Abstract
There is a growing awareness that inborn errors of metabolism can be a cause of non‐immune hydrops fetalis. The association between congenital disorders of glycosylation (CDG) and hydrops fetalis has been based on one case report concerning two sibs with hydrops fetalis and CDG‐Ik. Since then two patients with hydrops‐like features and CDG‐Ia have been reported. Two more unrelated patients with CDG‐Ia who presented with hydrops fetalis are reported here, providing definite evidence that non‐immune hydrops fetalis can be caused by CDG‐Ia. The presence of congenital thrombocytopenia and high ferritin levels in both patients was remarkable. These might be common features in this severe form of CDG. Both patients had one severe mutation in the phosphomannomutase 2 gene, probably fully inactivating the enzyme, and one milder mutation with residual activity, as had the patients reported in literature. The presence of one severe mutation might be required for the development of hydrops fetalis. CDG‐Ia should be considered in the differential diagnosis of hydrops fetalis and analysis of PMM activity in chorionic villi or amniocytes should also be considered.
Congenital disorders of glycosylation (CDG) are a heterogeneous group of autosomal recessive disorders, caused by defects in the glycosylation of proteins. CDG type Ia (CDG‐Ia) is the most frequent form of CDG and is caused by deficiency of phosphomannomutase (PMM). Many mutations have been found in the corresponding PMM2 gene located on chromosome 16p13,1,2 with two common mutations R141H and F119L accounting for, respectively, 37% and 16% of all mutant chromosomes.2
The clinical picture of CDG‐Ia is remarkably variable and ranges from mild to severe. Neurological symptoms include mental retardation, strabismus, hypotonia and cerebellar atrophy, which presents early in life, and neuropathy, retinitis pigmentosa and stroke‐like events that occur later in life. Characteristic dysmorphic features in CDG‐Ia are inverted, hypoplastic and widely spaced nipples, abnormal fat pads, orange peel skin and mild facial dysmorphisms including large ears, high forehead and thin upper lip. CDG‐Ia is a multisystem disease and can present with a wide range of systemic manifestations like cardiomyopathy, pericardial effusion, failure to thrive, liver disease, nephrotic syndrome and coagulation disorders.3,4 The mortality in the first years of life is around 20%.5
Pericardial effusion is quite common in CDG.6 Hypertrophic or dilated cardiomyopathy can be present.7,8,9 Severe presentation with prenatal hypertrophic cardiomyopathy and pericardial effusion has been reported.10 Even more severe presentation with hydrops fetalis was reported in two sibs with CDG,11 later identified as CDG‐Ik.12 Hydrops‐like features were since then reported in two more patients.13,14 We report on two patients who presented with non‐immune hydrops fetalis and were diagnosed with CDG‐Ia, providing definite evidence that non‐immune hydrops fetalis can be caused by CDG‐Ia.
Case reports
Patient 1
A boy, the first child of non‐consanguineous parents, was diagnosed by fetal ultrasonography at 29 weeks of gestation with hydrops fetalis, which consisted of severe skin oedema, pericardial effusion and ascites. The placenta was hydropic and there was ample amniotic fluid. The heart appeared large. A fetal umbilical blood sample showed thrombocytopenia (platelet count 13×109/l) but no anaemia (haemoglobin (Hb) concentration 8.8 mmol/l).
At 32 weeks of gestation a caesarean section was performed because of fetal distress. Birth weight was 3200 g (above 97th centile). Apgar scores were 0 at 1 and 5 min and 3 at 10 min. He was resuscitated and required artificial ventilation. He had severe skin oedema, and recurrent pericardial effusion and chylous ascites, both requiring drainage. There was only slight pleural effusion. He had inverted nipples, but no abnormal fat pads, and mild dysmorphic facial features including a short nose with a long philtrum, a thin upper lip and a short neck. He had a hepatomegaly. Echocardiography showed an open ductus Botalli. Cranial ultrasonography was normal. At birth he had a severe anaemia (Hb 1.8 mmol/l) and thrombocytopenia (9.0×109/l). He had a very low plasma albumin level (6 g/l).
During admission, his respiratory and myocardial function deteriorated and there was an increasing pericardial effusion. He died at 7 days during punction of the pericardial effusion.
Post‐mortem examination showed both left and right hypertrophic cardiomyopathy. The foramen ovale was small and either restrictive or prematurely closed. The ductus Botalli was open. Fibrin deposition was found in both cardiac atria, at the inflow of the superior vena cava and the pulmonary veins. Microscopic examination of the liver showed an increase of bile canaliculi with cholestasis and periportal fibrosis. However, electron microscopy did not show abnormal lysosomal inclusions in the hepatocytes.15 No cerebellar atrophy or other cerebral anomalies were found. Examination of the placenta showed a few hydropic villi and no signs of infection or ischaemia.
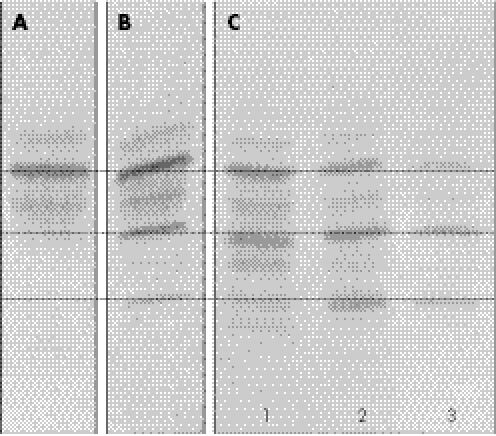
Isoelectric focusing of sialotransferrin in a blood sample taken at day 2, showed a pattern consistent with CDG‐I (fig 1). PMM activity in fibroblasts was decreased (0.12 mU/mg protein, normal controls (n = 29): 1.27–4.53 mU/mg protein, patients with CDG‐Ia (n = 13): 0.04–0.59 mU/mg protein). Mutation analysis of the PMM2 gene showed the common c.357C>A (F119L) mutation and a frameshift insertion (c.160_161insG), which results in a premature stopcodon at position p.59. The parents were heterozygotic for one of the mutations.
Figure 1 Isoelectric focusing of sialotransferrin in patient 1 and patient 2. (A) Normal control. (B) Patient 1, sample taken at day 2. (C) Patient 2, samples taken at day 2 (1), 22 (2) and 39 (3).
Ferritin was, retrospectively, measured in blood samples taken at day 6 and 8 and showed increased levels (445 and 1052 mg/l, respectively).
Patient 2
A girl, the first child of non‐consanguineous parents, was diagnosed by fetal ultrasonography at 35 weeks of gestation with hydrops fetalis with skin oedema, slight pericardial effusion and slight ascites. There was ample amniotic fluid and a “round” heart contour. The length of the femur was relatively short. She was born at 36 weeks by ceasarian section because of breech position. Birth weight was 3290 g (90th centile) and head circumference was 38 cm (above 97th centile). Apgar scores were 6 at 1 min, 7 at 5 min and 8 at 10 min. She required artificial ventilation for 1 day post partum. She had severe skin oedema and minimal ascites but no pericardial or pleural effusion was noticed. She had widely spaced hypoplastic nipples with inversion of the right nipple. She had abnormal fat pads and an orange peel skin. Mild dysmorphic facial features were a high forehead, small eyes and a thin upper lip. Echocardiography showed impaired myocardial function, which had improved after 1 day. Abdominal ultrasound showed mild dilatation of the renal pelvis, and some hyperechogenicity of the renal parenchyma was noticed. Cranial ultrasound at day 1 showed wide subarachnoid spaces, and lateral ventricles and calcifications in the basal ganglia. MRI of the brain at day 4 showed a recent cerebral infarction and confirmed the wide subarachnoid spaces. The cerebellar vermis was too small, but the cerebellar hemispheres appeared normal. MRI at 5 weeks showed evident progression of atrophy of the vermis and some atrophy of the cerebellar hemispheres had developed. No abnormalities were found at funduscopy and visual evoked potential testing was normal. Auditory brainstem responses were absent.
At birth she had a thrombocytopenia (33×109/l), which persisted and required several transfusions. Haemoglobin post partum was 10.2 mmol/l. Later on she developed mild anaemia (lowest Hb 5.9 mmol/l). She had a hyperbilirubinaemia and an increased level of thyroid stimulating hormone, and was started on thyrax at 4 weeks of age. She developed a combination of profuse watery stools, low albumin levels (15–20 g/l), hyponatraemia, hypokalaemia and low immunoglobulin levels, which was suspect for protein‐losing enteropathy. She died at 8 weeks because of severe failure to thrive and recurrent bacterial infections. The parents did not give permission for autopsy.
Isoelectric focusing of sialotransferrin in a blood sample at day 2 was abnormal with clearly increased disialotransferrin, but the pattern became more abnormal and more consistent with CDG‐I in a blood sample at day 22 and day 39 (fig 1) with both asialo and disialotransferrin increased. PMM activity was decreased in leucocytes (0.02 mU/mg protein, normal controls: 0.41–1.81 mU/mg protein (n = 49), patients with CDG‐Ia (n = 7): 0.0–0.17 mU/mg protein) and in fibroblasts (0.07 mU/mg protein). Mutation analysis of the PMM2 gene showed two missense mutations, c.357C>A (F119L) and c.470T>C (F157S). The parents were heterozygotic for one of the mutations.
Ferritin was measured, retrospectively, in blood samples taken at days 2, 3, 22 and 43 and showed highly increased levels (respectively 16 873, 4956, 2652 and 2170 mg/l).
Discussion
Hydrops fetalis is the abnormal accumulation of fluid in two or more fetal compartments, including skin oedema, ascites, pleural effusion and pericardial effusion. Prenatally this can be associated with polyhydramnios and a hydropic placenta. Non‐immune hydrops fetalis can have many causes. Inborn errors of metabolism, mostly lysosomal storage diseases, have been estimated to account for 1–2% of the cases.16 However, 25–50% of the cases of non‐immune hydrops fetalis are unexplained and a significant number could be the result of a metabolic disease.16,17 In 1998, CDG‐I was added to the extensive list of causes of non‐immune hydrops with the case report on two sibs with hydrops fetalis who were diagnosed with CDG‐Ik.11,12 Recently two CDG‐Ia cases were reported with hydrops or hydrops‐like features.13,14 The authors of the last case report question whether CDG is associated with true hydrops or just with hydrops‐like features. Their patient had skin oedema and pericardial effusion (with cardiomyopathy), but they state that they could not confirm the diagnosis of hydrops as ascites and pleural effusions were absent on ultrasound. However, they do mention ascites and pleural effusions in the autopsy of their patient.14 The other case reports11,13 just state the presence of hydrops and do not specify all the locations of the effusions. However when we specifically inquired about these symptoms, the presence of skin oedema, ascites and pleural effusions in these patients was confirmed, consistent with the diagnosis of true hydrops fetalis. True hydrops fetalis was also found in our patients. Furthermore, a limited description of a patient with CDG‐Ia presenting with hydrops fetalis at 27 weeks of gestation has appeared in an abstract.18 This confirms that CDG‐I can be a cause of hydrops fetalis. The clinical features of the patients with CDG‐I with hydrops fetalis in this study and literature are reviewed in table 1.
Table 1 Clinical features of patients with congenital disorders of glycosylation presenting with hydrops fetalis.
| Sex | De Koning et al11 | de Koning et al11 | Aronica et al13 | Noelle et al14 | Patient 1, this study | Patient 2, this study |
|---|---|---|---|---|---|---|
| M | M | M | F | M | F | |
| Hydrops | ||||||
| Diagnosis | At birth, 32 weeks | Ultrasound, 30 weeks | Ultrasound, 20 weeks | At birth, 40 weeks | Ultrasound, 29 weeks | Ultrasound, 35 weeks |
| Oedema | +* | +* | ++ | ++ | ++ | ++ |
| Ascites | +* | +* | +* | + (autopsy) | ++ | + |
| Pleural effusion | +* | +* | +* | + (autopsy) | + | − |
| Pericardial effusion | NS | NS | ++ | ++ | ++ | + |
| Dysmorphic features | ||||||
| Facial | + | + | + | NS | + | + |
| Inverted nipples | NS | NS | + | + | + | + |
| Fat pads | NS | NS | NS | NS | − | + |
| Peau d'orange | NS | NS | NS | + | − | + |
| Contractures | NS | NS | + | NS | − | − |
| Other symptoms | ||||||
| Cardiomyopathy | NS | Dilated | Hypertrophic | Hypertrophic | Hypertrophic | − |
| Hepatomegaly | NS | + | + | NS | + | − |
| Hypalbuminaemia | NS | NS | + | NS | + | + |
| Thrombocytopenia | NS | NS | +* | + | + | + |
| Anaemia | NS | NS | −* | + | + | +/− |
| Hyperferritinaemia | NS | NS | NS | + | + | + |
| Cerebellar atrophy | − (autopsy) | − (autopsy) | + (autopsy) | + (autopsy) | − (autopsy) | + (MRI) |
| Death | 2 days | 2 weeks | 1 month | 8 weeks | 7 days | 2 months |
| Mutations | CDG‐Ik | CDG‐Ik | T237R | F157S | c.160_161insG | F157S |
| V231M | F119L | F119L | F119L | |||
F, female; M, male; NS, not specified; +, present; −, absent.
*Information from personal communication (see Acknowledgements).
Hydrops fetalis can result from six mechanisms: (1) primary myocardial failure; (2) high output cardiac failure; (3) decreased plasma oncotic pressure; (4) increased capillary permeability; (5) obstruction of venous return and (6) obstruction of lymphatic flow.19 The hypalbuminaemia present in both our patients, and the cardiomyopathy present in patient 1 and in three patients from the literature, may be the main mechanisms in CDG‐I.
Persistent thrombocytopenia was present in both our patients and also in two hydrops patients from the literature.13,14 Remarkable in one reported patient14 were the highly increased ferritin levels, which had not been reported before in patients with CDG. We therefore measured, retrospectively, the ferritin levels in our patients and found them to be highly elevated too. The thrombocytopenia and high ferritin levels might be common features in this severe form of CDG‐Ia and provide an additional clue for the diagnosis of CDG‐I in hydrops fetalis. The cause of the hyperferritinaemia is unknown. Ferritin is secreted from the liver and lymphoid cells, and some of the ferritin is N‐glycosylated.20 It is speculative whether underglycosylation causes an enhanced secretion. Noelle et al14 describe macrophage activation in the post‐mortem bone marrow cytology in their patient and suggest this as a possible source of the hyperferritinaemia.
Since hydrops fetalis can be considered to be the most severe presentation of CDG‐Ia, the presence of severe mutations might be expected. However, homozygosity or compound heterozygosity for truncating mutations or the common severe R141H mutation, which virtually inactivates the enzyme21,22 have never been observed and a residual PMM activity seems to be required for survival.2,21,22 In fact, both our patients had the common F119L mutation, which is a relatively mild mutation with a residual activity of 25%.21,22 Patient 1 had in addition a frameshift insertion leading to a premature stopcodon, which can be expected to have no residual activity. Patient 2 had in addition the F157S mutation. This is considered to be a severe mutation, as it has never been found in combination with the common severe R141H mutation,2 and in combination with the D65Y mutation, is associated with a severe phenotype resulting in early death.2,23 The same combination of mutations (F119L/F157S) was found in one of the two other patients with CDG‐Ia with hydrops reported in the literature.14 The other reported patient13 had the T237R/V231M genotype. T237R is considered a severe mutation with virtually no residual activity22 although it has been found in combination with the common severe R141H mutation.24 The V231M has a significant residual activity of 38.5% but is extremely thermolabile,2,21 and in combination with the R141H mutation or in homozygotic form it has been associated with a severe phenotype with a high mortality rate.2,25,26 As all known patients with CDG‐Ia and hydrops fetalis had one severe mutation with probably no residual enzymatic activity and one milder mutation with some residual activity, the presence of one severe mutation may be required for the development of hydrops fetalis. Yet the R141H/F119L genotype, a combination of one severe and one milder mutation, is common in Northern Europe affecting 50% of patients with CDG‐Ia in the Netherlands,2 and hydrops fetalis has not yet been reported in patients with this genotype, although it has been suggested that these patients probably represent the severe end of the clinical spectrum.27 Thus, although one severe mutation might be required for a presentation with hydrops fetalis, this is clearly not sufficient to explain the severe presentation, and additional unknown factors must play a role. The frequency of CDG‐Ia may be as high as 1/20 000 and probably less than half of these patients are being diagnosed.2 It is probable that patients with CDG‐Ia presenting with hydrops fetalis have been missed. CDG‐Ia should be considered in the differential diagnosis of hydrops fetalis as the diagnosis involves a high recurrence risk with the possibility of prenatal diagnosis in future pregnancies of the parents. Investigation for lysosomal storage diseases has been advised in all cases of unexplained hydrops fetalis.16,17 We advise that testing for CDG is also considered in these cases. CDG‐Ia can be diagnosed by analysis of PMM activity in chorionic villi or amniocytes.28,29 Enzymatic measurements have to be interpreted with caution as low values have been obtained in poorly growing amniocytes with a normal genotype and patients with CDG can have intermediate PMM values. Mutation analysis is therefore the preferred method of prenatal diagnosis in families with a previous child with CDG‐Ia.29 In cases of unexplained hydrops fetalis, additional DNA analysis might be useful when the PMM activities are inconsistent and/or there is a high index of suspicion. These tests of course fail to diagnose other subtypes of CDG‐I that might also be associated with hydrops such as CDG‐Ik. When a fetal blood sample is available, sialotransferrin isoelectric focusing should be considered. This has been regarded as an unreliable test, as negative results have been found in fetuses with CDG‐Ia.30,31 Yet the isoelectric focusing of sialotransferrin was already abnormal in a blood sample at day 2 in both our patients who were born at 32 and 35 weeks, respectively (although the pattern became more abnormal over time in the second patient). It is undecided whether these 2‐day postnatal samples of prematurely born patients can be considered comparable to prenatal samples taken at these gestations. Interestingly, a clearly abnormal sialotransferrin pattern was found in a fetal blood sample at 27 weeks in the patient with hydrops described in the abstract of Fietz et al18 This suggests that the sialotransferrin pattern can indeed be already abnormal in fetal blood samples in patients with CDG‐I who present prenatally with hydrops fetalis. This test can therefore prove useful in the prenatal diagnosis of CDG‐I in patients with unexplained hydrops fetalis, although a negative result remains inconclusive.
Key Points:
Report on two patients with congenital disorders of glycosylation type Ia (CDG‐Ia) and hydrops fetalis.
Congenital thrombocytopenia and high ferritin levels might be associated features in patients with CDG‐Ia and hydrops fetalis.
CDG‐I should be considered in the differential diagnosis of unexplained hydrops fetalis.
Acknowledgements
We thank Professor Dr R Hennekam and Dr T de Koning for additional information on previously published cases. We also thank Professor Dr MSvan der Knaap for the assessment of the MRIs of patient 2.
Abbreviations
CDG - congenital disorders of glycosylation
PMM - phosphomannomutase
Footnotes
Competing interests: None declared.
References
- 1.Matthijs G, Schollen E, Pardon E, Veiga‐Da‐Cunha M, Jaeken J, Cassiman J J, Van Schaftingen E. Mutations in PMM2, a phosphomannomutase gene on chromosome 16p13, in carbohydrate‐deficient glycoprotein type I syndrome (Jaeken syndrome). Nat Genet 19971688–92. [DOI] [PubMed] [Google Scholar]
- 2.Matthijs G, Schollen E, Bjursell C, Erlandson A, Freeze H, Imtiaz F, Kjaergaard S, Martinsson T, Schwartz M, Seta N, Vuillaumier‐Barrot S, Westphal V, Winchester B. Mutations in PMM2 that cause congenital disorders of glycosylation, type Ia (CDG‐Ia). Hum Mutat 200016386–394. [DOI] [PubMed] [Google Scholar]
- 3.De Lonlay P, Seta N, Barrot S, Chabrol B, Drouin V, Gabriel B M, Journel H, Kretz M, Laurent J, Le Merrer M, Leroy A, Pedespan D, Sarda P, Villeneuve N, Schmitz J, van Schaftingen E, Matthijs G, Jaeken J, Korner C, Munnich A, Saudubray J M, Cormier‐Daire V. A broad spectrum of clinical presentations in congenital disorders of glycosylation I: a series of 26 cases. J Med Genet 20013814–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marquardt T, Denecke J. Congenital disorders of glycosylation: review of their molecular bases, clinical presentations and specific therapies. Eur J Pediatr 2003162359–379. [DOI] [PubMed] [Google Scholar]
- 5.Jaeken J, Carchon H. The carbohydrate‐deficient glycoprotein syndromes: an overview. J Inherit Metab Dis 199316813–820. [DOI] [PubMed] [Google Scholar]
- 6.Kristiansson B, Stibler H, Conradi N, Eriksson B O, Ryd W. The heart and pericardial effusions in CDGS‐I (carbohydrate‐deficient glycoprotein syndrome type I). J Inherit Metab Dis 199821112–124. [DOI] [PubMed] [Google Scholar]
- 7.Clayton P T, Winchester B G, Keir G. Hypertrophic obstructive cardiomyopathy in a neonate with the carbohydrate‐deficient glycoprotein syndrome. J Inherit Metab Dis 199215857–861. [DOI] [PubMed] [Google Scholar]
- 8.Marquardt T, Hülskamp G, Gehrmann J, Debus V, Harms E, Kehl H G. Severe transient myocardial ischemia caused by hypertrophic cardiomyopathy in a patient with congenital disorder of glycosylation type Ia. Eur J Pediatr 2002161524–527. [DOI] [PubMed] [Google Scholar]
- 9.Gehrmann J, Sohlbach K, Linnebank M, Bohles H J, Buderus S, Kehl H G, Vogt J, Harms E, Marquardt T. Cardiomyopathy in congenital disorders of glycosylation. Cardiol Young 200313345–351. [PubMed] [Google Scholar]
- 10.Garcia Silva M T, De Castro J, Stibler H, Simon R, Chasco Yrigoyen A, Mateos F, Ferrer I, Madero S, Velasco J M, Guttierrez‐Larraya F. Prenatal hypertrophic cardiomyopathy and pericardial effusion in carbohydrate‐deficient glycoprotein syndrome. J Inherit Metab Dis 199619257–259. [DOI] [PubMed] [Google Scholar]
- 11.De Koning T J, Toet M, Dorland L, de Vries L S, van den Berg I E, Duran M, Poll‐The B T. Recurrent nonimmune hydrops fetalis associated with carbohydrate‐deficient glycoprotein syndrome. J Inherit Metab Dis 199821681–682. [DOI] [PubMed] [Google Scholar]
- 12.Schwarz M, Thiel C, Lübbehusen J, Dorland B, de Koning T, von Figura K, Lehle L, Korner C. Deficiency of GDP‐Man:GlcNAc2‐PP‐Dolichol Mannosyltransferase causes congenital disorder of glycosylation type Ik. Am J Hum Genet 200474472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aronica E, van Kempen A A, van der Heide M, Poll‐The B T, van Slooten H J, Troost D, Rozemuller‐Kwakkel J M. Congenital disorder of glycosylation type Ia: a clinicopathological report of a newborn with cerebellar pathology. Acta Neuropathol 2005109433–442. [DOI] [PubMed] [Google Scholar]
- 14.Noelle V, Knuepfer M, Pulzer F, Schuster V, Siekmeyer W, Matthijs G, Vogtmann C. Unusual presentation of congenital disorder of glycosylation type Ia: congenital persistent thrombocytopenia, hypertrophic cardiomyopathy and hydrops‐like aspect due to marked peripheral oedema. Eur J Pediatr 2005164223–226. [DOI] [PubMed] [Google Scholar]
- 15.Grünewald S, de Vos R, Jaeken J. Abnormal lysosomal inclusions in liver hepatocytes but not in fibroblasts in congenital disorders of glycosylation (CDG). J Inherit Metab Dis 20032649–54. [DOI] [PubMed] [Google Scholar]
- 16.Kattner E, Schäfer A, Harzer K. Hydrops fetalis: manifestation in lysosomal storage diseases including Farber disease. Eur J Pediatr 1997156291–295. [DOI] [PubMed] [Google Scholar]
- 17.Burin M G, Scholz A P, Gus R, Sanseverino M T, Fritsh A, Magalhaes J A, Timm F, Barrios P, Chesky M, Coelho J C, Giugliani R. Investigation of lysosomal storage diseases in nonimmune hydrops fetalis. Prenat Diagn 200424653–657. [DOI] [PubMed] [Google Scholar]
- 18.Fietz M J, Fong B, Nicholls C, Edwards M, McKenzie F, Spilsbury J. Diagnosis of a fetus affected by CDG‐Ia using transferrin isoform analysis of fetal blood [abstract]. J Inherit Metab Dis 200528(suppl 1)P393 [Google Scholar]
- 19.Im S S, Rizos N, Joutsi P, Shime J, Benzie R J. Nonimmunologic hydrops fetalis. Am J Obstet Gynecol 1992148566–569. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh S, Hevi S, Chuck S L. Regulated secretion of glycosylated human ferritin from hepatocytes. Blood 20041032369–2376. [DOI] [PubMed] [Google Scholar]
- 21.Pirard M, Matthijs G, Heykants L, Schollen E, Grunewald S, Jaeken J, van Schaftingen E. Effect of mutations found in carbohydrate‐deficient glycoprotein syndrome type IA on the activity of phosphomannomutase 2. FEBS Lett 1999452319–322. [DOI] [PubMed] [Google Scholar]
- 22.Kjaergaard S, Skovby F, Schwartz M. Carbohydrate‐deficient glycoprotein syndrome type IA: expression and characterisation of wild type and mutant PMM2 in E.coli. Eur J Hum Genet 19997884–888. [DOI] [PubMed] [Google Scholar]
- 23.Briones P, Vilaseca A, Schollen E, Ferrer I, Maties M, Busquets C, Artuch R, Gort L, Marco M, van Schaftingen E, Matthijs G, Jaeken J, Chabas A. Biochemical and molecular studies in 26 Spanish patients with congenital disorder of glycosylation type Ia. J Inherit Metab Dis 200225635–646. [DOI] [PubMed] [Google Scholar]
- 24.Le Bizec C, Vuillaumier‐Barrot S, Barnier A, Dupre T, Durand G, Seta N. A new insight into PMM2 mutations in the French population. Hum Mutat. 2005; mutation in brief #807 online [DOI] [PubMed]
- 25.Imtiaz F, Worthington V, Champion M, Beesley C, Charlwood J, Clayton P, Keir G, Mian N, Winchester B. Genotypes and phenotypes of patients in the UK with carbohydrate‐deficient glycoprotein syndrome type 1. J Inherit Metab Dis 200023162–174. [DOI] [PubMed] [Google Scholar]
- 26.Erlandson A, Bjursell C, Stibler H, Kristiansson B, Wahlstrom J, Martinsson T. Scandinavian CDG‐Ia patients: genotype/phenotype correlation and geographic origin of founder mutations. Hum Genet 2001108359–367. [DOI] [PubMed] [Google Scholar]
- 27.Kjaergaard S, Schwartz M, Skovby F. Congenital disorder of glycosylation type Ia (CDG‐Ia): phenotypic spectrum of the R141H/F119L genotype. Arch Dis Child 200185236–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charlwood J, Clayton P, Keir G, Mian N, Young E, Winchester B. Prenatal diagnosis of the carbohydrate‐deficient glycoprotein syndrome type 1a (CDG1a) by a combination of enzymology and genetic linkage analysis after amniocentesis or chorionic villus sampling. Prenat Diagn 199818693–699. [PubMed] [Google Scholar]
- 29.Matthijs G, Schollen E, van Schaftingen E. The prenatal diagnosis of congenital disorders of glycosylation (CDG). Prenat Diagn 200424114–116. [DOI] [PubMed] [Google Scholar]
- 30.Clayton P, Winchester B, Di Tomaso E, Young E, Keir G, Rodeck C. Carbohydrate‐deficient glycoprotein syndrome: normal glycosylation in the fetus. Lancet 1993341956. [DOI] [PubMed] [Google Scholar]
- 31.Stibler H, Skovby F. Failure to diagnose carbohydrate‐deficient glycoprotein syndrome prenatally. Pediatr Neurol 19941171. [DOI] [PubMed] [Google Scholar]