Abstract
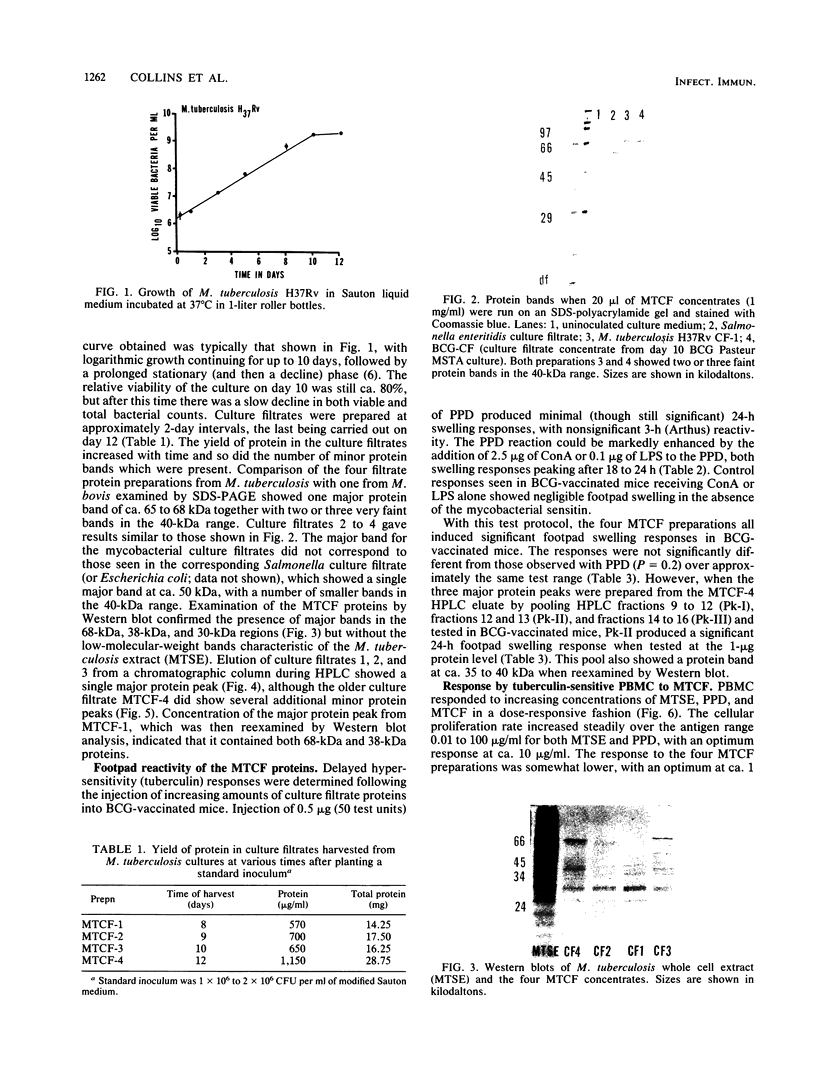
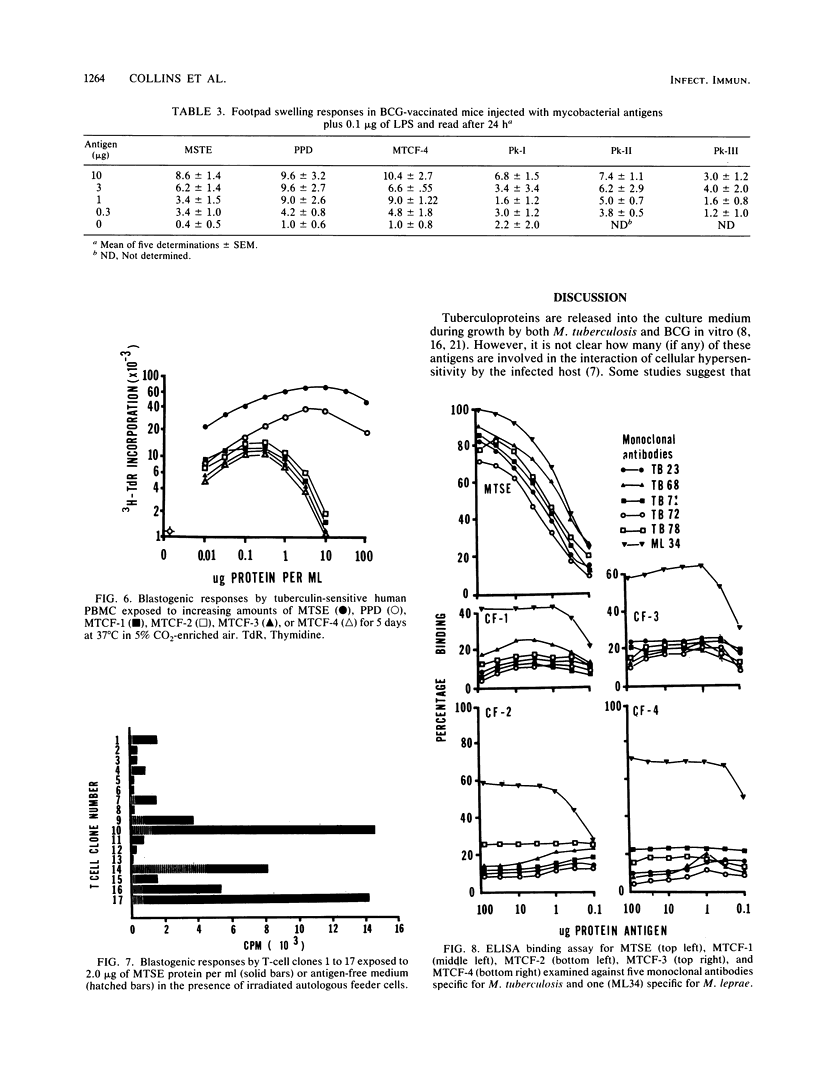
Mycobacterium tuberculosis culture filtrate (MTCF) protein antigens were isolated from mid-logarithmic-phase cultures grown in liquid medium and examined by high-pressure liquid chromatography and Western blot (immunoblot) analysis. A major protein band with a molecular mass of about 68 kilodaltons (kDa) and several fainter bands in the 38- and 24-kDa range were observed. The MTCF protein produced a significant delayed footpad hypersensitivity response in Mycobacterium bovis BCG-vaccinated C57BL/6 mice, comparable to that observed with standard purified protein derivative (PPD). The same proteins induced a blastogenic response in tuberculin-sensitive human peripheral blood monocytes and in T-cell clones developed from these cells. The proliferative responses to the MTCF antigens were equivalent to those observed following stimulation with PPD or M. tuberculosis sonic extracts. However, the MTCF sensitins were not recognized by five monoclonal antibodies directed against killed M. tuberculosis antigens in an enzyme immunoassay, although some response was seen with a monoclonal antibody (ML34) directed against M. leprae antigens. The ability of the MTCF to stimulate T-cell responses both in vivo and in vitro while not being recognized by antibodies directed against dead mycobacterial antigens suggests that they may be of interest as potential protective immunogens.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrew P. W., Rees A. D., Scoging A., Dobson N., Matthews R., Whittall J. T., Coates A. R., Lowrie D. B. Secretion of a macrophage-activating factor distinct from interferon-gamma by human T cell clones. Eur J Immunol. 1984 Oct;14(10):962–964. doi: 10.1002/eji.1830141018. [DOI] [PubMed] [Google Scholar]
- Collins F. M. Kinetics of the delayed-type hypersensitivity response in tuberculous guinea pigs and mice tested with several mycobacterial antigen preparations. Am Rev Respir Dis. 1983 May;127(5):599–604. doi: 10.1164/arrd.1983.127.5.599. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B. Delayed hypersensitivity and arthus reactivity in relation to host resistance in salmonella-infected mice. J Immunol. 1968 Nov;101(5):830–845. [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B. The relationship of delayed hypersensitivity to acquired antituberculous immunity. I. Tuberculin sensitivity and resistance to reinfection in BCG-vaccinated mice. Cell Immunol. 1970 Sep;1(3):253–265. doi: 10.1016/0008-8749(70)90047-x. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Wayne L. G., v Montalbine The effect of cultural conditions on the distribution of Mycobacterium tuberculosis in the spleens and lungs of specific pathogen-free mice. Am Rev Respir Dis. 1974 Aug;110(2):147–156. doi: 10.1164/arrd.1974.110.2.147. [DOI] [PubMed] [Google Scholar]
- De Bruyn J., Bosmans R., Turneer M., Weckx M., Nyabenda J., Van Vooren J. P., Falmagne P., Wiker H. G., Harboe M. Purification, partial characterization, and identification of a skin-reactive protein antigen of Mycobacterium bovis BCG. Infect Immun. 1987 Jan;55(1):245–252. doi: 10.1128/iai.55.1.245-252.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmrich F., Thole J., van Embden J., Kaufmann S. H. A recombinant 64 kilodalton protein of Mycobacterium bovis bacillus Calmette-Guerin specifically stimulates human T4 clones reactive to mycobacterial antigens. J Exp Med. 1986 Apr 1;163(4):1024–1029. doi: 10.1084/jem.163.4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe M., Nagai S., Patarroyo M. E., Torres M. L., Ramirez C., Cruz N. Properties of proteins MPB64, MPB70, and MPB80 of Mycobacterium bovis BCG. Infect Immun. 1986 Apr;52(1):293–302. doi: 10.1128/iai.52.1.293-302.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchal G., Milon G., Augier J. Apparent increased sensitivity of mice to tuberculin by adding a non-specific inflammatory agent to the antigen. Tubercle. 1986 Mar;67(1):61–67. doi: 10.1016/0041-3879(86)90033-4. [DOI] [PubMed] [Google Scholar]
- Matthews R., Scoging A., Rees A. D. Mycobacterial antigen-specific human T-cell clones secreting macrophage activating factors. Immunology. 1985 Jan;54(1):17–23. [PMC free article] [PubMed] [Google Scholar]
- Nagai S., Matsumoto J., Nagasuga T. Specific skin-reactive protein from culture filtrate of Mycobacterium bovis BCG. Infect Immun. 1981 Mar;31(3):1152–1160. doi: 10.1128/iai.31.3.1152-1160.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrazzini T., Louis J. A. Functional analysis in vitro and in vivo of Mycobacterium bovis strain BCG-specific T cell clones. J Immunol. 1986 Mar 1;136(5):1828–1834. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte R., Des Ormeaux Y. Influence of the age of mycobacterial cultures on the protein and carbohydrate composition of tuberculins. Can J Microbiol. 1972 May;18(5):637–645. doi: 10.1139/m72-101. [DOI] [PubMed] [Google Scholar]
- Turcotte R. Yield of non-dialyzable mycobacterial constituents during the growth cycle. Can J Microbiol. 1969 Jan;15(1):35–41. doi: 10.1139/m69-006. [DOI] [PubMed] [Google Scholar]
- Young D., Kent L., Rees A., Lamb J., Ivanyi J. Immunological activity of a 38-kilodalton protein purified from Mycobacterium tuberculosis. Infect Immun. 1986 Oct;54(1):177–183. doi: 10.1128/iai.54.1.177-183.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Bloom B. R., Grosskinsky C. M., Ivanyi J., Thomas D., Davis R. W. Dissection of Mycobacterium tuberculosis antigens using recombinant DNA. Proc Natl Acad Sci U S A. 1985 May;82(9):2583–2587. doi: 10.1073/pnas.82.9.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]





