Abstract
The Shwachman–Bodian–Diamond syndrome (SBDS) gene is a causative gene for Shwachman–Diamond syndrome, an autosomal recessive disorder with exocrine pancreatic insufficiency, bone marrow dysfunction and skeletal dysplasia. We report here on two patients with skeletal manifestations at the severest end of the phenotypic spectrum of SBDS mutations. An 11‐year‐old Japanese girl presented with neonatal respiratory failure necessitating lifelong ventilation support, severe short stature and severe developmental delay. She developed neutropenia in infancy, and decreased serum amylase was noted in childhood. A British boy was a stillbirth with pulmonary hypoplasia and hepatic fibrosis found on autopsy. Both cases had neonatal skeletal manifestations that included platyspondyly, lacy iliac crests and severe metaphysial dysplasia, and thus did not fall in the range of the known Shwachman–Diamond syndrome skeletal phenotype but resembled spondylometaphysial dysplasia (SMD) Sedaghatian type. The girl harboured a recurrent mutation (183TA→CT) and a novel missense mutation (79T→C), whereas the boy carried two recurrent mutations (183TA→CT and 258+2T→C). We also examined SBDS in one typical case with SMD Sedaghantian type and eight additional cases with neonatal SMD, but failed to discover SBDS mutations. Our experience expands the phenotypic spectrum of SBDS mutations, which, at its severest end, results in severe neonatal SMD.
The Shwachman–Bodian–Diamond syndrome (SBDS) gene (GeneBank AC079920) is a causative gene for Shwachman–Diamond syndrome (SDS; OMIM 260400), an autosomal recessive disorder with exocrine pancreatic insufficiency, haematological abnormalities and generalised skeletal dysplasia.1,2 Most SBDS mutations are caused by gene conversion between SBDS and its neighbouring pseudogene (SBDSP; GeneBank AC005236). The SBDS protein, which does not have sequence homology to known functional domains, is assumed to have a role in RNA metabolism.2,3,4 This presumption may account for pleiotropic effects of SBDS mutations and multi‐system involvement in SDS.
The clinical phenotype of SDS is diverse.5,6,7 Pancreatic insufficiency commonly presents with steatorrhoea in childhood, but it may develop first in the older age. Haematological abnormalities range from cyclic or persistent neutropenia through pancytopenia with a specific risk of evolution into myelodysplastic syndrome to acute myeloid leukaemia.8,9 Hepatic, renal and neurological dysfunctions are rare associations of SDS. SDS manifests radiologically as generalised metaphysial dysplasia, which causes only mild to moderate short stature in general. A small subset of affected individuals may present with respiratory distress due to a narrow thorax with short ribs, resembling Jeune syndrome (OMIM 208500), but the disorder is usually not lethal.10
We report here on two compound heterozygotes for SBDS mutations whose manifestations can be regarded as the severest end of the phenotypic spectrum known for mutations in the gene. One patient has survived respiratory failure with lifelong ventilation support, and showed severe short stature and developmental delay. The other was a stillbirth with pulmonary hypoplasia. Their extraskeletal manifestations may fit a diagnosis of SDS. However, their skeletal phenotype was not typical of SDS, but resembled a mild form of another skeletal dysplasia termed spondylometaphysial dysplasia (SMD) Sedaghatian type (OMIM 250220).11
Materials, methods and results
Clinical reports
Patient 1
The girl was born to a healthy, non‐consanguineous Japanese couple. She was delivered by caesarean section at 38 weeks gestation because of breech presentation. Her birth length was 40 cm (−4.9 SD), weight 1870 g (−3.1 SD) and head circumference 32.7 cm (−0.3 SD). Apgar scores at 1/5 min were 7/9. She had a narrow thorax and rhizomelic shortening of the limbs. Her profound hypotonia with poor breathing prompted assisted ventilation, which was enduringly continued. Laryngoscopy showed tracheomalacia with stenosis at 1 month of age. At 4 months of age, she developed intermittent neutropenia, which required granulocyte colony‐stimulating factor therapy that has been intermittently continued thereafter. At 30 months of age she presented with seizures, which were responsive to epilepsy medicines. Decreased serum isoamylase (4 IU/l) attracted attention at 5 years of age, but no overt symptoms of malabsorption were noted. She is currently 11 years old and is markedly retarded, along with severe hypotonia and bilateral hearing impairment (no reaction for 90 dB). Her height is 93 cm (−7.5 SD). A skeletal survey at birth showed a narrow thorax, short ribs with cupped anterior ends, severe dense platyspondyly, lacy iliac crests, delayed ossification of the caudal ilia, and metaphysial cupping and irregularity of the tubular bones (fig 1). A tentative diagnosis of SMD Sedaghatian type was made at that time. The skeletal alterations at 5 and 11 years of age comprised thoracic narrowing, posteriorly wedged vertebral bodies without platyspondyly, iliac hypoplasia, metaphysial irregularity with epiphysial plate widening and large epiphyses of the long bones, and metaphysial cupping of the short tubular bones (figs 2 and 3). The radiological findings in childhood were interpreted as an unclassifiable metaphysial dysplasia.
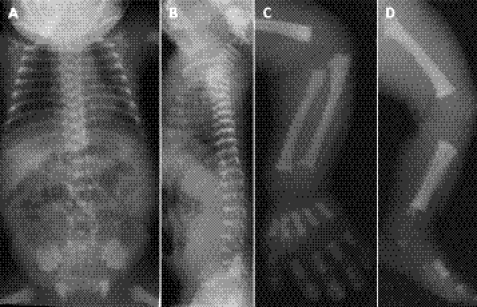
Figure 1 Radiographs of patient 1 at age 1 day: chest and abdomen (A), lateral spine (B), arm (C) and leg (D). Note the narrow thorax, short ribs with cupped anterior ends, severe dense platyspondyly, lacy iliac crests, delayed ossification of the caudal ilia, and metaphysial cupping and irregularity of the tubular bones. The proximal femoral metaphysis has a faded area with a rounded end, similar to what is seen in spondylometaphysial dysplasia Sedaghatian type.
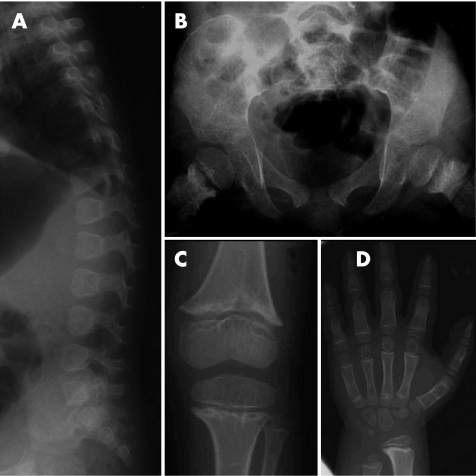
Figure 2 Radiographs of patient 1 at age 5 years: lateral spine (A), pelvis (B), knee (C) and hand (D). Note posteriorly wedged vertebral bodies without platyspondyly, iliac base hypoplasia, metaphysial irregularity with epiphysial plate widening and large epiphyses of the long bones, and metaphysial cupping of the short tubular bones.
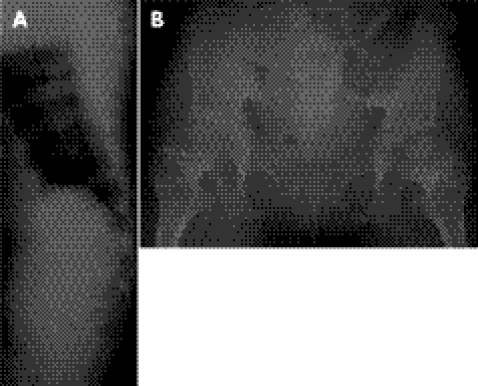
Figure 3 Radiographs of patient 1 at age 11 years: lateral spine (A) and pelvis (B). Note the flared, cupped anterior ends of the ribs, normal height of the vertebral bodies, iliac hypoplasia and continuing metaphysial changes of the proximal femora.
Patient 2
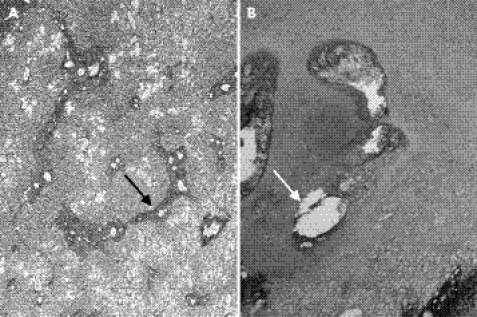
This boy was the product of the first pregnancy of a healthy, non‐consanguineous British couple. Fetal ultrasound at 17 weeks gestation was reported to be normal. A macerated stillbirth was delivered at full term. The weight was below the 1st centile for a full‐term infant. Physical findings included a narrow thorax and rhizomelic shortening of the limbs. A postmortem radiograph showed thoracic hypoplasia with horizontal short ribs, mild platyspondyly, ragged iliac crests, delayed ossification of the caudal ilia, hypoplastic pubic rami, and ragged, cupped metaphyses of the long bones (fig 4). A review at the European Skeletal Dysplasia Network review panel (www.esdn.org) concluded that the skeletal changes are suggestive of SMD Sedaghatian type. Autopsy showed atrial septal defect, hypoplasia of the gallbladder and distended ileal loops filled with meconium. Histological examination showed periportal hepatic fibrosis, calcifications in dilated renal tubules and pulmonary hypoplasia (lung:body weight ratio = 0.006; <0.012 indicates lethal hypoplasia) (fig 5a). The pancreas appeared unremarkable, although tissue preservation was poor. Chondro‐osseous histology showed cystic foci with neighbouring pyknotic chondrocytes in the resting zone. Cartilage columns in the hypertrophic zone were poorly organised, and cartilaginous spicules extended into the metaphysis (fig 5b). Elongation of the hypertrophic zones was modest.
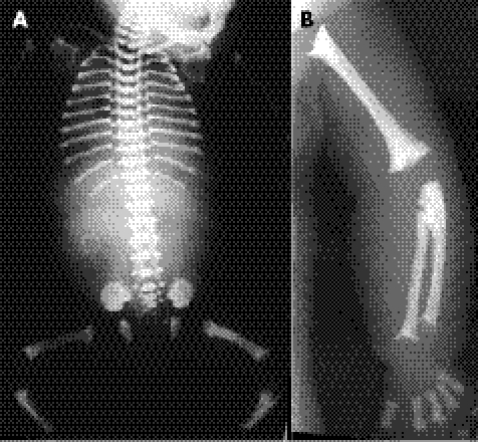
Figure 4 A postmortem radiograph of patient 2: babygram (A) and magnification view of the arm (B). Note the thoracic hypoplasia with horizontal short ribs, mild platyspondyly, ragged iliac crests, delayed ossification of the caudal ilia, hypoplastic pubic rami, and ragged, cupped metaphyses of the long bones.
Figure 5 Histological examination of patient 2: liver with reticulin staining (A) and chondro‐osseous junction of the femur with haematoxylin and eosin staining (B). Note periportal hepatic fibrosis in the liver (black arrow). The chondro‐osseous junction shows foci of cystic lesions in the resting zone (white arrow) and poorly organised cartilage columns of the hypertrophic zone.
Molecular analysis
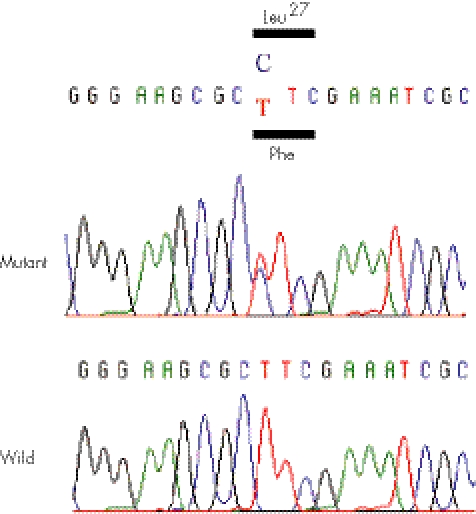
Peripheral blood from patient 1 and skin from patient 2 were obtained with informed consent from their parents. Genomic DNAs were extracted using standard procedures. SBDS mutation analysis was carried out as described previously.12 Patient 1 was a compound heterozygote for two SBDS mutations, 79T→C and 183TA→ CT, and patient 2 carried 183TA→CT and 258+2T→C mutations (fig 6). 79T→C is a novel missense mutation (F27L) that was not found in 70 Japanese control subjects. Codon 27 resides in the N‐terminal (FYSH) domain where SBDS mutations often occur,2,4 and it is highly conserved among various species from archaea to mammals. Both 183TA→CT (K62X) and 258+2T→C (C84fsX3) are recurrent mutations that are caused by gene conversion.2,12 No other sequence variations were found. The parents were unwilling to undergo molecular analysis. We also examined SBDS in neonatal SMD: five cases from the International Skeletal Dysplasia Registry (http://www.CSMS.edu/3805.html), including a previously reported case with SMD Sedaghatian type13 and four cases from the European Skeletal Dysplasia Network (http://www.esdn.org/). No SBDS mutations were found in these cases.
Figure 6 A novel missense mutation (79T→C) in exon 1 of Shwachman–Bodian–Diamond syndrome in patient 1.
Discussion
The skeletal phenotype of SDS is classified within the metaphysial dysplasia group.14 The cardinal skeletal findings include metaphysial irregularities of the long bones, particularly of the proximal femora, and short ribs with flared, cupped anterior ends.15 Both findings are generally mild. The former rarely manifests in the neonatal period.16 The latter may occasionally cause respiratory distress in the affected neonates, but they survive the neonatal period.10,16 The spine is normal in SDS. In classical SDS, the discrete skeletal signs are often sought to confirm a diagnosis that is suspected because of the extraskeletal features, namely, neutropenia or pancreatic insufficiency, and failure to thrive.
The radiological diagnosis of metaphysial dysplasia in childhood and the presence of neutropenia led us to investigate the SBDS gene in patient 1, which showed compound heterozygotic SBDS mutations in the girl. Her decreased serum isoamylase may have represented sub‐clinical exocrine pancreatic insufficiency. However, her skeletal phenotypes were much more severe than what is typically seen in SDS.17 The skeletal changes in childhood, including severe thoracic hypoplasia, iliac hypoplasia, mega‐epiphyses of the long bones and metaphysial cupping of the short tubular bones, were unusual for SDS. Furthermore, she presented with a form of SMD in the neonatal period causing severe respiratory distress. The phenotype was considered a mild form of SMD Sedaghatian type. Observation of the SBDS mutations in the girl led to investigation of the SBDS gene in other cases with a diagnosis of SMD Sedaghatian type, which discovered one more patient positive for SBDS mutations (patient 2). In retrospect, hepatic fibrosis in the boy was accounted for by SBDS mutations.
SMD Sedaghatian type is a rare lethal skeletal dysplasia inherited as an autosomal recessive trait.11,13,18,19,20,21,22 Most affected individuals succumbed in the perinatal period, and the longest survivor died 161 days after birth. The skeletal hallmarks of the disorder include a narrow thorax, moderate platyspondyly, metaphysial cupping and irregularity of the tubular bones, delayed ossification of the caudal ilia and, most distinctively, “lacy iliac crests”. These skeletal changes are reflected in the chondro‐osseous morphology of the disorder, that is, long hypertrophic zones with hypercellularity and little matrix extending into the metaphyses.13,18,19 The skeletal changes in the present patients were qualitatively similar to, but milder than, those of previously reported cases with SMD Sedaghatian type from the viewpoint of severity of metaphysial dysplasia, platyspondyly and delayed ossification of the caudal ilia. The somewhat milder skeletal changes seen in our two mutation‐positive patients may be correlated with the histological finding of moderate elongation of the hypertrophic zone found in patient 2, as opposed to the significant hypertrophic cell elongation seen in typical SMD Sedaghatian type. Extraskeletal abnormalities have been reported in SMD Sedaghatian type, and include cardiomyopathy and migration anomalies of the brain.11,13,21 However, neither haematological nor pancreatic abnormalities have been described in the disorder so far.
The present patients harboured one or both of the common SBDS mutations—that is, 183–184TA→CT and 258+2T→C. As no homozygote for 183–184TA→CT has been observed, the mutation may create complete loss of function of the SBDS protein with lethality in the homozygotic state—that is, a null mutation due to a stop codon. On the other hand, homozygosity of 258+2T→C and compound heterozygosity of 183–184TA→CT and 258+2T→C are prevalent in patients with SDS.2,23 While the 258+2T→C mutation predicts aberrant splicing with a premature termination codon, a small amount of the SBDS protein has been observed by immunoblotting with an antibody against the C‐terminus of the SBDS protein in a 258+2T→C homozygote.24 Thus, variable amounts of the functional protein in affected individuals determine variable biological consequences of 258+2T→C. In fact, the clinical phenotypes of compound heterozygotes of 183–184TA→CT and 258+2T→C are quite variable. For example, the height of the patients ranges from −1 SD to −4.5 SD.17 Thus, the severe phenotypes of patients 1 and 2 are attributable to a non‐functional allele (183–184TA→CT) and an allele with a trace amount of expression of the functional protein (258+2T→C).
We assume that 79T→C found in patient 1 is a disease‐causing mutation because it is predicted to cause a missense change of an evolutionally conserved amino acid; the amino acid is located in a mutation‐clustered domain of the SBDS gene.2,4 Our assumption is supported by the fact that it is not found in the polymorphism database and in a considerable number of ethnicity‐matched controls. However, a possibility that it is a functionally innocent polymorphism cannot be totally excluded at present, because the function of the SBDS protein is currently unknown, and hence no functional assay for the SBDS protein is available. The severe phenotype of the patient suggests that the biological consequence of 79T→C may be deleterious. Further studies are necessary to clarify its functional impact.
We also examined the SBDS gene in nine other cases with a diagnosis of neonatal SMD, including a case that had been previously reported as a typical SMD Sedaghatian type.13 However, no SBDS mutations were found in these patients. Implications of our experiences are as follows: SBDS mutations give rise to variable phenotypes, including typical SDS and neonatal SMD resembling SMD Sedaghatian type. The latter phenotype may be related to the heterozygosity of 183–184TA→CT, the homozygosity of which is assumed to cause the lethality. Classical SMD Sedaghatian type is a different entity in that no causal gene has been identified. The differences in extraskeletal manifestations may help in distinguishing neonatal SMD due to SBDS mutations from classical SMD Sedaghatian type, although these distinctions may be difficult in patients who do not survive the neonatal period.
Key points
We report on two patients with neonatal spondylometaphysial dysplasia (SMD) caused by compound heterozygotic mutations in the Shwachman–Bodian–Diamond syndrome (SBDS) gene. This is the second report of the disease caused by mutations in the SBDS gene.
Skeletal features of the patients resembled those of SMD Sedaghatian type, which is basically lethal in the neonatal period. This is the first report of the mutation in a neonatal form of SMD.
SBDS mutations were not found in nine additional cases with neonatal SMD including one typical case with SMD Sedaghantian type. SBDS mutations give rise to variable phenotypes, including typical Shwachman–Diamond syndrome and Sedaghatian‐like neonatal SMD. Classical SMD Sedaghatian type is a different entity in that no causal gene has been identified.
Acknowledgements
We thank Drs Kayoko Takahashi and Yukikatsu Ochiai (Department of Pediatrics, Tokyo Metropolitan Kita Medical Rehabilitation Center for the Handicapped, Tokyo, Japan) for giving us the clinical information of patient 1, and Dr Christine Hall (Department of Radiology, Great Ormond St Children's Hospital, London, UK) as well as the European Skeletal Dysplasia Network review panel for diagnostic review of patient 2. Supported in part by the following grants: Grant‐in‐Aid from Research on Child Health and Development (Contract Grant No: 17C‐1; H18‐005); EU grant QLGI‐CT2001‐02188 (European Union) and Swiss OFES‐BBW grant 01.258 (Switzerland; both to the ESDN network); a USPHS NIH Program Project Grant (HD‐ PO1‐HD 22657)(USA) and an NIH GCRC Grant M01‐RR00425 from the National Center for Research Resources (USA).
Abbreviations
SBDS - Shwachman–Bodian–Diamond syndrome
SDS - Shwachman–Diamond syndrome
SMD - spondylometaphysial dysplasia
Footnotes
Competing interests: None declared.
References
- 1.Dror Y, Freedman M H. Shwachman‐Diamond syndrome. Br J Haematol 2002118701–713. [DOI] [PubMed] [Google Scholar]
- 2.Boocock G R, Morrison J A, Popovic M, Richards N, Ellis L, Durie P R, Rommens J M. Mutations in SBDS are associated with Shwachman‐Diamond syndrome. Nat Genet 20033397–101. [DOI] [PubMed] [Google Scholar]
- 3.Savchenko A, Krogan N, Cort J R, Evdokimova E, Lew J M, Yee A A, Sanchez‐Pulido L, Andrade M A, Bochkarev A, Watson J D, Kennedy M A, Greenblatt J, Hughes T, Arrowsmith C H, Rommens J M, Edwards A M. The Shwachman‐Bodian‐Diamond syndrome protein family is involved in RNA metabolism. J Biol Chem 200528019213–19220. [DOI] [PubMed] [Google Scholar]
- 4.Shammas C, Menne T F, Hilcenko C, Michell S R, Goyenechea B, Boocock G R, Durie P R, Rommens J M, Warren A J. Structural and mutational analysis of the SBDS protein family: insight into the leukemia‐associated Shwachman‐Diamond syndrome. J Biol Chem 200528019221–19229. [DOI] [PubMed] [Google Scholar]
- 5.Mack D R, Forstner G G, Wilschanski M, Freedman M H, Durie P R. Shwachman syndrome: exocrine pancreatic dysfunction and variable phenotypic expression. Gastroenterology 19961111593–1602. [DOI] [PubMed] [Google Scholar]
- 6.Cipolli M, D'Orazio C, Delmarco A, Marchesini C, Miano A, Mastella G. Shwachman's syndrome: pathomorphosis and long‐term outcome. J Pediatr Gastroenterol Nutr 199929265–272. [DOI] [PubMed] [Google Scholar]
- 7.Ginzberg H, Shin J, Ellis L, Morrison J, Ip W, Dror Y, Freedman M, Heitlinger L A, Belt M A, Corey M, Rommens J M, Durie P R. Shwachman syndrome: phenotypic manifestations of sibling sets and isolated cases in a large patient cohort are similar. J Pediatr 199913581–88. [DOI] [PubMed] [Google Scholar]
- 8.Dror Y. Shwachman‐Diamond syndrome. Pediatr Blood Cancer 200545892–901. [DOI] [PubMed] [Google Scholar]
- 9.Hall G W, Dale P, Dodge J A. Shwachman‐Diamond syndrome: UK perspective. Arch Dis Child 200691521–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danks D M, Haslam R H A, Mayne V, Kaufmann H J, Holtzapple P G. Metaphyseal chondrodysplasia, neutropenia, and pancreatic insufficiency presenting with respiratory distress in the neonatal period. Arch Dis Child 197651697–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sedaghatian M R. Congenital lethal metaphyseal chondrodysplasia: a newly recognized complex autosomal recessive disorder. Am J Med Genet 19806269–274. [DOI] [PubMed] [Google Scholar]
- 12.Nakashima E, Mabuchi A, Makita Y, Masuno M, Ohashi H, Nishimura G, Ikegawa S. Novel SBDS mutations caused by gene conversion in Japanese patients with Shwachman‐Diamond syndrome. Hum Genet 2004114345–348. [DOI] [PubMed] [Google Scholar]
- 13.Peeden J N, Jr, Rimoin D L, Lachman R S, Dyer M L, Gerard D, Gruber H E. Spondylometaphyseal dysplasia, Sedaghatian type. Am J Med Genet 199244651–656. [DOI] [PubMed] [Google Scholar]
- 14.Superti‐Furga A, Unger S, the Nosology Group of the International Skeletal Dysplasia Society Nosology and classification of genetic skeletal disorders: 2006 revision. Am J Med Genet 20071431–18. [DOI] [PubMed] [Google Scholar]
- 15.Spranger J W, Brill P W, Poznanski A. eds. Bone dysplasias: an atlas of genetic disorders of skeletal development. 2nd edn. Oxford: Oxford University Press, 2002109–111.
- 16.Kozlowski K, Morris L. Shwachman's syndrome: unusual presentation as congenital rickets and asphyxiating thoracic dystrophy. Fortschr Roentgenstr 1991154344–345. [DOI] [PubMed] [Google Scholar]
- 17.Makitie O, Ellis L, Durie P R, Morrison J A, Sochett E B, Rommens J M, Cole W G. Skeletal phenotype in patients with Shwachman‐Diamond syndrome and mutations in SBDS. Clin Genet 200465101–112. [DOI] [PubMed] [Google Scholar]
- 18.Opitz J M, Spranger J W, Stoss H R, Pesch H ‐ J, Azadeh B. Sedaghatian congenital lethal metaphyseal chondrodysplasia‐‐observations in a second Iranian family and histopathological studies. Am J Med Genet 198726583–590. [DOI] [PubMed] [Google Scholar]
- 19.Campbell R S D, Ireland M, Bloxham C A, Chippindale A J. Platyspondylic lethal osteochondrodysplasia: Shiraz type with radiological–pathological correlation. Pediatr Radiol 19922290–92. [DOI] [PubMed] [Google Scholar]
- 20.Elcioglu N, Hall C M. Spondylometaphyseal dysplasia‐‐Sedaghatian type. Am J Med Genet 199876410–414. [PubMed] [Google Scholar]
- 21.Koutouby A, Habibullah J, Moinuddin F A. Spondylometaphyseal dysplasia: Sedaghatian type. Am J Med Genet 200090199–202. [DOI] [PubMed] [Google Scholar]
- 22.Foulds N, Fairhurst J, Temple I K, Cade S, Groves C, Lancaster T. A female case of Sedaghatian type spondylometaphyseal dysplasia. Am J Med Genet 2003118377–381. [DOI] [PubMed] [Google Scholar]
- 23.Kujipers T W, Alders M, Tool A T J, Mellink C, Roos D, Hennekam C M. Hematologic abnormalities in Shwachman Diamond syndrome: lack of genotype–phenotype relationship. Blood 2005106356–361. [DOI] [PubMed] [Google Scholar]
- 24.Austin K M, Leary R J, Shimamura A. The Shwachman‐Diamond SBDS protein localizes to the nucleolus. Blood 20051061253–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]