Abstract
X‐linked retinoschisis is the leading cause of macular degeneration in males and leads to splitting within the inner retinal layers leading to visual deterioration. Many missense and protein truncating mutations have now been identified in the causative retinoschisis gene (RS1) which encodes a 224 amino acid secreting retinal protein, retinoschisin. Retinoschisin octamerises is implicated in cell–cell interactions and cell adhesion perhaps by interacting with β2 laminin. Mutations cause loss of retinoschisin function by one of the three mechanisms: by interfering with protein secretion, by preventing its octamerisation or by reducing function in the secreted octamerised protein. The development of retinoschisis mouse models have provided a model system that closely resembles the human disease. Recent reports of RS1 gene transfer to these models and the sustained restoration of some retinal function and morphology suggest gene replacement may be a possible future therapy for patients.
X‐linked retinoschisis (XLRS) is a retinal dystrophy caused by mutations in the RS1 gene in Xp22.1, which leads to schisis (splitting) of the neural retina leading to reduced visual acuity in affected men (OMIM #312700). The condition accounts for almost all congenital retinoschisis with occasional reports of autosomal dominant retinoschisis making up the remainder.1 The split in the retina occurs predominantly within the inner retinal layers and is very different from retinal detachment, which is a split between the neural retina and the retinal pigment epithelium.
XLRS was first described in the 19th century2 and documented as X linked in 1913.3 Several cases were then described but were given alternative names including neuroretinal disease in men4 and congenital vascular veils in the retina.5 The term “X linked retinoschisis” was first used in 19536 and this, together with “juvenile retinoschisis,”7 is now the generally accepted term.
The causative gene RS1 was identified in 19978 and numerous inactivating mutations have since been found9 (http://www.dmd.nl/rs/index.html). Investigation of the protein retinoschisin, encoded by RS1, has revealed it to be a secretory protein, containing a discoidin domain and functioning as an octamer.10,11,12,13 Three retinoschisis mouse models have been developed and they have similar retinal pathology to the human disease.14,15,16 This article aims to review the clinical, pathological and electrophysiological features of XLRS, our current understanding of its molecular basis and to consider future therapy.
Prevalence and epidemiology
XLRS is the leading cause of juvenile macular degeneration in males17 with an estimated prevalence of between 1 in 15 000 and 1 in 30 000.18 These figures, based on the Finnish population, are similar to the data from a clinical study of XLRS in The Netherlands.19 Many of the mutations described in the gene have been identified in more than one family9 with some indication of founder effect. This is particularly marked in Finland, with three mutations accounting for almost all cases,20 illustrating the allelic homogeneity in Finland.21 The wider worldwide genetic heterogenity suggests that the worldwide prevalence may be lower than these estimates. However, it is likely that XLRS is still underdiagnosed.
Clinical features
There is a great variation in disease severity even among individuals who have the same causative RS1 mutation,22,23,24,25 and no correlation has been identified between mutation type and disease severity or progression.23 Patients often present at school age with poor vision and reading difficulties, although this can vary with patients presenting as young as 3 months.26 The age of onset follows a bimodal distribution with patients presenting in infancy with squint and nystagmus and those with only poor vision presenting at school age.26 Visual impairment is variable with best‐corrected visual acuity from 20/20 to 20/60017,27 and marked differences are found at all ages even within a family or in patients with the same mutation.23 Foveal schisis (retinal splitting), seen as a cartwheel pattern of folds radiating out from the fovea (fig 1), is the characteristic sign of XLRS and is present in 98–100% of cases.27,28,29 However, over time this may become less distinct.27 Peripheral retinoschisis is often noted in the inferotemporal region. During infancy, these cavities may be very large bullous retinoschisis,26 and this generally regresses leaving lines of pigment in older patients.26,27 More than half the patients have some peripheral retinoschisis,27 which can vary from shallow schisis to marked elevation in the inner leaflet over a large retinal area. Breaks occur within the inner layer varying from small holes to large tears,27 and fragmentation of the inner leaf can lead to membranous remnants referred to as vitreous veils. Vessels crossing between the walls of the schisis may be unsupported and at risk of haemorrhage. Additional peripheral changes may include pigmentation, which can resemble retinitis pigmentosa, sublinear retinal fibrosis, white retinal flecks and vascular attenuation or sheathing.27 In a proportion of patients an inner retinal reflex resembling a tapetal reflex is observed.27
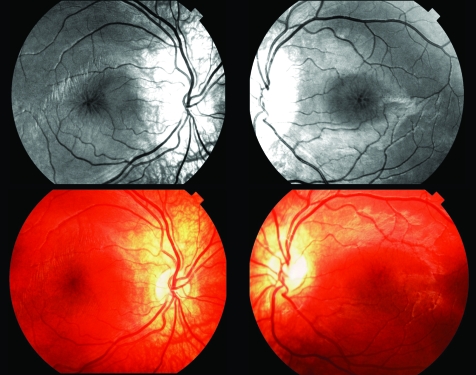
Figure 1 Red‐free (top) and colour photographs (bottom) of macular region in X‐linked retinoschisis. The radial cystic lesions at the macula are more obvious on the red‐free image.
Visual function is often stable from childhood until the 40s when deterioration occurs,17,23,27 but complications, including vitreous haemorrhage (up to a third of patients)27,28 and retinal detachment (up to 20% patients27,29), may lead to severe visual impairment. Most retinal detachments associated with XLRS are rhematogenous in origin owing to the development of peripheral retinal breaks. Bilateral macular detachments possibly caused by abnormal vitreomacular traction have also been reported.30 Sudden visual loss secondary to vitreous haemorrhage is an occasional presenting feature in older children. Leucocoria associated with tractional retinal detachment caused by an organised vitreous haemorrhage has been reported in a 9 month old infant.31 In such cases it is of importance to exclude other causes of leucocoria, such as retinoblastoma, Coats' disease, Norrie's disease, retinal detachment and retinopathy of prematurity and vitreoretinopathies. Axial hypermetropia also appears to be a consistent feature of XLRS.32
In general females who are heterozygous for an RS1 mutation remain asymptomatic and have no clinical features of the condition,28,33 although we have recently seen a young girl with the clinical features of XLRS1 and a reduced b‐wave on electroretinogram (ERG).34 The patient has an affected father and is heterozygous for his mutation with no other RS1 mutation. It is likely that she has skewed X inactivation accounting for her clinical features. The only other case of affected females in the literature is from one highly consanguineous family from Columbia in which three females are affected and all are homozygous for a frameshift mutation (639delG).35
Investigations
The clinical diagnosis of XLRS can be challenging and a delay in diagnosis averaging 8 years after the onset of symptoms has been documented.27 Subtle foveal schisis can be difficult to observe ophthalmoscopically, but may be more apparent on red‐free illumination (fig 1). To this end, digital fundus photography with colour and red‐free illumination can be very helpful. Electrodiagnostic testing is useful in both supporting or suggesting the diagnosis.
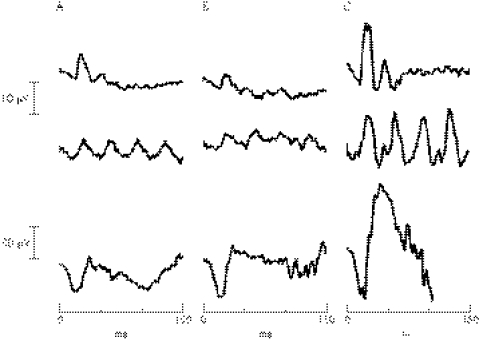
The ERG, (fig 2) is the electrical response of the retina to a flash of light that can be recorded at the cornea. It is recorded with the retina in a dark‐adapted (scotopic) or light‐adapted (photopic) state. The International Society for Clinical Electrophysiology of Vision publishes standard protocols for adult ERG examination, although often there has to be some compromise when testing children.36 The ERG comprises several component potentials that originate from different stages of retinal processing, which overlap in time. Although there are many components of the ERG, it is the relative contributions of the a‐wave and b‐wave that are of particular interest in XLRS. The a‐wave arises by suppression of a circulating dipole current generated by photoreceptors by a light stimulus and produces a negative going a‐wave. Although the early (12–15 ms) portion of the a‐wave is thus directly related to photoreceptor function, there is a postreceptoral element as the wave progresses.37 The larger corneo‐positive b‐wave, which truncates the negative a‐wave, is largely generated by the activity of depolarising bipolar cells within the inner retina.38 Patients with XLRS show a characteristic pattern on the ERG (fig 2), which is best detected after dark adaptation and using a standard, ganzfeld, bright white flash stimulus. A reduction in the amplitude of the b‐wave and a relative preservation of the negative a‐wave gives rise to the so‐called electronegative ERG. Reduced b‐wave amplitudes indicate an inner retinal abnormality.28,29,39,40 Further evidence for the selective effect on ON‐bipolars may be seen by separating on and off responses using long‐duration (200 ms) stimuli.41
Figure 2 Electroretinogram (ERG) recordings from the left eye of two other patients with X‐linked retinoschisis (A) aged 8 years and (B) aged 6 years, and one normal control individual (C) aged 6 years. All used International Society for Clinical Electrophysiology of Vision‐standard stimulation but followed a paediatric protocol,36 using a gold‐mylar skin electrode (Burden Neuroscience, Bristol, UK) mounted on the lower eyelid, with outer canthus reference. (A) and (B) exhibit reduced and delayed scotopic flash and flicker ERGs (top and middle rows), but the key feature is the reduced dark‐adapted b‐wave (bottom row) with b:a ratios of 1.27 and 1.43, in comparison with the C's b:a ratio of 2.37. For adult ERG findings see Holder et al and Stanga et al.41,48
The characteristic negative ERG is not unique to XLRS and is seen in a variety of other hereditary and acquired retinal disorders, most notably congenital stationary night blindness (CSNB). Electrophysiology can show some differences between XLRS and the complete and incomplete forms of CSNB,41 but an important factor in making the differential diagnosis is their quite different presentations (see Differential diagnosis). Nevertheless, the variation of b:a ratio is considered to be an important diagnostic parameter.42 In the early stage of disease, the a‐wave is often normal but the amplitude may reduce with disease progression and we have found that up to a third of patients do have a reduction in their a‐wave,43 indicating the photoreceptor involvement in the disease. However, it is clear from a number of studies that not all individuals with XLRS show the classic electronegative ERG, and b‐wave amplitudes may not be significantly different from normal.43,44,45,46 The ERG phenotype shows a wide variability between, as well as within, families with different genotypes, indicating considerable heterogeneity of ERG response without clinical, age or genetic correlations,22 thus it cannot be relied on as the sole investigation for XLRS.
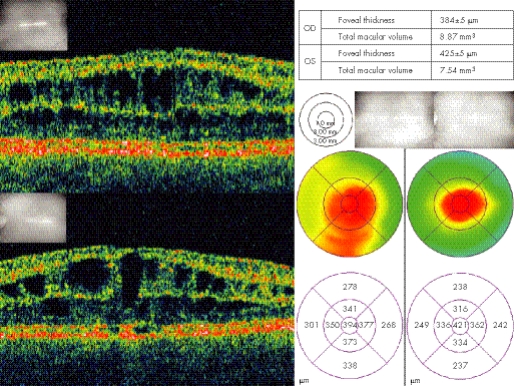
A recent addition to the armamentarium of useful investigations for XLRS is optical coherence tomography (OCT) (fig 3). This is a non‐invasive, non‐contact procedure that uses low coherence interferometry to detect relative reflection changes and different optical surfaces. The wavelength is close to infrared and is thus well tolerated. With resolutions approaching 10 μm it can be used to diagnose and monitor retinal disease. Its value in retinoschisis has been well demonstrated in many reports.47,48,49,50,51 Typically, it can produce a two‐dimensional cross‐sectional image of structures in the eye. The OCT can scan across the macular and perimacular region in a variety of orientations. The images produced clearly show the splitting of retina, which in many cases involves more than one layer. Cleavage can be seen in or just below the superficial nerve fibre layer and also, to a variable extent, in deeper layers. The other characteristic features that are seen are thin walled, vertical palisades spanning the cleft between the split retinal layers and giving rise to the cystic‐like spaces in the perifoveal region (fig 3). These cystic‐like spaces have a tendency to enlarge and become confluent as they approach the fovea. An advantage of the OCT is that it can show splitting of retinal layers even when this is not clinically observed. Later stages of the disease are associated with atrophic changes in the macula. These can be observed with OCT as a generalised reduction of the foveal thickness.
Figure 3 Optical coherence tomography images of the macular region from the same patient as fig 1. Top and bottom left showing schisis through multiple inner layers of the retina in right and left eyes, respectively. Macular thickness maps showing central macular thickening diffuse in right and more focal in the left eye.
A recent adaptation combining scanning laser ophthalmoscopy and OCT‐termed three‐dimensional OCT, can produce transverse and longitudinal images of the retina and demonstrates that splitting can occur in any layer in the retina.52 Although the investigation is useful, there does not appear to be any correlation between the OCT characteristics of the central macular region and the visual acuity.53
The cystic‐like spaces do not demonstrate hyperfluorescence when undertaking fluroescein angiography, in contrast with that observed in cystoid macular oedema. Indocyanin green angiography on the other hand is capable of demonstrating the cystic‐like spaces centred on the foveola,54 although this modality is more invasive than OCT.
Genetic testing can now be performed to confirm a diagnosis. Mutations can be detected in 90–95% of patients who have a clinical diagnosis when all six exons and splice junctions are sequenced (see below). Identifying the causative mutation in an affected man is very helpful, both for confirmation of the diagnosis and in genetic counselling as females who are at risk of carrying the mutation can be offered genetic testing.
Management
It is often helpful for patients to have an explanation of the usual disease progression, which may stabilise in the teens until middle age, and of the remarkable differences in disease severity among family members,22,23,24,25 which indicates that disease onset and rate of progression cannot be predicted by either mutation analysis or by comparisons with other affected relatives. Many affected children benefit from correction of refractive correction, low vision aids and educational support. Currently, there is no treatment of the retinal degeneration and treatment of the schisis cavities is usually not indicated. One recent report describes successful treatment of schisis cavities with topical dorzolamide (carbonic anhydrase inhibitor).55 Seven out of eight patients treated had an improvement in the degree of cystic foveal lesions in at least one eye when measured using OCT and six of these patients had a modest improvement in visual acuity. These are interesting results but additional studies are required to assess how long the effects are maintained and whether there is a sustained improvement in functional vision.
Vitreous haemorrhage when not associated with retinal detachment usually resolves spontaneously. In the event of severe complications such as retinal detachment and vitreous haemorrhage, surgical intervention may be required. Regillo et al56 evaluated surgical management of six eyes from four patients with XLRS1 using scleral buckling for retinal detachment and vitrectomy for vitreous haemorrhage or proliferative vitretinopathy. Anatomical success and ambulatory vision was achieved in five of the six eyes with a mean follow‐up of 3.8 years. However, two of the four eyes treated by primary scleral buckling eventually required vitrectomy. Recent reports of using perfluorocarbon liquid or perfluorodecalin during vitrectomy to repair retinoschisis‐associated retinal detachments has shown promising results.57,58
Families often benefit from genetic counselling to explain the X‐linked inheritance pattern and recurrence risks in future offsprings. If a genetic diagnosis has been made with the identification of the causative mutation, then women who are at risk of carrying the mutation can be offered genetic testing. It is particularly important to explain the extreme variation in severity even within families since, for example, affected brothers might have very different disease.23
Differential diagnosis
The identification of foveal schisis in a male, associated with a reduced b‐wave on ERG and a family history consistent with X‐linked inheritance, makes the diagnosis very likely. This can be confirmed by molecular genetic studies. X‐linked inheritance and electronegative dark‐adapted ERG, as previously stated, is not confined to XLRS. The chief differentials are X linked CSNB type 1 (MIM #310500) and type 2 (MIM #300071). Ophthalmoscopically visible fundus changes may not be visible in either XLRS or XLCSNB and both may present with nystagmus, although it is more common in CSNB. A clear history of nyctalopia would direct one to the correct diagnosis. Furthermore, myopia is typical of XLCSNB in contrast with the hypermetropia which is frequent in XLRS.
Flat b‐waves on ERG testing are also associated with a variety of postphototransduction disorders of the inner retina representing between 2.9% and 4.8% of all ERGs recorded in tertiary referral centres.59,60 The ERG findings should be taken in the context of other clinical features to support the diagnosis.
Cystic changes in the macula may be due to a variety of causes. Most frequent of these is macular oedema, which is often owing to conditions such as retinal vein occlusion, diabetic retinopathy, uveitis, retinitis pigmentosa and even dominantly inherited cystoid macular oedema,61 although the associated clinical features and leakage on fluorescein angiography accompanying these disorders rarely lead to diagnostic confusion. There are also descriptions of possible autosomal recessive foveal schisis.62,63 The second of these descriptions describes female patients with foveal schisis but no additional retinal abnormalities and normal electrodiagnostic testing. The foveal findings looked somewhat different from those in XLRS.63 A more recently recognised syndrome of macular retinoschisis in highly myopic eyes with posterior staphyloma has been characterised by OCT, demonstrating splitting of the inner and outer retinal layers within the macular region.64 OCT performed for optic nerve pit maculopathy demonstrates foveal retinoschisis that may be secondary to posterior vitreous traction.65 In this condition a small pit is visible at the temporal edge of the optic disc.
Degenerative retinoschisis tends to involve the peripheral retina with splitting of the outer retinal layers. The condition tends to be unilateral, occurring in an older age group and is not associated with ERG abnormalities or RS1 mutations.66
Other conditions which should be differentiated from XLRS1 include the rare autosomal recessive condition, Goldmann–Favre syndrome, caused by mutations in the gene NR2E3,67 which can lead to foveal schisis, but the associated nyctalopia and pigmentary clumping should help to differentiate this from XLRS. In addition, the ERG is usually extinguished. Niacin, occasionally prescribed for familial hyperlipidaemia, has been shown to cause a reversible cystic maculopathy.68 As in XLRS, these cysts fail to show leakage on flourescein angiography, but are demonstrated on OCT affecting both the outer plexiform and inner nuclear layers.69,70 An unusual autosomal dominant retinoschisis with both macular and peripheral involvement has been reported in which male‐to‐male transmission was documented. The ERG responses in six out of eight failed to demonstrate any abnormality.1
Pathology
Few affected eyes have been available for study, although investigation of retinoschisis mouse models has greatly assisted these investigations14,15,16. Condon et al71 examined one surgically enucleated and two postmortem eyes from two related men with XLRS and this was followed up with investigation of the globes from three further patients (two of whom were related).72 These studies delineated the pathology in the inner retina describing the characteristic abnormality: a split (or schisis) within the superficial retinal layers, the inner limiting membrane, the nerve fibre layer and the ganglion cell layer, the inner leaflet of the schisis consisting of inner limiting membrane, fragments of Müller cells (glial cells) and blood vessels. The ganglion cell layer is thinned with marked degeneration of the overlying photoreceptors associated with thinning of the inner nuclear layer.72 The schisis cavity, the inner and outer schisis layers, and the surrounding retina are described as containing an amorphous eosinophilic PAS‐positive material that is filamentous and thought to originate from Müller cells.72
Molecular genetics
The RS1 gene (OMIM# 312700), which maps to Xp22, was identified late in 1997 after an extensive positional cloning effort by a number of groups8 and has six exons with a cDNA of 3.1 kb. RS1 is expressed exclusively in the retina8 by photoreceptors and bipolar cells10,73 and encodes retinoschisin, a 224 amino acid secretory protein of 24 kDa, which is detected throughout all layers in the neural retina despite the restricted pattern of gene expression.10,73,74
Retinoschisin has a signal peptide allowing transport into the endoplasmic reticulum for trafficking through the secretory pathway and a single discoidin domain which contains 10 cysteine residues.8,10 Discoidin domains (also known as the F5/8 type C domains) are found in a family of extracellular cell surface proteins and are involved in cell adhesion and signalling.75 The discoidin domain receptors, for example, which are transmembrane tyrosine kinase receptors, interact through their discoidin domains with collagens and regulate cell adhesion and extracellular matrix remodelling.75 Other proteins containing a discoidin domain include blood coagulation factors 5 and 8, milk fat globule protein, neuropilins 1 and 2 and neurexin IV.76 The cysteine residues within the retinoschisis discoidin domain are critical for folding and the formation of functional retinoschisin dimers and ultimately octamers.11 Disulphide bonds between two pairs of cysteine residues (Cys63‐Cys219 and Cys110‐Cys142) stablilise the folded monomeric retinoschisin subunits and additional disulphide bonded pairs of cysteines link the subunits into dimers (Cys40‐Cys40) and octamers (Cys59‐Cys223).11 Disruption of these bonds interferes with dimer and octamer formation.11
Retinoschisin is believd to function in cell adhesion in the development and maintenance of retinal architecture. This is in keeping with other proteins containing discoidin domains and is supported by the observations in patients and in the mouse models. A wave of expression begins during retinal development immediately after neuronal birth and terminal differentiation as neuronal cell type is born77 and is then maintained in adult life.
The molecular interactions of retinoschisin and its molecular role in maintenance of retinal integrity have yet to be fully elucidated. Recent data suggest retinoschisin might interact with β2 laminin within the extracellular space and with αB crystallin intracellularly.78 Laminins are large heterotrimeric extracellular glycoproteins thought to play a role in the development and stablility of synapses.79,80 Deletion of the β2 chain leads to a reduction in the amplitude of the b‐wave in mice reminiscent of XLRS81 supporting a possible molecular interaction between retinoschisin and β2 laminin. αB crystallin is an intracellular molecule which functions as a chaperone82 and may interact with retinoschisin as it moves through the secretory pathway. The physiological role of these potential molecular interactions and the implications for XLRS require further investigation.
Molecular pathology
Numerous RS1 mutations associated with XLRS have now been described9,22,25 (http://www.dmd.nl/rs/index.html). The mutations are predominantly missense and are clustered in exons 4–6, which encode the discoidin domain, although deletions, insertions and splice site mutations have been described. Studies of mutant retinoschisin indicate missense mutations lead to disease pathology by at least one of the following three mechanisms:12,13 interfering with retinoschisis secretion, allowing secretion but interfering with retinoschisin octamerisation or allowing secretion and octamerisation but interfere with protein function. The position of these mutations within the protein has helped predictions of these mechanisms.13,83 There is no correlation between the molecular mechanism of disease and its severity,23 which suggests there may be other factors influencing disease severity such as genetic modifiers or environmental influences.84
Animal models
To date, three mouse models exist for XLRS. In 2002, Weber et al14 replaced exon 3 of the murine homologue of human XLRS1 (retinoschisis‐1 homologue, XLRS1h) in‐frame with a LacZ/Neor cassette to create null mouse model (XLRS1h−/Y) with no protein expression. Similarly in 2004, Zeng et al15 replaced exon 1 and 1.6 kb of intron 1 of XLRS1h with a Neor cassette to produce a second null‐allele mouse model. More recently, the Tennessee Mouse Genome Consortium produced a mouse pedigree (44TNJ) with a retinal phenotype using an ENU‐based mutagenesis screen.16 Subsequent mutation analysis of this pedigree revealed a T→C substitution within intron 2 of XLRS1h which created an alternative splice site leading to three transcripts in the affected male mouse: wild type, 10bp insertion after exon 1, 26bp deletion (exon 2). Virtual protein translation showed that both alternative transcripts would form premature stop codons in XLRS1h, although further investigation is required to determine if these truncated XLRS1h peptides are expressed in the 44TNJ mouse.16
All three XLRS1h knockout models displayed morphological and functional retinal phenotypes similar to human XLRS. Fundus examination revealed the presence of small cyst‐like structures in the inner retina of the XLRS1h−/Y mouse14 and intraretinal flecks in 44TNJ mouse.16 Similarly, ERG analysis of all three mouse models showed a characteristic reduced dark‐adapted b‐wave. Histologically, the mice displayed disorganisation of retinal layers due to mislocalisation of cells within the inner plexiform, inner nuclear and outer plexiform layers; focal areas of retinal splitting or “schisis” were also evident within the inner nuclear layer and structural abnormalities of synapses occur within the outer plexiform layer.14,15,16
Gene replacement
The developmental and subsequent continued expression of RS177 and the pathology described in the mouse retina indicate that retinoschisin has an important role in both retinal development and maintenance. This suggests that XLRS is, at least initially, a developmental abnormality of retina and that gene replacement might therefore be a therapeutic possibility. This has been used with some success on both knock‐out mouse XLRS models.15,85 In each case, the RS1 gene was delivered to the affected male mice using an adeno‐associated viral vector and intraocular injection. Subsequent investigation of the retina in these animals indicated successful expression of retinoschisin in all retinal layers.15,85 The ERG recording was taken as a measure of retinal function and in each of these models replacement of the RS1 gene led to restoration of the b‐wave amplitude.15,85 Min et al85also describe an improvement in the rod‐mediated a‐wave. These effects were maintained until at least 5 months after the injection. This group also reported an improvement in the morphology of the inner retina and photoreceptors after injection.85 But there was no similar improvement documented in the study by Zenn et al,15 perhaps reflecting a difference in the viral vector and promoter used which may have resulted in increased levels of protein in the Min et al's study. These results indicate sustained restoration of some retinal function and morphology and suggest that gene replacement might be a possible future treatment for patients.
However, these results should be treated with some caution. The modelling of retinoschisis in the mouse is limited as mice do not have a fovea and many patients have the disease restricted to the fovea or with only mild peripheral changes. The benefits of gene therapy for these patients may be limited and, in addition, the developmental anomalies of the retina are unlikely to be corrected by gene therapy in childhood or adult life. Futhermore the mouse models are both null mutants expressing no retinoschisin which is a different scenario to that in most patients who have missense mutations,9,22,25 many of which lead to intracellular retention of mutant retinoschisin. Therefore, there is a risk that gene therapy might not be effective in these patients as the presence of mutant retinoschisin might have a dominant negative effect on the wild‐type molecule, interfering with its function either by causing sequestration of the wild‐type protein within the cell or by the formation of octamers of wild‐type and mutant protein. Expressing two alleles in one cell will be a novel situation because although women carriers have both mutant and wild‐type alleles, X‐inactivation will silence one of these alleles in each cell. This needs further investigation.
Summary
In summary, XRLS is an important cause of male visual loss in childhood and should be considered as a possible diagnosis in boys with reduced visual acuity and men with macular changes. Recent advances in methods of investigation, including OCT and genetic testing, complement the results of clinical examination and ERG, which is beneficial particularly given its clinical variability. To date there has been no treatment for the retinal degeneration of XLRS although the complications can be treated as they occur. The recent descriptions of gene replacement restoring some retinal function in two knockout XLRS mouse models gives hope that gene therapy may be an option for treatment in the future.
Acknowledgements
The authors are grateful to the MRC for funding the research work that led in part to this review (MRC G0000089/51849).
Abbreviations
CSNB - congenital stationary night blindness
ERG - electroretinogram
OCT - optical coherence tomography
RS1 - retinoschisis gene
XLRS - X‐linked retinoschisis
Footnotes
Competing interests: None.
References
- 1.Yassur Y, Nissenkorn I, Ben‐Sira I, Kaffe S, Goodman R M. Autosomal dominant inheritance of retinoschisis. Am J Ophthalmol 198294338–343. [DOI] [PubMed] [Google Scholar]
- 2.Haas J. Ueber das Zusammenvorkommen von Veranderungen der Retina und Choroidea. Arch Augenheilkd 189837343–348. [Google Scholar]
- 3.Pagenstecher H. Uebereine unterdemBildeder Natzhauterblosung verlaufende,erbicheErkankungderRetina. Graefes Arch Clin Exp Ophthalmol 191386457–462. [Google Scholar]
- 4.Thomson E. Memorandum regarding a family in which neuroretinal disease of an unusal kind occurred in only males. Brit J Ophthalmol 193216681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mann I, Macrae A. Congenital vascular veils in the vitreous. Brit J Ophthalmol 1938221–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jager G M. A hereditary retinal disease. Trans Ophthalmol Soc UK 195373617–619. [Google Scholar]
- 7.Sabates F N. Juvenile retinoschisis. Am J Ophthalmol 196662683–689. [DOI] [PubMed] [Google Scholar]
- 8.Sauer C G, Gehrig A, Warneke‐Wittstock R, Marquardt A, Ewing C C, Gibson A, Lorenz B, Jurklies B, Weber B H. Positional cloning of the gene associated with X‐linked juvenile retinoschisis. Nat Genet 199717164–170. [DOI] [PubMed] [Google Scholar]
- 9.The Retinoschisis Consortium Functional implications of the spectrum of mutations found in 234 cases with X‐linked juvenile retinoschisis. Hum Mol Genet 199871185–1192. [DOI] [PubMed] [Google Scholar]
- 10.Grayson C, Reid S N, Ellis J A, Rutherford A, Sowden J C, Yates J R, Farber D B, Trump D. Retinoschisin, the X‐linked retinoschisis protein, is a secreted photoreceptor protein, and is expressed and released by Weri‐Rb1 cells. Hum Mol Genet 200091873–1879. [DOI] [PubMed] [Google Scholar]
- 11.Wu W W, Molday R S. Defective discoidin domain structure, subunit assembly, and endoplasmic reticulum processing of retinoschisin are primary mechanisms responsible for X‐linked retinoschisis. J Biol Chem 200327828139–28146. [DOI] [PubMed] [Google Scholar]
- 12.Wu W W, Wong J P, Kast J, Molday R S. RS1, a discoidin domain‐containing retinal cell adhesion protein associated with X‐linked retinoschisis, exists as a novel disulfide‐linked octamer. J Biol Chem 200528010721–10730. [DOI] [PubMed] [Google Scholar]
- 13.Wang T, Zhou A, Waters C T, O'Connor E, Read R J, Trump D. Molecular pathology of X linked retinoschisis: mutations interfere with retinoschisin secretion and oligomerisation. Br J Ophthalmol 20069081–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weber B H, Schrewe H, Molday L L, Gehrig A, White K L, Seeliger M W, Jaissle G B, Friedburg C, Tamm E, Molday R S. Inactivation of the murine X‐linked juvenile retinoschisis gene, Rs1h, suggests a role of retinoschisin in retinal cell layer organization and synaptic structure. Proc Natl Acad Sci USA 2002996222–6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeng Y, Takada Y, Kjellstrom S, Hiriyanna K, Tanikawa A, Wawrousek E, Smaoui N, Caruso R, Bush R A, Sieving P A. RS‐1 Gene delivery to an adult Rs1h knockout mouse model restores ERG b‐wave with reversal of the electronegative waveform of X‐linked retinoschisis. Invest Ophthalmol Vis Sci 200445(9)3279–3285. [DOI] [PubMed] [Google Scholar]
- 16.Jablonski M M, Dalke C, Wang X, Lu L, Manly K F, Pretsch W, Favor J, Pardue M T, Rinchik E M, Williams R W, Goldowitz D, Graw J. An ENU‐induced mutation in Rs1h causes disruption of retinal structure and function. Mol Vis 200511569–581. [PubMed] [Google Scholar]
- 17.Forsius H, Krause U, Helve J, Voupala V, Mustonen E, Vainio‐Mattila B, Fellman J. Visual acuity in 183 cases of X‐chromosomal retinoschisis. Can J Ophthalmol 19738385–393. [PubMed] [Google Scholar]
- 18.De La Chappelle A, Alitalo T, Forsius H. X‐linked juvenile retinoschisis. In: Wright AF, Jay B, eds. Molecular Genet Inherited Eye Disorders. Switzerland: Harwood Academic Publishers, 1994;339–57,
- 19.van Schooneveld M J. X‐linked juvenile retinoschisis. Amsterdam: Netherlands Ophthalmic Research Institut, 1997113
- 20.Huopaniemi L, Rantala A, Forsius H, Somer M, de la Chapelle A, Alitalo T. Three widespread founder mutations contribute to high incidence of X‐ linked juvenile retinoschisis in Finland. Eur J Hum Genet 19997368–376. [DOI] [PubMed] [Google Scholar]
- 21.Peltonen L, Jalanko A, Varilo T. Molecular genetics of the Finnish disease heritage. Hum Mol Genet 199981913–1923. [DOI] [PubMed] [Google Scholar]
- 22.Eksandh L C, Ponjavic V, Ayyagari R, Bingham E L, Hiriyanna K T, Andreasson S, Ehinger B, Sieving P A. Phenotypic expression of juvenile X‐linked retinoschisis in Swedish families with different mutations in the XLRS1 gene. Arch Ophthalmol 20001181098–1104. [DOI] [PubMed] [Google Scholar]
- 23.Pimenides D, George N D, Yates J R, Bradshaw K, Roberts S A, Moore A T, Trump D. X‐linked retinoschisis: clinical phenotype and RS1 genotype in 86 UK patients. J Med Genet 200542e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shinoda K, Ishida S, Oguchi Y, Mashima Y. Clinical characteristics of 14 japanese patients with X‐linked juvenile retinoschisis associated with XLRS1 mutation. Ophthalmic Genet 200021171–180. [PubMed] [Google Scholar]
- 25.Simonelli F, Cennamo G, Ziviello C, Testa F, de Crecchio G, Nesti A, Manitto M P, Ciccodicola A, Banfi S, Brancato R, Rinaldi E. Clinical features of X linked juvenile retinoschisis associated with new mutations in the XLRS1 gene in Italian families. Br J Ophthalmol 2003871130–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.George N D, Yates J R, Bradshaw K, Moore A T. Infantile presentation of X linked retinoschisis. Br J Ophthalmol 199579653–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.George N D, Yates J R, Moore A T. Clinical features in affected males with X‐linked retinoschisis. Arch Ophthalmol 1996114274–280. [DOI] [PubMed] [Google Scholar]
- 28.Deutmann A F.The hereditary dystrophies of the posterior pole of the eye. Assen: Van Gorcum, 1971
- 29.Kellner U, Brummer S, Foerster M H, Wessing A. X‐linked congenital retinoschisis. Graefe's Archive Ophthalmol 1990228432–437. [DOI] [PubMed] [Google Scholar]
- 30.Garg S J, Lee H C, Grand M G. Bilateral macular detachments in X‐linked retinoschisis. Arch Ophthalmol 20061241053–1055. [DOI] [PubMed] [Google Scholar]
- 31.Prasad A, Wagner R, Bhagat N. Vitreous hemorrhage as the initial manifestation of X‐linked retinoschisis in a 9‐month‐old infant. J Pediatr Ophthalmol Strabismus 20064356–58. [DOI] [PubMed] [Google Scholar]
- 32.Kato K, Miyake Y, Kachi S, Suzuki T, Terasaki H, Kawase Y, Kanda T. Axial length and refractive error in X‐linked retinoschisis. Am J Ophthalmol 2001131812–814. [DOI] [PubMed] [Google Scholar]
- 33.Vainio‐Mattila B, Eriksson A W, Forsius H. X‐chromosomal recessive retinoschisis in the region of Pori. Acta Ophthalmologia 1969471135–1148. [DOI] [PubMed] [Google Scholar]
- 34.Saldana M, Sheridan E, Thompson J, Monk E, Doran R D, Trump D, Long V. X‐linked retinoschisis in the female with a heterozygous RS1 missense mutation. Am J of Med Genetics. 2006;in press [DOI] [PubMed]
- 35.Mendoza‐Londono R, Hiriyanna K T, Bingham E L, Rodriguez F, Shastry B S, Rodriguez A, Sieving P A, Tamayo M L. A Colombian family with X‐linked juvenile retinoschisis with three affected females finding of a frameshift mutation. Ophthalmic Genet 19992037–43. [DOI] [PubMed] [Google Scholar]
- 36.Marmor M F, Zrenner E. Standard for clinical electroretinography (1999 update). Doc Ophthalmol 199897143–156. [DOI] [PubMed] [Google Scholar]
- 37.Bush R A, Sieving P A. A proximal retinal component in the primate photopic ERG a‐wave. Invest Ophthalmol Vis Sci 199435635–645. [PubMed] [Google Scholar]
- 38.Forrester J, Dick A, McMenamin P, Lee W.The eye basic sciences in practice. London: Saunders, 1996
- 39.Tanino T, Katsumi O, Hirose T. Electrophysiological similarities between two eyes with X‐linked retinoschisis. Doc Ophthalmol 198560149–161. [DOI] [PubMed] [Google Scholar]
- 40.Peachey N S, Fishman G A, Derlacki D J, Brigell M G. Psychophysical and electroretinographic findings in X‐linked juvenile retinoschisis. Arch Ophthalmol 1987105513–516. [DOI] [PubMed] [Google Scholar]
- 41.Holder G E, Robson A G. Genetically determined disorders of retinal function. In: Celesia GG, ed. Disorders of visual processing. Vol 5: Elsevier, 2005;271–194,
- 42.Tanimoto N, Usui T, Takagi M, Hasegawa S, Abe H, Sekiya K, Miyagawa Y, Nakazawa M. Electroretinographic findings in three family members with X‐linked juvenile retinoschisis associated with a Novel Pro192Thr mutation of the XLRS1 gene. Jpn J Ophthalmol 200246568–576. [DOI] [PubMed] [Google Scholar]
- 43.Bradshaw K, George N, Moore A, Trump D. Mutations of the XLRS1 gene cause abnormalities of photoreceptor as well as inner retinal responses of the ERG. Doc Ophthalmol 199998153–173. [DOI] [PubMed] [Google Scholar]
- 44.Bradshaw K, Allen L, Trump D, Hardcastle A, George N, Moore A. A comparison of ERG abnormalities in XLRS and XLCSNB. Doc Ophthalmol 2004108135–145. [DOI] [PubMed] [Google Scholar]
- 45.Sieving P A, Bingham E L, Kemp J, Richards J, Hiriyanna K. Juvenile X‐linked retinoschisis from XLRS1 Arg213Trp mutation with preservation of the electroretinogram scotopic b‐wave. Am J Ophthalmol 1999128179–184. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura M, Ito S, Terasaki H, Miyake Y. Japanese X‐linked juvenile retinoschisis: conflict of phenotype and genotype with novel mutations in the XLRS1 gene. Arch Ophthalmol 20011191553–1554. [PubMed] [Google Scholar]
- 47.Azzolini C, Pierro L, Codenotti M, Brancato R. OCT images and surgery of juvenile Macular retinoschisis. Eur J Ophthalmol 19977196–200. [DOI] [PubMed] [Google Scholar]
- 48.Stanga P E, Chong N H, Reck A C, Hardcastle A J, Holder G E. Optical coherence tomography and electrophysiology in X‐linked juvenile retinoschisis associated with a novel mutation in the XLRS1 gene. Retina 20012178–80. [DOI] [PubMed] [Google Scholar]
- 49.Muscat S, Fahad B, Parks S, Keating D. Optical coherence tomography and multifocal electroretinography of X‐linked juvenile retinoschisis. Eye 200115796–799. [DOI] [PubMed] [Google Scholar]
- 50.Eriksson U, Larsson E, Holmstrom G. Optical coherence tomography in the diagnosis of juvenile X‐linked retinoschisis. Acta Ophthalmol Scand 200482218–223. [DOI] [PubMed] [Google Scholar]
- 51.Chan W M, Choy K W, Wang J, Lam D S, Yip W W, Fu W, Pang C P. Two cases of X‐linked juvenile retinoschisis with different optical coherence tomography findings and RS1 gene mutations. Clin Experiment Ophthalmol 200432429–432. [DOI] [PubMed] [Google Scholar]
- 52.Minami Y, Ishiko S, Takai Y, Kato Y, Kagokawa H, Takamiya A, Nagaoka T, Kinouchi R, Yoshida A. Retinal changes in juvenile X linked retinoschisis using three dimensional optical coherence tomography. Br J Ophthalmol 2005891663–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Apushkin M A, Fishman G A, Janowicz M J. Correlation of optical coherence tomography findings with visual acuity and macular lesions in patients with X‐linked retinoschisis. Ophthalmology 2005112495–501. [DOI] [PubMed] [Google Scholar]
- 54.Souied E H, Goritsa A, Querques G, Coscas G, Soubrane G. Indocyanine green angiography of juvenile X‐linked retinoschisis. Am J Ophthalmol 2005140558–561. [DOI] [PubMed] [Google Scholar]
- 55.Apushkin M A, Fishman G A. Use of dorzolamide for patients with X‐linked retinoschisis. Retina 200626741–745. [DOI] [PubMed] [Google Scholar]
- 56.Regillo C D, Tasman W S, Brown G C. Surgical management of complications associated with X‐linked retinoschisis. Arch Ophthalmol 19931111080–1086. [DOI] [PubMed] [Google Scholar]
- 57.Lomeo M D, Diaz‐Rohena R, Lambert H M. Use of perfluorocarbon liquid in the repair of retinoschisis retinal detachments. J Ophthalmic Nurs Technol 19971618–21. [PubMed] [Google Scholar]
- 58.Aslan O, Batman C, Cekic O, Ozalp S. The use of perfluorodecalin in retinal detachments with retinoschisis. Ophthalmic Surg Lasers 199829818–821. [PubMed] [Google Scholar]
- 59.Koh A H, Hogg C R, Holder G E. The incidence of negative ERG in clinical practice. Doc Ophthalmol 200110219–30. [DOI] [PubMed] [Google Scholar]
- 60.Renner A B, Kellner U, Cropp E, Foerster M H. Dysfunction of transmission in the inner retina: incidence and clinical causes of negative electroretinogram. Graefes Arch Clin Exp Ophthalmol 2006 [DOI] [PubMed]
- 61.Deutman A F, Pinckers A J, Aan de Kerk A L. Dominantly inherited cystoid macular edema. Am J Ophthalmol 197682540–548. [DOI] [PubMed] [Google Scholar]
- 62.Lewis R A, Lee G B, Martonyi C L, Barnett J M, Falls H F. Familial foveal retinoschisis. Arch Ophthalmol 1977951190–1196. [DOI] [PubMed] [Google Scholar]
- 63.Kabanarou S A, Holder G E, Bird A C, Webster A R, Stanga P E, Vickers S, Harney B A. Isolated foveal retinoschisis as a cause of visual loss in young females. Br J Ophthalmol 200387801–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takano M, Kishi S. Foveal retinoschisis and retinal detachment in severely myopic eyes with posterior staphyloma. Am J Ophthalmol 1999128472–476. [DOI] [PubMed] [Google Scholar]
- 65.Hirakata A, Hida T, Ogasawara A, Iizuka N. Multilayered retinoschisis associated with optic disc pit. Jpn J Ophthalmol 200549414–416. [DOI] [PubMed] [Google Scholar]
- 66.Gehrig A, White K, Lorenz B, Andrassi M, Clemens S, Weber B H. Assessment of RS1 in X‐linked juvenile retinoschisis and sporadic senile retinoschisis. Clin Genet 199955461–465. [DOI] [PubMed] [Google Scholar]
- 67.Sharon D, Sandberg M A, Caruso R C, Berson E L, Dryja T P. Shared mutations in NR2E3 in enhanced S‐cone syndrome, Goldmann‐Favre syndrome, and many cases of clumped pigmentary retinal degeneration. Arch Ophthalmol 20031211316–1323. [DOI] [PubMed] [Google Scholar]
- 68.Gass J D. Nicotinic acid maculopathy. Am J Ophthalmol 197376500–510. [DOI] [PubMed] [Google Scholar]
- 69.Spirn M J, Warren F A, Guyer D R, Klancnik J M, Spaide R F. Optical coherence tomography findings in nicotinic acid maculopathy. Am J Ophthalmol 2003135913–914. [DOI] [PubMed] [Google Scholar]
- 70.Dajani H M, Lauer A K. Optical coherence tomography findings in niacin maculopathy. Can J Ophthalmol 200641197–200. [DOI] [PubMed] [Google Scholar]
- 71.Condon G P, Brownstein S, Wang N S, Kearns J A, Ewing C C. Congenital hereditary (juvenile X‐linked) retinoschisis. Histopathologic and ultrastructural findings in three eyes. Arch Ophthalmol 1986104576–583. [DOI] [PubMed] [Google Scholar]
- 72.Kirsch L S, Brownstein S, de Wolff‐Rouendaal D. A histopathological, ultrastructural and immunohistochemical study of congenital hereditary retinoschisis. Can ophthalmol 199631301–310. [PubMed] [Google Scholar]
- 73.Molday L L, Hicks D, Sauer C G, Weber B H, Molday R S. Expression of X‐linked retinoschisis protein RS1 in photoreceptor and bipolar cells. Invest Ophthalmol Vis Sci 200142816–825. [PubMed] [Google Scholar]
- 74.Reid S N, Yamashita C, Farber D B. Retinoschisin, a photoreceptor‐secreted protein, and its interaction with bipolar and muller cells. J Neurosci 2003236030–6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vogel W F, Abdulhussein R, Ford C E. Sensing extracellular matrix: An update on discoidin domain receptor function. Cell Signal 2006181108–1116. [DOI] [PubMed] [Google Scholar]
- 76.Vogel W. Discoidin domain receptors: structural relations and functional implications. The FASEB Journal 199913S77–S82. [DOI] [PubMed] [Google Scholar]
- 77.Takada Y, Fariss R N, Tanikawa A, Zeng Y, Carper D, Bush R, Sieving P A. A retinal neuronal developmental wave of retinoschisin expression begins in ganglion cells during layer formation. Invest Ophthalmol Vis Sci 2004453302–3312. [DOI] [PubMed] [Google Scholar]
- 78.Steiner‐Champliaud M F, Sahel J, Hicks D. Retinoschisin forms a multi‐molecular complex with extracellular matrix and cytoplasmic proteins: interactions with beta2 laminin and alphaB‐crystallin. Mol Vis 200612892–901. [PubMed] [Google Scholar]
- 79.Hunter D D, Shah V, Merlie J P, Sanes J R. A laminin‐like adhesive protein concentrated in the synaptic cleft of the neuromuscular junction. Nature 1989338229–234. [DOI] [PubMed] [Google Scholar]
- 80.Noakes P G, Gautam M, Mudd J, Sanes J R, Merlie J P. Aberrant differentiation of neuromuscular junctions in mice lacking s‐laminin/laminin beta 2. Nature 1995374258–262. [DOI] [PubMed] [Google Scholar]
- 81.Libby R T, Lavallee C R, Balkema G W, Brunken W J, Hunter D D. Disruption of laminin beta2 chain production causes alterations in morphology and function in the CNS. J Neurosci 1999199399–9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Horwitz J. Alpha‐crystallin can function as a molecular chaperone. Proc Natl Acad Sci USA 19928910449–10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fraternali F, Cavallo L, Musco G. Effects of pathological mutations on the stability of a conserved amino acid triad in retinoschisin. FEBS Lett 200354421–26. [DOI] [PubMed] [Google Scholar]
- 84.Iannaccone A, Mura M, Dyka F M, Ciccarelli M L, Yashar B M, Ayyagari R, Jablonski M M, Molday R S. An unusual X‐linked retinoschisis phenotype and biochemical characterization of the W112C RS1 mutation. Vision Res 2006463845–3852. [DOI] [PubMed] [Google Scholar]
- 85.Min S H, Molday L L, Seeliger M W, Dinculescu A, Timmers A M, Janssen A, Tonagel F, Tanimoto N, Weber B H, Molday R S, Hauswirth W W. Prolonged recovery of retinal structure/function after gene therapy in an Rs1h‐deficient mouse model of X‐linked juvenile retinoschisis. Mol Ther 200512644–651. [DOI] [PubMed] [Google Scholar]