of GJA8 knockout mice, suggesting that it is required for proper growth and development of the eye.16 This study adds to the range of phenotypes associated with GJA8 mutations and to our knowledge, describes the first mutation in this gene to be associated with autosomal recessive inheritance of cataract.
KEY POINTS
Candidate gene analysis on an Indian family with autosomal recessive cataract showed an insertion (c.670insA) in GJA8 that segregated with disease in the family and was consistent with recessive inheritance.
The mutation is predicted to lead to a frameshift at codon 203 of GJA8/connexin 50 with termination after 46 amino acids, giving rise to a protein of 248 residues.
This study is the first to demonstrate the involvement of connexin 50 in recessive cataract.
ELECTRONIC DATABASE INFORMATION
Ensembl database: http://www.ensembl.org
ACKNOWLEDGEMENTS
We thank all the patients and their family members for their consent to participate in the project. Thanks are also due to Drs Archana Bhargava and Sheik Fazal Hussain for systemic evaluation of patients and Dr Ravi Thomas for his valuable suggestions. This study was supported by Hyderabad Eye Research Foundation. S. P. G. Ponnam was supported by junior research fellowships from the ICMR and CSIR, Government of India.
Abbreviations
SSCP - single‐strand conformation polymorphism
References
- 1.Dandona L, Williams J D, Williams B C, Rao G N. Population‐based assessment of childhood blindness in southern India. Arch Ophthalmol 1998116545–546. [PubMed] [Google Scholar]
- 2.Eckstein M, Vijayalakshmi P, Killedar M, Gilbert C, Foster A. Aetiology of childhood cataract in south India. Br J Ophthamol 199680628–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Online Mendelian Inheritance in Man, OMIM (TM) McKusick‐Nathans Institute of Genetic Medicine, Johns Hopkins University (Baltimore, MD) and National Center for Biotechnology Information, National Library of Medicine (Bethesda, MD), Apr 2007. http://www.ncbi.nlm.nih.gov/omim
- 4.Pras E, Frydman M, Levy‐Nissenbaum E, Bakhan T, Raz J, Assia E I, Goldman B, Pras E. A nonsense mutation (W9X) in CRYAA causes autosomal recessive cataract in an inbred Jewish Persian family. Invest Opthalmol Vis Sci 2000413511–3515. [PubMed] [Google Scholar]
- 5.Pras E, Levy‐Nissenbaum E, Bakhan T, Lahat H, Assia E, Geffen‐Carmi N, Frydman M, Goldman B, Pras E. A missense mutation in the LIM2 gene is associated with autosomal recessive presenile cataract in an inbred Iraqi Jewish family. Am J Hum Genet 2002701363–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pras E, Raz J, Yahalom V, Frydman M, Garzozi H J, Pras E, Hejtmancik J F. A nonsense mutation in the glucosaminyl (N‐acetyl) transferase 2 gene (GCNT2): association with autosomal recessive congenital cataracts. Invest Ophthalmol Vis Sci 2004451940–1945. [DOI] [PubMed] [Google Scholar]
- 7.Smaoui N, Beltaief O, BenHamed S, M'Rad R, Maazoul F, Ouertani A, Chaabouni H, Hejtmancik J F. A homozygous splice mutation in the HSF4 gene is associated with an autosomal recessive congenital cataract. Invest Ophthalmol Vis Sci 2004452716–2721. [DOI] [PubMed] [Google Scholar]
- 8.Riazuddin S A, Yasmeen A, Yao W, Seergeev Y V, Zhang Q, Zulfiqar F, Riaz A, Riazuddin S, Hejtmancik J F. Mutations in betaB3‐crystallin associated with autosomal recessive cataract in two Pakistani families. Invest Opthalmol Vis Sci 2005462100–2106. [DOI] [PubMed] [Google Scholar]
- 9.Ramachandaran R D, Perumalsamy V, Hejtmancik J F. Autosomal recessive juvenile onset cataract associated with mutations in BFSP1. Hum Genet 2007121475–482. [DOI] [PubMed] [Google Scholar]
- 10.Kistler J, Kirkland B, Bullivant S. Identification of a 70,000‐D protein in lens membrane junctional domains. J Cell Biol 198510128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musil L S, Beyer E C, Goodenough D A. Expression of the gap junction protein connexin43 in the embryonic chick lens: molecular cloning, ultrastuctural localization, and post‐translational phosporylation. J Membr Biol 1990116163–175. [DOI] [PubMed] [Google Scholar]
- 12.Paul D L, Ebihara L, Takemoto L J, Swenson K I, Goodenough D A. Connexin46, a novel lens gap junction protein, induces voltage‐gated currents in nonjunctional plasma membrane of Xenopus oocytes. J Cell Biol 19911151077–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White T W, Bruzzone R, Goodenough D A, Paul D L. Mouse Cx50, a functional member of the connexin family of gap junction proteins, is the lens fiber protein Mp 70. Mol Biol Cell 19923711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, Deutsch U, Sohl G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem 2002383725–737. [DOI] [PubMed] [Google Scholar]
- 15.Gong X, Li E, Klier G, Huang Q, Wu Y, Lei H, Kumar N M, Horwitz J, Gilula N B. Disruption of aplha3‐connexin gene leads to proteolysis and cataractogenesis in mice. Cell 199791833–843. [DOI] [PubMed] [Google Scholar]
- 16.White T W, Goodenough D A, Paul D L. Targeted ablation of connexin50 in mice results in microphthalmia and zonular pulverulent cataracts. J Cell Biol 1998143815–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeRosa A M, Mui R, Srinivas M, White T W. Functional characterization of a naturally occurring Cx50 truncation. Invest Ophthalmol Vis Sci 2006474474–4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Universal Protein Resource http://www.expasy.uniprot.org (accessed Apr 2007)
- 19.Shiels A, Mackay D, Ionides A, Berry V, Moore A, Bhattacharya S. A missense mutation in the human connexin50 gene (GJA8) underlies autosomal dominant “zonular pulverulent” cataract, on chromosome 1q. Am J Hum Genet 199862526–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pal J D, Berthoud V M, Beyer E C, Mackay D, Shiels A, Ebihara L. Molecular mechanism underlying a Cx50‐linked congenital cataract. Am J Physiol Cell Physiol 1999276C1443–C1446. [DOI] [PubMed] [Google Scholar]
- 21.Arora A, Minogue P J, Liu X, Reddy M A, Ainsworth J R, Bhattacharya S S, Webster A R, Hunt D M, Ebihara L, Moore A T, Beyer E C, Berthoud V M. A novel GJA8 mutation is associated with autosomal dominant lamellar pulverulent cataract: further evidence for gap junction dysfunction in human cataract. J Med Genet. 2006;43: e2, http://jmg.bmj.com/cgi/content/full/43/1/e2 (accessed April 2007) [DOI] [PMC free article] [PubMed]
- 22.Chang B, Wang X, Hawes N L, Ojakian R, Davisson M T, Lo W K, Gong X. A Gja8 (Cx50) point mutation causes an alteration of alpha 3 connexin (Cx46) in semi‐dominant cataracts of Lop10 mice. Hum Mol Genet 200211507–513. [DOI] [PubMed] [Google Scholar]
- 23.Xu X, Ebihara L. Characterization of a mouse Cx50 mutation associated with the No2 mouse cataract. Invest Ophthalmol Vis Sci 1999401844–1850. [PubMed] [Google Scholar]
- 24.Pal J D, Liu X, Mackay D, Shiels A, Berthoud V M, Beyer E C, Ebihara L. Connexin 46 mutations linked to congenital cataract show loss of gap junction channel function. Am J Physiol Cell Physiol 2000279C596–C602. [DOI] [PubMed] [Google Scholar]
- 25.Mackay D, Ionides A, Kibar Z, Rouleau G, Berry V, Moore A, Shiels A, Bhattarcharya S. Connexin46 mutations in autosomal dominant congenital cataract. Am J Hum Genet 1999641357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minogue P J, Liu X, Ebihara L, Beyer E C, Berthoud V M. An aberrant sequence in a connexin46 mutant underlies congenital cataracts. J Biol Chem 200528040788–40795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez‐Wittinghan F J, Sellitto C, Li L, Gong X, Brink P R, Mathias R T, White T W. Dominant cataracts result from incongruous mixing of wild‐type lens connexins. J Cell Biol 2003161969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willoughby C E, Arab S, Gandhi R, Zeinali S, Arab S, Luk D, Billingsley G, Munier F L, Heon E. A novel GJA8 mutation in an Iranian family with progressive autosomal dominant congenital nuclear cataract. J Med Genet. 2003;40: e124, http://jmg.bmj.com/cgi/content/full/40/11/e124 (accessed Apr 2007) [DOI] [PMC free article] [PubMed]
- 29.Devi R R, Vijayalakshmi P. Novel mutations in GJA8 associated with autosomal dominant congenital cataract and microcornea. Mol Vis 200612190–195. [PubMed] [Google Scholar]
- 30.Berry V, Mackay D, Khaliq S, Francis P J, Hameed A, Anwar K, Mehdi S Q, Newbold R J, Ionides A, Shiels A, Moore T, Bhattacharya S S. Connexin 50 mutation in a family with congenital “zonular nuclear” pulverulent cataract of Pakistani origin. Hum Genet 1999105168–170. [DOI] [PubMed] [Google Scholar]
- 31.Vanita V, Hennies H C, Singh D, Nurnberg P, Sperling K, Singh J R. A novel mutation in GJA8 associated with autosomal dominant congenital cataract in a family of Indian origin. Mol Vis 2006121217–1222. [PubMed] [Google Scholar]
- 32.Polyakov A V, Shagina I A, Khlebnikova O V, Evgrafov O V. Mutation in the connexin 50 gene (GJA8) in a Russian family with zonular pulverulent cataract. Clin Genet 200160476–478. [DOI] [PubMed] [Google Scholar]