Abstract
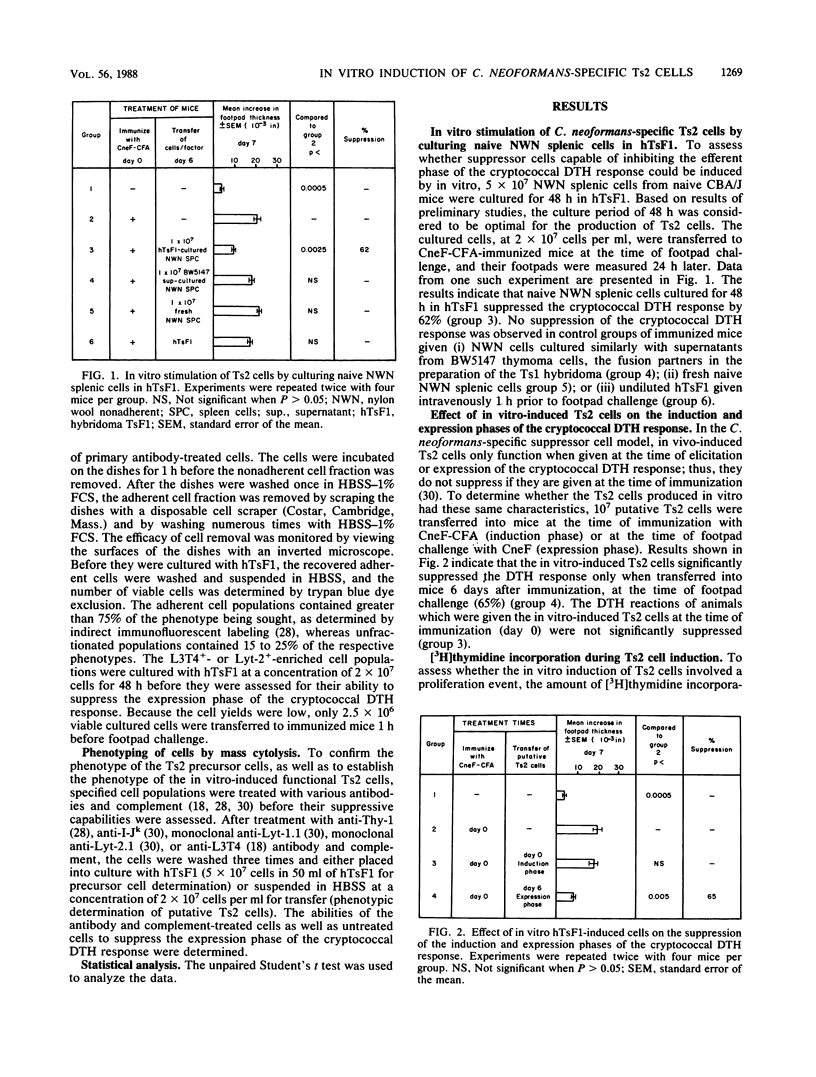
Using a cryptococcal culture filtrate antigen (CneF) in a murine model, we have demonstrated previously that a cascade of Cryptococcus neoformans-specific suppressor T cells and soluble factors function in suppressing the cryptococcal delayed-type hypersensitivity (DTH) response. In addition, we have successfully hybridized the C. neoformans-specific, first-order T-suppressor (Ts1) cell and have established that the culture supernatant (hTsF1) from this hybridoma induces second-order T-suppressor (Ts2) cells in vivo. Here we report the in vitro induction of expression-phase suppressor cells. The suppressor cells were induced by culturing nylon wool-nonadherent splenic cells from naive mice with hTsF1 in the absence of CneF. Nylon wool-nonadherent splenic cells similarly cultured with supernatants from the BW5147 thymoma cells, the fusion partners of the hybridoma, did not significantly suppress the cryptococcal DTH response. The suppressor cells were designated Ts2 cells based on their similarities in function, specificity, and phenotype, i.e. L3T4-, Lyt-2+, and I-J+, to the in vivo-induced Ts2 cells. By employing the in vitro culture technique, we demonstrated that the precursors of the functional Ts2 cells were L3T4- Lyt-1-2+ I-J- cells. The induction of Ts2 cells was not associated with [3H]thymidine incorporation; therefore, we concluded that hTsF1 induces the Lyt-2+ I-J- cells to differentiate into Lyt-2+ I-J+ functional Ts2 cells without a significant amount of proliferation. From the results of this study, a better understanding of the processes involved in the regulation of the DTH response to CneF was achieved. The in vitro culture technique will allow for further detailed studies of the interactions between the various cell populations and the Ts1 cell-derived soluble factor during the induction of Ts2 cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asherson G. L., Colizzi V., Zembala M. An overview of T-suppressor cell circuits. Annu Rev Immunol. 1986;4:37–68. doi: 10.1146/annurev.iy.04.040186.000345. [DOI] [PubMed] [Google Scholar]
- Beckwith M., Rich S. S. Suppressor-target interaction in alloantigen induced responses: induction of a second cell in the suppressive pathway. J Immunol. 1982 Feb;128(2):791–796. [PubMed] [Google Scholar]
- Cauley L. K., Murphy J. W. Response of congenitally athymic (nude) and phenotypically normal mice to Cryptococcus neoformans infection. Infect Immun. 1979 Mar;23(3):644–651. doi: 10.1128/iai.23.3.644-651.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbert E. J., Kontiainen S., Douglas L. M., Feldmann M. Definition of function-related isotypic markers on T cells. Curr Top Microbiol Immunol. 1982;100:19–32. doi: 10.1007/978-3-642-68586-6_3. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Bennett J. E. Prognostic factors in cryptococcal meningitis. A study in 111 cases. Ann Intern Med. 1974 Feb;80(2):176–181. doi: 10.7326/0003-4819-80-2-176. [DOI] [PubMed] [Google Scholar]
- Dorf M. E., Benacerraf B. Suppressor cells and immunoregulation. Annu Rev Immunol. 1984;2:127–157. doi: 10.1146/annurev.iy.02.040184.001015. [DOI] [PubMed] [Google Scholar]
- Eng R., Chmel H., Corrado M., Smith S. M. The course of cryptococcal capsular polysaccharide antigenemia/human cryptococcal polysaccharide elimination kinetics. Infection. 1983 May-Jun;11(3):132–136. doi: 10.1007/BF01641291. [DOI] [PubMed] [Google Scholar]
- Germain R. N., Theze J., Waltenbaugh C., Dorf M. E., Benacerraf B. Antigen-specific T cell-mediated suppression. II. In vitro induction by I-J-coded L-glutamic acid50-L-tyrosine50 (GT)-specific T cell suppressor factor (GT-T8F) of suppressor T cells (T82) bearing distinct I-J determinants. J Immunol. 1978 Aug;121(2):602–607. [PubMed] [Google Scholar]
- Germain R. N., Thèze J., Kapp J. A., Benacerraf B. Antigen-specific T-cell-mediated suppression. I. Induction of L-glutamic acid60-L-alanine30-L-tyrosine10 specific suppressor T cells in vitro requires both antigen-specific T-cell-suppressor factor and antigen. J Exp Med. 1978 Jan 1;147(1):123–136. doi: 10.1084/jem.147.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman J. S., Kaufman L., Koenig M. G. Diagnosis of cryptococcal meningitis. Value of immunologic detection of cryptococcal antigen. N Engl J Med. 1971 Aug 19;285(8):434–436. doi: 10.1056/NEJM197108192850804. [DOI] [PubMed] [Google Scholar]
- Gordon M. A., Vedder D. K. Serologic tests in diagnosis and prognosis of cryptococcosis. JAMA. 1966 Sep 19;197(12):961–967. [PubMed] [Google Scholar]
- Graybill J. R., Alford R. H. Cell-mediated immunity in Cryptococcosis. Cell Immunol. 1974 Oct;14(1):12–21. doi: 10.1016/0008-8749(74)90164-6. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kauffman C. A., Bergman A. G., Severance P. J., McClatchey K. D. Detection of cryptococcal antigen. Comparison of two latex agglutination tests. Am J Clin Pathol. 1981 Jan;75(1):106–109. doi: 10.1093/ajcp/75.1.106. [DOI] [PubMed] [Google Scholar]
- Kearns R. J., DeFreitas E. C. In vitro propagation of antigen-specific T lymphocytes that adoptively transfer resistance to Listeria monocytogenes. Infect Immun. 1983 May;40(2):713–719. doi: 10.1128/iai.40.2.713-719.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakpour F. R., Murphy J. W. Characterization of a third-order suppressor T cell (Ts3) induced by cryptococcal antigen(s). Infect Immun. 1987 Jul;55(7):1657–1662. doi: 10.1128/iai.55.7.1657-1662.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lim T. S., Murphy J. W., Cauley L. K. Host-etiological agent interactions in intranasally and intraperitoneally induced Cryptococcosis in mice. Infect Immun. 1980 Aug;29(2):633–641. doi: 10.1128/iai.29.2.633-641.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim T. S., Murphy J. W. Transfer of immunity to cryptococcosis by T-enriched splenic lymphocytes from Cryptococcus neoformans-sensitized mice. Infect Immun. 1980 Oct;30(1):5–11. doi: 10.1128/iai.30.1.5-11.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mage M. G., McHugh L. L., Rothstein T. L. Mouse lymphocytes with and without surface immunoglobulin: preparative scale separation in polystyrene tissue culture dishes coated with specifically purified anti-immunoglobulin. J Immunol Methods. 1977;15(1):47–56. doi: 10.1016/0022-1759(77)90016-3. [DOI] [PubMed] [Google Scholar]
- Miller S. D., Butler L. D., Claman H. N. Suppressor T cell circuits in contact sensitivity. I. Two mechanistically distinct waves of suppressor T cells occur in mice tolerized with syngeneic DNP-modified lymphoid cells. J Immunol. 1982 Aug;129(2):461–468. [PubMed] [Google Scholar]
- Mosley R. L., Murphy J. W., Cox R. A. Immunoadsorption of Cryptococcus-specific suppressor T-cell factors. Infect Immun. 1986 Mar;51(3):844–850. doi: 10.1128/iai.51.3.844-850.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., Cozad G. C. Immunological unresponsiveness induced by cryptococcal capsular polysaccharide assayed by the hemolytic plaque technique. Infect Immun. 1972 Jun;5(6):896–901. doi: 10.1128/iai.5.6.896-901.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W. Effects of first-order Cryptococcus-specific T-suppressor cells on induction of cells responsible for delayed-type hypersensitivity. Infect Immun. 1985 May;48(2):439–445. doi: 10.1128/iai.48.2.439-445.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., Moorhead J. W. Regulation of cell-mediated immunity in cryptococcosis. I. Induction of specific afferent T suppressor cells by cryptococcal antigen. J Immunol. 1982 Jan;128(1):276–283. [PubMed] [Google Scholar]
- Murphy J. W., Mosley R. L., Moorhead J. W. Regulation of cell-mediated immunity in cryptococcosis. II. Characterization of first-order T suppressor cells (Ts1) and induction of second-order suppressor cells. J Immunol. 1983 Jun;130(6):2876–2881. [PubMed] [Google Scholar]
- Murphy J. W., Mosley R. L. Regulation of cell-mediated immunity in cryptococcosis. III. Characterization of second-order T suppressor cells (Ts2). J Immunol. 1985 Jan;134(1):577–584. [PubMed] [Google Scholar]
- Phanuphak P., Moorhead J. W., Claman H. N. Tolerance and contact sensitivity to DNFB in mice. I. In vivo detection by ear swelling and correlation with in vitro cell stimulation. J Immunol. 1974 Jan;112(1):115–123. [PubMed] [Google Scholar]
- Poon R. Y., Kapp J. A., Pierce C. W., Sorensen C. M. Characterization of two monoclonal idiotype-binding suppressor T cell factors specific for the antibody response to L-glutamic acid60-L-alanine30-L-tyrosine10 (GAT). J Immunol. 1986 Dec 15;137(12):3709–3716. [PubMed] [Google Scholar]
- Sherr D. H., Minami M., Okuda K., Dorf M. E. Analysis of T cell hybridomas. III. Distinctions between two types of hapten-specific suppressor factors that affect plaque-forming cell responses. J Exp Med. 1983 Feb 1;157(2):515–529. doi: 10.1084/jem.157.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M., Tokuhisa T. Cellular consequences in the suppression of antibody response by the antigen-specific T-cell factor. J Exp Med. 1980 Mar 1;151(3):517–527. doi: 10.1084/jem.151.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold L. E., Roberts G. D., Brewer N. S., Zeller D. J., Fineman L. D., Paschall C. L., Rosenlof R. C. Massive antigenemia during disseminated cryptococcosis. Mayo Clin Proc. 1980 Aug;55(8):513–515. [PubMed] [Google Scholar]
- Young E. J., Hirsh D. D., Fainstein V., Williams T. W. Pleural effusions due to Cryptococcus neoformans: a review of the literature and report of two cases with cryptococcal antigen determinations. Am Rev Respir Dis. 1980 Apr;121(4):743–747. doi: 10.1164/arrd.1980.121.4.743. [DOI] [PubMed] [Google Scholar]



