Abstract
Background
Non‐ketotic hyperglycinaemia (NKH) is an inborn error of metabolism characterised by accumulation of glycine in body fluids and various neurological symptoms. NKH is caused by deficiency of the glycine cleavage multienzyme system with three specific components encoded by GLDC, AMT and GCSH. Most patients are deficient of the enzymatic activity of glycine decarboxylase, which is encoded by GLDC. Our recent study has suggested that there are a considerable number of GLDC mutations which are not identified by the standard exon‐sequencing method.
Methods
A screening system for GLDC deletions by multiplex ligation‐dependent probe amplification (MLPA) has been developed. Two distinct cohorts of patients with typical NKH were screened by this method: the first cohort consisted of 45 families with no identified AMT or GCSH mutations, and the second cohort was comprised of 20 patients from the UK who were not prescreened for AMT mutations.
Results
GLDC deletions were identified in 16 of 90 alleles (18%) in the first cohort and in 9 of 40 alleles (22.5%) in the second cohort. 14 different types of deletions of various lengths were identified, including one allele where all 25 exons were missing. Flanking sequences of interstitial deletions in five patients were determined, and Alu‐mediated recombination was identified in three of five patients.
Conclusions
GLDC deletions are a significant cause of NKH, and the MLPA analysis is a valuable first‐line screening for NKH genetic testing.
Keywords: glycine cleavage system, multiplex ligation‐dependent probe amplification, GLDC deletion, Alu repeats, mutation spectrum
Non‐ketotic hyperglycinaemia (NKH), also called glycine encephalopathy, is an inborn error of glycine metabolism caused by deficiency of the glycine cleavage system (GCS).1,2,3 Classically, NKH presents in the first few days of life with progressive lethargy, hypotonia, myoclonic jerks, hiccups and apnoea, usually leading to coma and death unless the patient is treated adequately.4 Patients with atypical glycine encephalopathy often lack neonatal symptoms, but manifest aggressive behaviour, cognitive impairment, and impaired work or school performance.5,6 Atypical patients manifest only non‐specific clinical symptoms with most patients remaining undiagnosed and thus without the benefit of early diagnosis and treatment.7 The fundamental defect of NKH lies in the mitochondrial GCS (EC2.1.2.10)8 that consists of four individual proteins:9 glycine decarboxylase encoded (also called P‐protein) by GLDC; aminomethyltransferase (T‐protein) encoded by AMT; hydrogen carrier protein (H‐protein) encoded by GCSH; and dihydrolipoamide dehydrogenase encoded by GCSL. Dihydrolipoamide dehydrogenase is a housekeeping enzyme that serves as an E3 component of other enzyme complexes such as pyruvate dehydrogenase. The three GCS‐specific genes are mapped on different chromosomes: GLDC on chromosome 9p24,10AMT on 3p21.1–21.211 and GCSH on 16q24.12 Enzymatic analysis has shown that approximately 80% of patients with NKH are deficient of glycine decarboxylase activity.13
In Finnish patients we reported a common missense mutation, S564I, that accounts for 70% of mutant alleles.14 Toone et al15 reported a missense mutation, R515S, in 5% of Caucasian mutant alleles. Most of the reported mutations are, however, private, found in only a single family,16,17,18,19,20 thus making DNA analysis difficult. Recently, we have undertaken a comprehensive mutation screening of the three genes, GLDC, AMT and GCSH, in patients with neonatal, infantile and late‐onset types of NHK.21 Various GLDC and AMT mutations were identified in patients with neonatal and infantile types of NHK, but not in those with the late onset type. Among 56 patients with the neonatal type, GLDC mutations were found in 36 patients, whereas AMT mutations were identified in 11 patients. In 14 of 36 patients, GLDC mutations were identified in only one allele, suggesting that some mutations are not detected by the exon‐sequencing method. We have reported several patients with deletion of GLDC exon 1,22 and Sellner et al20 have reported a patient with deletion of the GLDC exons 2–15. These studies suggest that a considerable number of deletions may remain unidentified in GLDC.
The purpose of the present study was to establish a method of screening for deletions within GLDC and determine their frequency in patients with NKH. A multiplex ligation‐dependent probe amplification (MLPA) method23 was used to screen 65 patients with NKH. Using this method, 14 different types of exonic deletions were found in 25 of 130 alleles (19%) in patients with NKH. Our results suggest that deletions in the GLDC gene are a common cause of NKH, and that MLPA analysis is a useful first‐line screening in NKH genetic testing.
Methods
Patients with NKH
DNA samples were obtained from two cohorts of patients with typical NKH with a neonatal onset. Our original cohort of 56 patients with neonatal‐type NKH21 was found to contain 11 patients with AMT mutations. We excluded those 11 patients and defined a new cohort of the remaining 45 patients with NKH (the AMT‐mutation negative cohort). The second cohort contained 20 patients (14 Caucasian and 6 from the Indian subcontinent) with neonatal‐type NKH, who were referred to the Birmingham Children's Hospital, Birmingham, UK, for enzymatic and genetic confirmation of the clinical diagnosis of NKH. In the second cohort, screening for only the R515S and A389V mutations in the GLDC gene was conducted. The study was approved by the Ethics Committee of Tohoku University School of Medicine, Sendai, Japan, and all patients or their legal representatives gave informed consent for DNA analysis.
Synthetic MLPA probes
In all, 29 pairs of MLPA probes were designed for analysis (table 1). As there is a processed pseudogene (GLDCP) which is 98% homologous with GLDC exons,22 probes for the GLDC gene were placed at the 5′ or 3′ junction of each exon. No probe for exon 14 was used, as it lies only 175 bp from exon 13. Probes for AMT exons 1, 4 and 9, EXT2 exon 13, and GLDCP were used as gene dose controls for estimation of GLDC copy number. The length of the synthetic MLPA probes ranged from 41 to 112 bp in size. Table 2 shows their nucleotide sequences. The probe for EXT2 exon 13 was synthesised as reported previously.24 We first tested the 3′ end of each exon as the target site. However, this did not work for GLDC exon 5, 18, 24 and AMT exon 9, so probes were designed at these 5′ regions of the exons. All downstream MLPA probes were 5′ phosphorylated for ligation with the upstream probes. The MLPA probe mixture was prepared by mixing 2 nmol/l of each MLPA probe, and used as described below.
Table 1 Multiplex ligation‐dependent probe amplification probes and polymerase chain reaction products in control participants.
| Target | Upstream probe | Downstream probe | PCR product size (bp) | Control values (n = 18) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Exon | Name | Location | Length (base) | Name | Location | Length (base) | Peak area (mean (SD)) arbitrary unit | Relative peak area (mean (SD))* | SD/mean (%) | |
| GLDC | Exon 1 | M‐GLDC‐E1U | Exon 1 | 50 | M‐GLDC‐E1D | Intron 1 | 50 | 100 | 26557 (1944) | 11.52 (0.57) | 5 |
| Exon 2 | M‐GLDC‐E2U | Exon 2 | 54 | M‐GLDC‐E2D | Intron 2 | 54 | 108 | 41534 (1673) | 18.04 (0.47) | 3 | |
| Exon 3 | M‐GLDC‐E3U | Exon 3 | 58 | M‐GLDC‐E3D | Intron 3 | 58 | 116 | 44796 (1710) | 19.46 (0.31) | 2 | |
| Exon 4 | M‐GLDC‐E4U | Exon 4 | 62 | M‐GLDC‐E4D | Intron 4 | 62 | 124 | 36206 (1716) | 15.72 (0.42) | 3 | |
| Exon 5 | M‐GLDC‐E5U‐2 | Intron 4 | 66 | M‐GLDC‐E5D‐2 | Exon 5 | 66 | 132 | 43086 (2700) | 18.72 (1.07) | 6 | |
| Exon 6 | M‐GLDC‐E6U | Exon 6 | 70 | M‐GLDC‐E6D | Intron 6 | 70 | 140 | 42903 (1261) | 18.64 (0.30) | 2 | |
| Exon 7 | M‐GLDC‐E7U | Exon 7 | 74 | M‐GLDC‐E7D | Intron 7 | 74 | 148 | 36397 (1739) | 15.80 (0.33) | 2 | |
| Exon 8 | M‐GLDC‐E8U | Exon 8 | 78 | M‐GLDC‐E8D | Intron 8 | 78 | 156 | 26102(1643) | 11.34 (0.71) | 6 | |
| Exon 9 | M‐GLDC‐E9U | Exon 9 | 84 | M‐GLDC‐E9D | Intron 9 | 80 | 164 | 30208 (1499) | 13.15 (0.37) | 3 | |
| Exon 10 | M‐GLDC‐E10U | Exon 10 | 92 | M‐GLDC‐E10D | Intron 10 | 80 | 172 | 19567 (1174) | 8.49 (0.25) | 3 | |
| Exon 11 | M‐GLDC‐E11U | Exon 11 | 100 | M‐GLDC‐E11D | Intron 11 | 80 | 180 | 24892 (1987) | 10.80 (0.61) | 6 | |
| Exon 12 | M‐GLDC‐E12U | Exon 12 | 108 | M‐GLDC‐E12D | Intron 12 | 80 | 188 | 16336 (949) | 7.09 (0.21) | 3 | |
| Exon 13 | M‐GLDC‐E13U‐2 | Exon 13 | 112 | M‐GLDC‐E13D | Intron 13 | 80 | 192 | 23856 (1097) | 10.36 (0.26) | 2 | |
| Exon 15 | M‐GLDC‐E15U | Exon 15 | 52 | M‐GLDC‐E15D | Intron 15 | 52 | 104 | 45980 (1888) | 19.97 (0.54) | 3 | |
| Exon 16 | M‐GLDC‐E16U | Exon 16 | 56 | M‐GLDC‐E16D | Intron 16 | 56 | 112 | 40630 (1275) | 17.66 (0.53) | 3 | |
| Exon 17 | M‐GLDC‐E17U | Exon 17 | 60 | M‐GLDC‐E17D | Intron 17 | 60 | 120 | 43310 (1651) | 18.82 (0.57) | 3 | |
| Exon 18 | M‐GLDC‐E18U‐2 | Intron 17 | 64 | M‐GLDC‐E18D‐2 | Exon 18 | 64 | 128 | 20771 (1002) | 9.02 (0.31) | 3 | |
| Exon 19 | M‐GLDC‐E19U | Exon 19 | 68 | M‐GLDC‐E19D | Intron 19 | 68 | 136 | 33266 (1049) | 14.45 (0.28) | 2 | |
| Exon 20 | M‐GLDC‐E20U | Exon 20 | 72 | M‐GLDC‐E20D | Intron 20 | 72 | 144 | 27145 (1199) | 11.79 (0.32) | 3 | |
| Exon 21 | M‐GLDC‐E21U | Exon 21 | 76 | M‐GLDC‐E21D | Intron 21 | 76 | 152 | 26832 (1246) | 11.65 (0.24) | 2 | |
| Exon 22 | M‐GLDC‐E22U | Exon 22 | 80 | M‐GLDC‐E22D | Intron 22 | 80 | 160 | 23008 (1083) | 10.00 (0.51) | 5 | |
| Exon 23 | M‐GLDC‐E23U | Exon 23 | 88 | M‐GLDC‐E23D | Intron 23 | 80 | 168 | 20375 (1428) | 8.84 (0.38) | 4 | |
| Exon 24 | M‐GLDC‐E24U‐2 | Intron 24 | 96 | M‐GLDC‐E24D‐2 | Exon 24 | 80 | 176 | 20051 (609) | 8.72 (0.31) | 4 | |
| Exon 25 | M‐GLDC‐E25U | Exon 25 | 104 | M‐GLDC‐E25D | Intron 25 | 80 | 184 | 29957 (1235) | 13.10 (0.25) | 2 | |
| GLDCP | Processed pseudogene | M‐GLDC‐E1U† | Pseudogene | 50 | M‐GLDCP‐1D | Pseudogene | 47 | 97 | 33495 (1483) | – | – |
| EXT2 | Exon 13 | M‐EXT2‐E13U | Exon 13 | 41 | M‐EXT2‐E13D | Exon 13 | 44 | 85 | 67822 (4013) | – | – |
| AMT | Exon 1 | M‐AMT‐E1U | Exon 1 | 44 | M‐AMT‐E1D | Intron 1 | 44 | 88 | 51455 (2056) | – | – |
| Exon 4 | M‐AMT‐E4U | Exon 4 | 45 | M‐AMT‐E4D | Intron 4 | 46 | 91 | 27010 (1212) | – | – | |
| Exon 9 | M‐AMT‐E9U‐2 | Intron 8 | 47 | M‐AMT‐E9D‐2 | Exon 9 | 47 | 94 | 50490 (2102) | – | – | |
PCR, polymerase chain reaction.
*Peak area of each GLDC exon/sum of peak areas of GLDCP, EXT2, AMT exon 1, AMT exon 4 and AMT exon 9; †shared with upstream primer for GLDC exon 1.
Table 2 Nucleotide sequences of the MPLA probes for detection of GLDC deletions.
| Target gene | Probe name | Nucleotide sequences (5′ to 3′) |
|---|---|---|
| GLDC | M‐GLDC‐E1U | GGGTTCCCTAAGGGTTGGAGAGAGAGATGCTGCAGACCTTGGGGCTGGCG |
| M‐GLDC‐E1D | *GTAAGGACCTCCACCCGGCCCTCCGCGTCTAGATTGGATCTTGCTGGCAC | |
| M‐GLDC‐E2U | GGGTTCCCTAAGGGTTGGATTTGAAAAGACCCTTGAAAATGGAAGACCCTGTTT | |
| M‐GLDC‐E2D | *GTAAGTGGCCGGGAGGGCTCCCTTGGACTTATCTAGATTGGATCTTGCTGGCAC | |
| M‐GLDC‐E3U | GGGTTCCCTAAGGGTTGGAACAGACGATTTTGCGGAACTTACTGGAGAACTCAGGATG | |
| M‐GLDC‐E3D | *GTAATGTATTTCTCAGTTCAGGAACAGGATGACTGTCTAGATTGGATCTTGCTGGCAC | |
| M‐GLDC‐E4U | GGGTTCCCTAAGGGTTGGAATGAGGGGACTGCAGCCGCAGAGGCACTGCAGCTGTGCTACAG | |
| M‐GLDC‐E4D | *GTGAGAGGCCTCTCAAAGTGCTGGAATTCCAGTTGTGGGTCTAGATTGGATCTTGCTGGCAC | |
| M‐GLDC‐E5U‐2 | GGGTTCCCTAAGGGTTGGACTATTTATTTAATGTTCACGTTGGAATGTGCTTTTTCTTTTCAACAG | |
| M‐GLDC‐E5D‐2 | *ACACAACAAGAGGAGGAAATTTCTCGTTGATCCCCGTTGCCACTCTAGATTGGATCTTGCTGGCAC | |
| M‐GLDC‐E6U | GGGTTCCCTAAGGGTTGGAGGGAAGGTGGAAGACTTTACGGAACTCGTGGAGAGAGCTCATCAGAGTGGG | |
| M‐GLDC‐E6D | *GTAGGTATACCTTTCTTGTGGGGGGTCCGTGGAGGCGTATCCCAACTTCTAGATTGGATCTTGCTGGCAC | |
| M‐GLDC‐E7U | GGGTTCCCTAAGGGTTGGACTGTCCGAGAAAGCTTGGTGAGAATGATGCCTGGAAGAATGGTGGGGGTAACAAG | |
| M‐GLDC‐E7D | *GTAAAGGGGCTCATGTTTCTCTACTTTTATTGTGATTATGATTTCCCTGATTCTAGATTGGATCTTGCTGGCAC | |
| M‐GLDC‐E8U‐2 | GGGTTCCCTAAGGGTTGGACCATTTTCTCAGTGGGAACTAAGGGCGGGCCTCTTCAGTTCCCAC | |
| M‐GLDC‐E8D‐2 | *CTGAGCATTCATATTTGCCCCGTCTAGGTAGACCTGTCCTCTCTAGATTGGATCTTGCTGGCAC | |
| M‐GLDC‐E9U | GGGTTCCCTAAGGGTTGGAATGTTCCCATGGGCTGGAGCATATTGCTAGGAGGGTACATAATGCCACTTTGATTTTGTCAGAAG | |
| M‐GLDC‐E9D | *GTGAGTTGGTAATCTGTCTAAAACATTTGGGCATAATAAAATTGATAAATTTGAGTATCTAGATTGGATCTTGCTGGCAC | |
| M‐GLDC‐E10U | GGGTTCCCTAAGGGTTGGAGCTGCTAGTGAAGGAGGTCTTGGGCAGGGCCGCTCAGCGGCAGATCAATTTTCGGCTTTTTGAGGATGGCACA | |
| M‐GLDC‐E10D | *GTAAGTCAAATTTTCAGTATTTTTACCAGTTTTTCAAATTTTCACATTGTTTCTCATTCTAGATTGGATCTTGCTGGCAC | |
| M‐GLDC‐E11U | GGGTTCCCTAAGGGTTGGACTTGGTATTTCTCTTGATGAaACAGTCAATGAAAAAGATCTGGACGATTTGTTGTGGATCTTTGGTTGTGAGTCATCTGCA | |
| M‐GLDC‐E11D | *GTAAGTAAAATAAAAACATGCGTTCCTCAtCATAACTATTGGAGGTGGTAGCAAAAGTCTAGATTGGATCTTGCTGGCAC | |
| M‐GLDC‐E12U | GGGTTCCCTAAGGGTTGGAGCTGAAAGCATGGGAGAGGAGTGCAGAGGTATTCCAGGGTCTGTGTTCAAGAGGACCAGCCCGTTCCTCACCCATCAAGTGTTCAACAG | |
| M‐GLDC‐E12D | *GTTTGTGTGTCTTGTGTGACTTCTGCGTTTTGTGCTTTGGTAATCAGCTAGGGAGTTTCTAGATTGGATCTTGCTGGCAC | |
| M‐GLDC‐E13U‐2 | GGGTTCCCTAAGGGTTGGATGTTCACAGCTACCACTCTGAAACAAACATTGTCCGGTACATGAAGAAACTGGAAAATAAAGACATTTCCCTTGTTCACAGCATGATTCCACT | |
| M‐GLDC‐E13D | *GGTAGTTATTTGTGGCCTTTTTTCTCATTTCCAAGCTACCCCAATCCCACGTCTCTTTCTAGATTGGATCTTGCTGGCAC | |
| M‐GLDC‐E15U | GGGTTCCCTAAGGGTTGGAAGGTTATGACCAGGTCTGTTTCCAGCCAAACAG | |
| M‐GLDC‐E15D | *GTAAGGGCATTTCTTTTCTTATTGTTTCATCTAGATTGGATCTTGCTGGCAC | |
| M‐GLDC‐E16U | GGGTTCCCTAAGGGTTGGAAGCCTACTTAAACCAGAAAGGAGAGGGGCACAGAACG | |
| M‐GLDC‐E16D | *GTGAGTATGGCAGGAGGTGGCGCTTGCTCACCATCTAGATTGGATCTTGCTGGCAC | |
| M‐GLDC‐E17U | GGGTTCCCTAAGGGTTGGAATAAATATGGGAATATCGATGCAGTTCACCTCAAGGCCATG | |
| M‐GLDC‐E17D | *GTACTTGTCTTCTCCTTAGCAGATGGGAGAGGCCGGATCTAGATTGGATCTTGCTGGCAC | |
| M‐GLDC‐E18U‐2 | GGGTTCCCTAAGGGTTGGACCATTTTCTCAGTGGGAACTAAGGGCGGGCCTCTTCAGTTCCCAC | |
| M‐GLDC‐E18D‐2 | *CTGAGCATTCATATTTGCCCCGTCTAGGTAGACCTGTCCTCTCTAGATTGGATCTTGCTGGCAC | |
| M‐GLDC‐E19U | GGGTTCCCTAAGGGTTGGATCTGCATTCCCCACGGAGGAGGTGGTCCTGGCATGGGGCCCATCGGAGT | |
| M‐GLDC‐E19D | *GTAAGTTCTGGGCTGCTGGTTTCAGGATGGCTTTGGAGACAGAATTCTAGATTGGATCTTGCTGGCAC | |
| M‐GLDC‐E20U | GGGTTCCCTAAGGGTTGGACGGCCCCATGGGGCTCCAGTTCCATCTTGCCCATTTCCTGGGCTTATATCAAG | |
| M‐GLDC‐E20D | *GTGAGGCCTGGGAGTATGTGCAGGTGTGCAGGTGGGTGGGGGCGTCAGGTCTAGATTGGATCTTGCTGGCAC | |
| M‐GLDC‐E21U | GGGTTCCCTAAGGGTTGGAACTACATGGCCAAGCGATTAGAAACACACTACAGAATTCTTTTCAGGGGTGCAAGAG | |
| M‐GLDC‐E21D | *GCAAGTATCAACTTTAATCTATCATTACTTGGTTTTTTTCTTGGCCAAACTAATCTAGATTGGATCTTGCTGGCAC | |
| M‐GLDC‐E22U | GGGTTCCCTAAGGGTTGGACCCTTCAAAAAGTCTGCAAATATTGAGGCTGTGGATGTGGCCAAGAGACTCCAGGATTATG | |
| M‐GLDC‐E22D | *GTAAGTGGCTTTTGACATTCATGCCGCCGCCCATGCTGGCTGTGGACCACTTCCTAATCTAGATTGGATCTTGCTGGCAC | |
| M‐GLDC‐E23U | GGGTTCCCTAAGGGTTGGAATCAGCATTCGGCAGGAAATTGCTGACATTGAGGAGGGCCGCATCGACCCCAGGGTCAATCCGCTGAAG | |
| M‐GLDC‐E23D | *GTGCGTAGGCCCTGGAACATTGCTTGAAATGTTCCTTAAACTAGAAAATGATGTCTGTCTAGATTGGATCTTGCTGGCAC | |
| M‐GLDC‐E24U‐2 | GGGTTCCCTAAGGGTTGGAGCTAAGAGCGTACACCCGTCAGGATAGGAGCTGGCCCATGCCTTCCCAGCTGGCACATTCAGATTCAGAGAACTTAC | |
| M‐GLDC‐E24D‐2 | *GAGTGGGAATGCTGCCACCTCTCTGGAATAAGGCCGGTCCCAGTGGGAAGATGTAACTCTAGATTGGATCTTGCTGGCAC | |
| M‐GLDC‐E25U | GGGTTCCCTAAGGGTTGGATGTGGGACTAGCATTGCCACCTCCTTTGCCCTAAGAGAAACCTCCCAGAACATCTCACAGCATTTCCATCTTTTGTCCTTTGCAG | |
| M‐GLDC‐E25D | *CCCTTCGTGAAACCAGAGAACAAATTCTGGCCAACGATTGCCCGGATTGATGACATATCTAGATTGGATCTTGCTGGCAC | |
| GLDCP | M‐GLDCP‐1D | *AGCATTGATGAATTGATCGAGAAGTCTAGATTGGATCTTGCTGGCAC |
| EXT2 | M‐EXT2‐E13U | GGGTTCCCTAAGGGTTGGACAGCCATAGATGGGCTTTCACT |
| M‐EXT2‐E13D | *AGACCAAACACACATGGTGGATCTAGATTGGATCTTGCTGGCAC | |
| AMT | M‐AMT‐E1U | GGGTTCCCTAAGGGTTGGAGATGCAGAGGGCTGTAAGTGTGGTG |
| M‐AMT‐E1D | *GCCCGTCTGGGCTTTCGCCTGTCTAGATTGGATCTTGCTGGCAC | |
| M‐AMT‐E4U | GGGTTCCCTAAGGGTTGGAAGGGCCACCTGTATGTGGTGTCCAAC | |
| M‐AMT‐E4D | *GCTGGCTGCTGGGAGAAAGATTTTCTAGATTGGATCTTGCTGGCAC | |
| M‐AMT‐E9U‐2 | GGGTTCCCTAAGGGTTGGATGATGCGTGGCTTATGCTTGCTTGACAG | |
| M‐AMT‐E9D‐2 | *GTACTGTGACTAGTGGCTGCCCCTTCTAGATTGGATCTTGCTGGCAC |
Single‐underlined and double‐underlined sequences are binding sites for forward and reverse polymerase chain reaction (PCR) primers, respectively.
*5′ phosphorylation.
MLPA procedures
An MLPA P0 FAM detection kit (MRC Holland, Amsterdam, The Netherlands) was used in this study. This kit contains all the necessary reagents except the MLPA probe mixture. MLPA was performed essentially according to the manufacturer's instructions (www.mrc‐holland.com). Briefly, 50–250 ng of genomic DNA was used as the starting material, and after hybridisation, ligation and amplification, the PCR products were size‐separated by an ABI 310 Genetic Analyzer (Applied Biosystems, Foster City, California, USA). For normalisation, relative peak areas were calculated by dividing each measured peak area by the sum of the five control peak areas (table 1). Mean and SD were obtained by testing 18 control DNA samples.
Long‐range PCR
To clarify the boundary sequences of the deleted fragments, we used nested and long‐range PCR with the LA PCR kit (TaKaRa Co Ltd, Tokyo, Japan) for PCR across the breakpoints. PCR fragments containing the boundary sequences of the deletions were size‐separated on 1% agarose gel and bands with the expected sizes were cut out for purification by the QIAquick Gel Extraction kit (Qiagen, Hilden, Germany). Purified PCR fragments were subjected to the dye‐terminator‐sequencing analysis with the BigDye Terminator Sequencing Kit (Applied Biosystems).
Results
MPLA analysis in control DNA
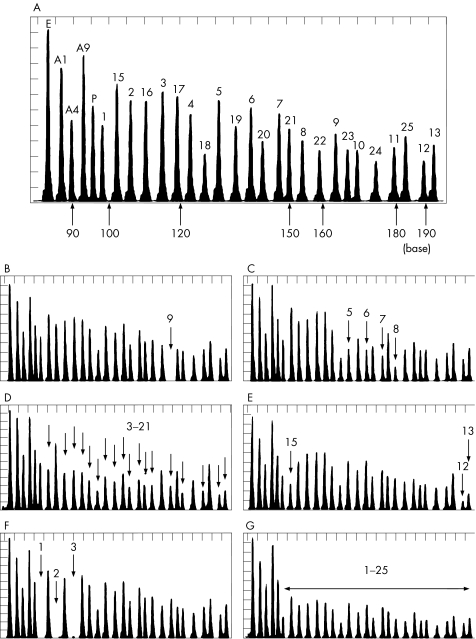
Eighteen control DNA samples were tested to estimate the deviation of each peak area. Figure 1A shows a representative chromatogram with all 29 peaks. Intervals between the peaks correspond to a difference of three or four bases in size of DNA fragments. Each peak area was measured and the mean (SD) was calculated (table 1). The sum of the five control peak areas (EXT2 exon 13, AMT exons 1, 4 and 9, and GLDCP) was used to normalise the relative peak area of each GLDC exon. As a result, the mean (SD) ranged from 2% to 6%. We therefore set a screening threshold for deletion as <80%, <–3 SD from the mean value.
Figure 1 Multiplex ligation‐dependent probe amplification (MLPA) analysis of a control subject and patients with non‐ketotic hyperglycinaemia (NKH) with neonatal onset. A representative MLPA chromatogram of a control participant (A). The five control peaks include EXT2 exon 13 (E), AMT exons 1, 4 and 9 (A1, A4, A9), and GLDCP (P). The number on each peak indicates the exon number of the GLDC gene. MLPA probe for GLDC exon 14 was not used in this assay. MLPA analysis of patients with NKH: homozygotic deletion of exon 9 (B), heterozygotic deletion of exons 5–8 (C), heterozygotic deletion of exons 3–21 (D), heterozygotic deletion of exons 12–15 (E), homozygotic deletion of exons 1–3 (F) and heterozygotic deletion of all 25 GLDC exons (G).
MPLA analysis in patients with NKH
Two independent cohorts of patients with neonatal‐onset NKH were screened by our MLPA system. Nine different types of GLDC deletions were detected in the first AMT‐mutation negative cohort of patients, whereas six different types of deletions were found in the second cohort of patients, in which no prescreening of AMT mutation had been performed (table 3). Figure 1 shows six representative results of GLDC deletions: homozygotic deletion of exon 9 (fig 1B), heterozygotic deletion of exons 5–8 (fig 1C), heterozygotic deletion of exons 3–21 (fig 1D), heterozygotic deletion of exons 12–15 (fig 1E), homozygotic deletion of exons 1–3 (fig 1F) and heterozygotic deletion involving all 25 GLDC exons (fig 1G). In the first cohort, a total of 16 deletion alleles were identified in 90 mutant alleles (18%). In the second cohort, 9 of 40 (22.5%) alleles were positive for deletion screening. No deletions of AMT exons 1, 4 and 9 were detected in this study. MLPA analysis of family P41 suggested that the patient was homozygotic for a deletion of exon 7 (data not shown). Subsequent sequencing analysis of the probe binding sites disclosed a one‐bp deletion, c.1054delA, in the M‐GLDC‐E7U binding site. Similarly, in the MLPA analysis of family B5, both parents appeared to be heterozygotic for a deletion of exon 5 (data not shown). Sequencing of the probe binding sites showed that this was due to a single base substitution in the M‐GLDC‐E5U‐2 binding site on one allele. Unfortunately, no DNA was available from the index case, but the patient from family B5 was presumed to be homozygotic for this c.636‐1G→C mutation, which was deduced to abolish the conserved consensus AG at the splicing acceptor sites.
Table 3 GLDC deletions identified in patients with NKH.
| Deletion | Missing exons | Number of alleles | Family | Ethnicity | Other allele | Comment |
|---|---|---|---|---|---|---|
| First cohort (AMT‐mutation negative, 45 families) | ||||||
| 1 | Exons 1–2 | 2 | P14 | Caucasian | c.2714T→G (p.V905G) | |
| P36 | Caucasian | Deletion (exons 1–17) | ||||
| 2 | Exons 1–3 | 3 | P5 | Oriental | Deletion (exons 1–3) | Homzygote, consanguinity (−) |
| P70 | Oriental | Unidentified | ||||
| 3 | Exons 1–17 | 2 | P36 | Caucasian | Deletion (exons 1–2) | |
| P40 | Caucasian | Unidentified | ||||
| 4 | Exons 1–25 | 1 | P32 | Caucasian | c.1786C→T (p.R596X) | |
| 5 | Exons 3–4 | 1 | P69 | Oriental | c.2311G.A (p.G771R) | |
| 6 | Exons 3–8 | 1 | P120 | Oriental | c.2574T→G (p.Y858X) | |
| 7 | Exons 3–9 | 1 | P47 | Oriental | c.2519T→A (p.M840K) | |
| 8 | Exons 3–22 | 1 | P48 | Caucasian | c.2665+1G→C | |
| 9 | Exons 12–15 | 4 | P7 | Oriental | c.2266_2268del TTC | |
| P8 | Oriental | c.2080G→C (p.A694P) | ||||
| P22 | Oriental | Unidentified | ||||
| P74 | Oriental | c.2311G→A (p.G771R) | ||||
| Second cohort (not prescreened for AMT mutation, 20 families) | ||||||
| 1 | Exon 1 | 1 | B3 | Caucasian | Unidentified | |
| 2 | Exons 1–2 | 2 | B8 | Caucasian | c.1545G→C (p.R515S) | |
| B13 | Caucasian | c.1545G→C (p.R515S) | ||||
| 3 | Exons 1–16 | 2 | B10 | Pakistani | Deletion (exons 1–16) | Homozygote, consanguinity (+) |
| 4 | Exons 3–21 | 1 | B6 | Caucasian | Unidentified | |
| 5 | Exon 9 | 2 | B7 | Caucasian | Deletion (exon 9) | Homozygote, consanguinity (+) |
| 6 | Exons 5–8 | 1 | B18 | Caucasian | c.1545G→C (p.R515S) | |
Identification of boundary sequences of the deletions
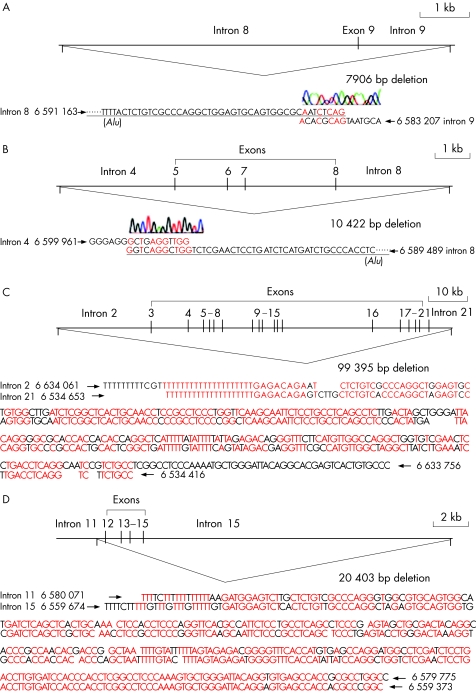
To confirm the deletions identified by the MPLA study and elucidate the mechanisms of the deletions, we examined the boundary sequences of four interstitial deletions within GLDC. We examined the patient homozygotic for a deletion of exon 9 (family B7 in the second cohort), the patient with heterozygotic deletion of exons 5–8 (family B18 in the second cohort), the patient with heterozygotic deletion of exons 3–21 (family B6 in the second cohort) and the patients with heterozygotic deletions of exons 12–15 (families P74 and P8 in the first cohort; fig 2). Nested long‐range PCR was employed in this analysis, which was followed by direct sequencing analysis. In the patient of family B7, a 7906‐bp deletion was identified, extending from the 3′ end of intron 8 (∼6 kb) to the 5′ end of intron 9 (∼2 kb) as shown in fig 2A. In the patient in family B18, we found a 10 422‐bp deletion beginning at the 3′ end of intron 4 (∼3 kb) and including exons 5–8, up to the 5′ end of intron 8 with ∼3 kb (fig 2B). The patient in family B6 had the longest deletion, 99 395 bp, among the four patients. Both 5′ and 3′ fragments shared near identical sequences as shown in fig 2C. In a Japanese patient (P74) with heterozygotic deletion of exons 12–15, a 20 403‐bp deletion was identified (fig 2D). The deleted fragment consisted of a short 3′ end of intron 11 (∼0.6 kb), exons 12–15 and the 5′ end of intron 15 (∼18 kb). The identical breakpoint was also found in the patient from family P8 (data not shown). Thus far, the deleted fragments flanked with Alu motifs in B6, P74, and P8 patients, but not in patients from families B7 or B18. Four Caucasian patients (from P14, P36, B13 and B8) had a deletion of exons 1 and 2. However, as the two patients, B13 and B8, from the UK had different haplotypes (data not shown), this deletion has occurred more than once. This observation agrees with our previous finding that the deletions of the GLDC exon 1 had multiple origins.21
Figure 2 Boundary sequences of the deleted fragments. The boundary sequences of four different types of GLDC deletions were identified: the deletion of exon 9 in family B7 (A), the deletion of exons 5–8 in family B18 (B), the deletion of exons 3–21 in family B6 (C) and the deletion of exons 12–15 in family P74 (D). The boundary sequence found in family P74 was the same as that in family P8. Nucleotide sequences in red indicate the identical bases in the 5′ and 3′ flanks of the deletions.
Distribution of deletions in the GLDC gene
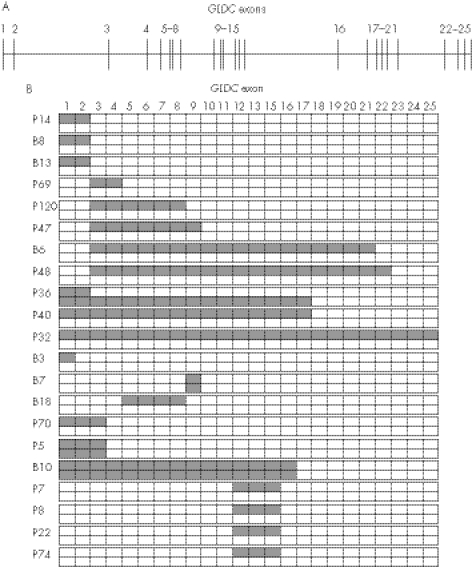
Figure 3 shows the distribution of the missing exons by GLDC deletions. The lengths of the GLDC deletions were heterogeneous, ranging from a single exon to all 25. Of the 50 breakpoints of the deletions, 26 (52%) were found 5′ upstream of the GLDC gene or in introns 1–3, suggesting that deletions tend to occur in the 5′ part of the GLDC gene, a region relatively rich in Alu repeats.
Figure 3 Distribution of missing exons by the GLDC deletions. The exon–intron organisation of the GLDC gene is illustrated (A). Patients with non‐ketotic hyperglycinaemia with GLDC deletions were classified by their missing exons (B). Hatched boxes indicate GLDC exons involved in the deletions. Note the clustering of the deletion breakpoints in the 5′ end of GLDC.
Discussion
We established a detection system for GLDC deletions by using the MLPA method, and showed that deletions within this gene are a common cause of NKH. Fourteen different types of GLDC deletions were identified in screening 65 patients with neonatal‐onset NKH. GLDC deletions were identified in 21 of 65 patients with NKH (32.3%), and in 25 of 130 NKH alleles (19.2%) by MLPA analysis. The MLPA method provides a good first‐line screen in a condition where there are no common mutations and full sequencing of 25 exons of the GLDC gene is a lengthy process. The deletion detection rates by MLPA analysis were 18% and 22.5% in the first and second cohorts, respectively. In our previous study, the exon‐sequencing analysis has shown GLDC mutations in 41 of 90 alleles (45%).21 Thus, this MLPA test improved the sensitivity of mutation detection from 45% to 63%. Mutations for NKH are highly heterogeneous: the prevalent mutations previously reported are Finnish S564I mutation (70%)14 and Caucasian R515S mutation (5%),15 hampering the genetic testing in diagnosis of NKH. In contrast, GLDC deletions seem to be prevalent in different ethnic groups. In a previous study, we analysed the relative allele number of the GLDC exon 1 by using GLDCP as a copy number control.22 As MLPA analysis covers the whole gene in one simple assay, it is highly recommended for the first screening in the genetic testing of NKH.
Point mutations in MLPA‐probe binding sites may cause false positives in MLPA analysis, notably where a single exon is deleted. A mismatching in the binding site of the MLPA probes is known to reduce the ligation efficiency. In our study, we encountered four single‐exon deletions in the analysis of families P41, B3, B5 and B7. Subsequent sequencing analysis of the probe binding sites showed that the patient in family P41 had a 1‐bp deletion and that the patient from B5 carried a 1‐base substitution at the splicing accepter site of intron 4. Both mutations are predicted to be disease causing. No base change was found in the patient from B3. In the patient from B7, exon 9 of the patient failed to be amplified by PCR and a single‐exon deletion was confirmed by subsequent sequencing across the breakpoint (fig 2A). As the MLPA probes for GLDC were designed to bind an exon–intron boundary to avoid detection of the pseudogene of GLDC, GLDCP, the MLPA method can also detect some mutations that cause aberrant splicing. Sequencing the probe‐binding regions of the GLDC gene where MLPA analysis suggests a single‐exon deletion is therefore necessary before making a diagnosis of GLDC deletion.
Key points
A screening system for genomic deletions within GLDC has been developed by the multiplex‐ligation‐dependent probe amplification (MLPA) method.
GLDC deletions were identified in approximately 20% of non‐ketotic hyperglycinaemia (NKH) mutant alleles.
The MLPA analysis is useful for first‐line screening in the genetic testing of NKH.
In a previous study, we diagnosed the patient of family P32 as a homozygote of a nonsense mutation, c.1786C→T (p.R596X), although there was no history of consanguinity.21 A familial study was not possible because no parental DNA was available. The present study showed that he was heterozygotic for a deletion containing all 25 GLDC exons (table 3, fig 1G), indicating that he was a compound heterozygote of the nonsense mutation c.1786C→T and the deletion of exons 1–25. As this deletion was the biggest one so far identified, we looked to see whether it involved any adjacent genes. We performed a microarray analysis to determine the genotypes of many single‐nucleotide polymorphisms (SNPs) by using the GeneChip Human Mapping 100 k Set (Affymetrix, Santa Clara, California, USA). GLDC is located between base positions 6635650 and 6522467 bp in chromosome 9 (GenBank, NT_008413). The JMJD2C gene (6748083–7165647 bp) is located 5′ upstream of GLDC whereas the UHRF2 gene (6403151–6497051 bp) lies 3′ downstream of GLDC. The SNP at the base position 6606648 bp, which is located within the GLDC gene, was indeed homozygotic in this patient (data not shown). In contrast, two SNPs at the base positions of 6513056 and 6759229 bp were heterozygotic, suggesting that the deletion is <246 kb, and thus that the two adjacent genes are unlikely to be involved in the deletion.
We determined flanking sequences of interstitial deletions in five patients, and Alu‐mediated recombination was identified in three of five patients. The Alu elements, approximately 300 bp in length, compose about 10% of the whole human genome.25 There are several inherited disorders in which Alu‐mediated recombination/deletion is a common cause: hereditary angioedema, C1‐INH;26 α‐thalassemia, α‐globin gene;27 and Ehlers–Danlos syndrome, PLOD.28 Recently, Alu‐mediated genomic recombination has also been reported in non‐inherited human cancer, hepatoma.29 A total of 120 copies of Alu repeats are present in the GLDC gene, which has a length of 113 kb, resulting in one Alu of 1.1 kb on average. This is much higher than the average density of one Alu every 3–4 kb over the whole human genome.30 The GLDC deletions tend to be located in the 5′ end of the GLDC gene, which may be explained by the fact that the region contains a high number of Alu repeats.
The diagnosis of NKH is difficult to establish on clinical and biochemical grounds alone, and typically requires a liver biopsy for enzyme analysis or DNA studies to confirm a diagnosis. However, the complex nature of the genetics of NKH (three genes and no common mutations) makes DNA analysis a lengthy and difficult process. Our finding that deletions within the GLDC gene are one of the most common causes of NKH and the development of a simple assay for such mutations will make genetic analysis for this disorder much more straightforward. Such analysis will reduce the need for a liver biopsy in a sick child, make diagnosis easier, and improve the ease and reliability of antenatal diagnosis.
Acknowledgements
We thank all the families who participated in this study.
Abbreviations
GCS - glycine cleavage system
MLPA - multiplex ligation‐dependent probe amplification
NKH - non‐ketotic hyperglycinaemia
PCR - polymerase chain reaction
SNP - single‐nucleotide polymorphism
Footnotes
Funding: This work was supported by a grant from the Ministry of Education, Culture, Sports, Science, and Technology and the Ministry of Health, Labor and Welfare in Japan. Funding was also provided by the Robert Gaddie Memorial Fund and from the Tohoku University 21st COE Program “Comprehensive Research and Education Center for Planning of Drug Development and Clinical Evaluation”, Sendai, Japan.
Competing interests: None declared.
Electronic databases: NKH (GCE)‐OMIM, 605899. GLDC‐OMIM: 238300, GenBank: NT_008413.
References
- 1.Hamosh A, Johnston M V. Nonketotic hyperglycinemia. In: Scriver CR, Beaudet AL, Sly WS, et al eds. The metabolic and molecular bases of inherited disease. 8th edn, Vol 2. New York: McGraw‐Hill, 20012065–2078.
- 2.Kure S, Tada K, Narisawa K. Nonketotic hyperglycinemia: biochemical, molecular, and neurological aspects. J Hum Genet 19974213–22. [DOI] [PubMed] [Google Scholar]
- 3.Tada K, Kure S. Nonketotic hyperglycinemia: pathophysiological studies. Proc Japan Acad 200581(Ser B)411–417. [Google Scholar]
- 4.Hoover‐Fong J E, Shah S, Van Hove J L, Applegarth D, Toone J, Hamosh A. Natural history of nonketotic hyperglycinemia in 65 patients. Neurology 2004631847–1853. [DOI] [PubMed] [Google Scholar]
- 5.Dinopoulos A, Kure S, Chuck G, Sato K, Gilbert D L, Matsubara Y, Degrauw T. Glycine decarboxylase mutations: a distinctive phenotype of nonketotic hyperglycinemia in adults. Neurology 2005641255–1257. [DOI] [PubMed] [Google Scholar]
- 6.Flusser H, Korman S H, Sato K, Matsubara Y, Galil A, Kure S. Mild glycine encephalopathy (NKH) in a large kindred due to a silent exonic GLDC splice mutation. Neurology 2005641426–1430. [DOI] [PubMed] [Google Scholar]
- 7.Korman S H, Wexler I D, Gutman A, Rolland M O, Kanno J, Kure S. Treatment from birth of nonketotic hyperglycinemia due to a novel GLDC mutation. Ann Neurol 200659411–415. [DOI] [PubMed] [Google Scholar]
- 8.Tada K, Narisawa K, Yoshida T, Yokoyama K, Nakagawa H, Tanno K, Mochizuki K, Arakawa T, Yoshida T, Kikuchi G. Hyperglycinemia: a defect in glycine cleavage reaction. Tohoku J Exp Med 196998289–296. [DOI] [PubMed] [Google Scholar]
- 9.Kikuchi G. The glycine cleavage system: composition, reaction mechanism, and physiological significance. Mol Cell Biochem 19731169–187. [DOI] [PubMed] [Google Scholar]
- 10.Isobe M, Koyata H, Sakakibara T, Momoi‐Isobe K, Hiraga K. Assignment of the true and processed genes for human glycine decarboxylase to 9p23–24 and 4q12. Biochem Biophys Res Commun 19942031483–1487. [DOI] [PubMed] [Google Scholar]
- 11.Nanao K, Takada G, Takahashi E, Seki N, Komatsu Y, Okamura‐Ikeda K, Motokawa Y, Hayasaka K. Structure and chromosomal localization of the aminomethyltransferase gene (AMT). Genomics 199419 pp 27-30 (published erratum appears in Genomics, 199420519. [DOI] [PubMed] [Google Scholar]
- 12.Kure S, Kojima K, Kudo T, Kanno K, Aoki Y, Suzuki Y, Shinka T, Sakata Y, Narisawa K, Matsubara Y. Chromosomal localization, structure, single‐nucleotide polymorphisms, and expression of the human H‐protein gene of the glycine cleavage system (GCSH), a candidate gene for nonketotic hyperglycinemia. J Hum Genet 200146378–384. [DOI] [PubMed] [Google Scholar]
- 13.Tada K. Nonketotic hyperglycinemia: clinical and metabolic aspects. Enzyme 19873827–35. [DOI] [PubMed] [Google Scholar]
- 14.Kure S, Takayanagi M, Narisawa K, Tada K, Leisti J. Identification of a common mutation in Finnish patients with nonketotic hyperglycinemia. J Clin Invest 199290160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toone J R, Applegarth D A, Coulter‐Mackie M B, James E R. Biochemical and molecular investigations of patients with nonketotic hyperglycinemia. Mol Genet Metab 200070116–121. [DOI] [PubMed] [Google Scholar]
- 16.Boneh A, Korman S H, Sato K, Kanno J, Matsubara Y, Lerer I, Ben‐Neriah Z, Kure S. A single nucleotide substitution that abolishes the initiator methionine codon of the GLDC gene is prevalent among patients with glycine encephalopathy in Jerusalem. J Hum Genet 200550230–234. [DOI] [PubMed] [Google Scholar]
- 17.Kure S, Mandel H, Rolland M O, Sakata Y, Shinka T, Drugan A, Boneh A, Tada K, Matsubara Y, Narisawa K. A missense mutation (His42Arg) in the T‐protein gene from a large Israeli‐Arab kindred with nonketotic hyperglycinemia. Hum Genet 1998102430–434. [DOI] [PubMed] [Google Scholar]
- 18.Kure S, Ichinohe A, Kojima K, Sato K, Kizaki Z, Inoue F, Yamanaka C, Matsubara Y. Mild variant of nonketotic hyperglycinemia with typical neonatal presentations: mutational and in vitro expression analyses in two patients. J Pediatr 2004144827–829. [DOI] [PubMed] [Google Scholar]
- 19.Kure S, Shinka T, Sakata Y, Osamu N, Takayanagi M, Tada K, Matsubara Y, Narisawa K. A one‐base deletion (183delC) and a missense mutation (D276H) in the T‐ protein gene from a Japanese family with nonketotic hyperglycinemia. J Hum Genet 199843135–137. [DOI] [PubMed] [Google Scholar]
- 20.Sellner L, Edkins E, Greed L, Lewis B. Detection of mutations in the glycine decarboxylase gene in patients with nonketotic hyperglycinaemia. Mol Genet Metab 200584167–171. [DOI] [PubMed] [Google Scholar]
- 21.Kure S, Kato K, Dinopoulos A, Gail C, Degrauw T J, Christodoulou J, Bzduch V, Kalmanchey R, Fekete G, Trojovsky A, Plecko B, Breningstall G, Tohyama J, Aoki Y, Matsubara Y. Comprehensive mutation analysis of GLDC, AMT, and GCSH in nonketotic hyperglycinemia. Hum Mutat 200627343–352. [DOI] [PubMed] [Google Scholar]
- 22.Takayanagi M, Kure S, Sakata Y, Kurihara Y, Ohya Y, Kajita M, Tada K, Matsubara Y, Narisawa K. Human glycine decarboxylase gene (GLDC) and its highly conserved processed pseudogene (psiGLDC): their structure and expression, and the identification of a large deletion in a family with nonketotic hyperglycinemia. Hum Genet 2000106298–305. [DOI] [PubMed] [Google Scholar]
- 23.Schouten J P, McElgunn C J, Waaijer R, Zwijnenburg D, Diepvens F, Pals G. Relative quantification of 40 nucleic acid sequences by multiplex ligation‐dependent probe amplification. Nucleic Acids Res 200230e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White S J, Vink G R, Kriek M, Wuyts W, Schouten J, Bakker B, Breuning M H, den Dunnen J T. Two‐color multiplex ligation‐dependent probe amplification: detecting genomic rearrangements in hereditary multiple exostoses. Hum Mutat 20042486–92. [DOI] [PubMed] [Google Scholar]
- 25.Batzer M A, Deininger P L. Alu repeats and human genomic diversity. Nat Rev Genet 20023370–379. [DOI] [PubMed] [Google Scholar]
- 26.Stoppa‐Lyonnet D, Carter P E, Meo T, Tosi M. Clusters of intragenic Alu repeats predispose the human C1 inhibitor locus to deleterious rearrangements. Proc Natl Acad Sci USA 1990871551–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicholls R D, Fischel‐Ghodsian N, Higgs D R. Recombination at the human alpha‐globin gene cluster: sequence features and topological constraints. Cell 198749369–378. [DOI] [PubMed] [Google Scholar]
- 28.Heikkinen J, Toppinen T, Yeowell H, Krieg T, Steinmann B, Kivirikko K I, Myllyla R. Duplication of seven exons in the lysyl hydroxylase gene is associated with longer forms of a repetitive sequence within the gene and is a common cause for the type VI variant of Ehlers‐Danlos syndrome. Am J Hum Genet 19976048–56. [PMC free article] [PubMed] [Google Scholar]
- 29.Hsieh S Y, Chen W Y, Yeh T S, Sheen I S, Huang S F. High‐frequency Alu‐mediated genomic recombination/deletion within the caspase‐activated DNase gene in human hepatoma. Oncogene 2005246584–6589. [DOI] [PubMed] [Google Scholar]
- 30.Rowold D J, Herrera R J. Alu elements and the human genome. Genetica 200010857–72. [DOI] [PubMed] [Google Scholar]