Abstract
Recently, several reports have been published that showed a higher incidence of assisted reproductive technologies (ART) in patients with Beckwith–Wiedemann syndrome compared with the general population, and in most of these patients, aberrant methylation imprints of KvDMR1 have been found. This has led to the concern that ART might increase the incidence of imprinting syndromes such as Beckwith–Wiedemann syndrome. Not much is known on environmental or genetic factors that may interfere with the processes of imprint maintenance or resetting. A methylation analysis of KvDMR1 was performed in human oocytes at different stages of nuclear maturity and in sperm cells. The results indicate that the maternal methylation imprints were already established at the germinal vesicle stage, whereas all sperm cells were unmethylated, thereby showing that the KvDMR1 carries a germline methylation imprint. For one of the oocytes analysed, an unmethylated pattern was found, which highlights the need for further molecular studies that consider the safety of ART.
A small subset of genes, the so called “imprinted genes”, escape the classic bi‐allelic expression, and are predominantly or exclusively expressed from one parental allele.1 Monoallelic expression relies on epigenetic mechanisms, and DNA methylation of CpG dinucleotides at differentially methylated regions (DMRs) is the best‐studied epigenetic mark so far. The epigenetic marks or “imprints” are reset with every reproductive cycle. Imprint resetting involves erasure in the primordial germ cells and the acquisition of new sex‐specific imprints during later stages of germ cell development. The imprints of the gametes are maintained stable in the early embryo despite overall epigenetic reprogramming.2 Deregulation of imprinted genes can lead to several imprinting syndromes, including Angelman syndrome (OMIM 105830), Prader–Willi syndrome (OMIM 176270) and Beckwith–Wiedemann syndrome (BWS; OMIM 130650).3 BWS is a rare (1/13000) syndrome characterised by prenatal and postnatal overgrowth, macroglossia, abdominal wall defects and a predisposition to embryonic tumours. BWS is associated with genetic and epigenetic changes in an imprinting cluster of about 1 Mb on chromosome 11p15. This cluster includes two imprinted domains, each controlled by an imprinting control region (ICR), which is differentially methylated.4 The more telomeric domain contains two reciprocally imprinted genes, insulin‐like growth factor 2 (IGF2) and H19, which are regulated by the paternally methylated ICR1 (H19 DMR).5,6 The second, more centromeric domain, contains the maternally expressed potassium voltage gated channel subfamily Q member 1 gene (KCNQ1), the paternally expressed KCNQ1 overlapping transcript KCNQ1OT1 (also called LIT1) and several other maternally expressed genes, including the cyclin‐dependent kinase inhibitor 1C (CDKN1C). The ICR2 of this domain is located in a CpG island in intron 10 of the KCNQ1 gene and contains the KvDMR1, which is maternally methylated in somatic tissues.7,8,9
Key points
We performed a methylation analysis of the human imprinted KvDMR1 in oocytes at different stages of nuclear maturity (germinal vesicles, metaphase I and metaphase II oocytes).
An overall methylated pattern was found in 15 of 16 oocytes analysed. However, one oocyte had an unmethylated pattern. We also verified the methylation status of sperm cells, and an overall unmethylated pattern was detected.
We have shown that the human KvDMR1 is a germline DMR, for which the maternal imprints are already established at the germinal vesicle stage.
Recently, some papers showed a higher incidence of assisted reproductive technologies (ART) in patients with BWS compared with the general population.10,11,12,13,14,15 Molecular analysis showed that most cases were due to epigenetic rather than genetic changes. In all but one patient with BWS born after ART, loss of maternal methylation at KvDMR1 was observed, whereas imprinting mutations at KvDMR1 occur usually only in 50% of patients with BWS. It has been hypothesised that in vitro culture systems or hormonal stimulation of the ovaries during ART may cause these epigenetic defects by interfering with maternal imprint establishment during gametogenesis and/or with imprint maintenance during the pre‐implantation period.12,14,16 Another hypothesis is that the epigenetic defects after ART are related to the fertility problems of the couples.14,17 To clarify the timing of imprint acquisition of the human KvDMR1, we analysed the methylation pattern in oocytes at different stages of nuclear maturity using a bisulphite sequencing technique.
Materials and methods
Samples
Genomic DNA was directly isolated from human blood samples using a QIAmp Blood Maxikit (Qiagen Benelux BL, Venlo, The Netherlands). The oocytes were donated for research by patients of the Centre for Reproductive Medicine after they had given informed consent, and with the approval of the institutional ethics committee. The collected research oocytes were immature, at the germinal vesicle or metaphase I stage on the day of oocyte retrieval, or they spontaneously matured in vitro to the metaphase II stage, having been left overnight in the incubator. All the oocytes were denuded of their surrounding cumulus and corona cells using a combination of mechanical (pipetting) and enzymatic (40 IU/ml hyaluronidase; Sigma Chemical Company, St Louis, Missouri, USA) methods. Special attention was paid to the complete removal of the zona pellucida using an acidic tyrode solution. Removing the first polar body so that only the epialleles of the mature metaphase II oocyte could be studied was not always successful. Oocyte numbers 4, 5 and 12 were obtained from the same intracytoplasmic sperm injection (ICSI)/in vitro fertilisation cycle, as were oocyte numbers 6 and 11. Several independent experiments of single oocytes were performed for each of the three stages, except for the metaphase I oocytes, where on one occasion a pool of two oocytes was used (number 7). Semen samples with normal semen parameters were obtained from male donors at our fertility centre.
Bisulphite sequencing technique
The methylation patterns were studied by a bisulphite sequencing protocol based on the chemical conversion of unmethylated cytosines to uracil by the bisulphite, whereas the methylated cytosines remained unaltered. After polymerase chain reaction (PCR) amplification of the bisulphite‐converted DNA, the PCR products were directly sequenced or first cloned and then sequenced. For bisulphite conversion of the single cells, a bisulphite protocol based on low melting point agarose beads was used.18 A specific heminested PCR protocol was developed for the amplification of 12 CpG sites (10 CpGs that were also studied by Cerrato et al19 and 2 CpGs directly upstream) of the human KvDMR1 on the upper strand of the bisulphite‐converted DNA. In the first round of PCR, the forward primer LITBisF (5′‐GGG GGT TTT TTA GTA TGG TTT TTT TT‐3′ nucleotide position 67934–67959 in GenBank accession number U90095) labelled with 5′‐indocarbocyanin was used, together with the reverse primer LITRV (5′‐CAC TAC CCA AAC CAA ACT ACA CTA C‐3′; 68202–68225). In the second round of PCR, the reverse primer LITRV was combined with the 5′‐indocarbocyanin‐labelled forward primer LITFI (5′‐GGT TTT TTT TAT TTT TTT GGG AGG GTT TG‐3′ 68070–68098). A PCR mix containing 0.4 µM of each primer, 0.2 mM dNTPs (Amersham Pharmacia Biotech, Roosendaal, The Netherlands), 1× PCR buffer (Applied Biosystems, Nieuwerkerk a/d IJsel, The Netherlands), 2 mM MgCl2 (Applied Biosystems), 1.25 U AmpliTaq DNA Polymerase (Applied Biosystems) in a total volume of 25 µl was used. The following PCR programme was used for genomic DNA: a denaturation step of 5 min at 94°C followed by 20 cycles of 30 s at 94°C, 30 s at 68°C and 30 s at 72°C, and a final extension for 5 min at 72°C. A volume of 3 µl of the first round was used as DNA input for amplification in the second round of PCR with the following programme: 5 min of denaturation at 94°C followed by 25 cycles of 30 s at 94°C, 30 s at 63°C and 30 s at 72°C, and a final extension step for 5 min at 72°C. The PCR programme for single or a few germ cells has an additional five cycles in the first round and ten cycles in the second round. The PCR fragments were analysed on an ALFExpress automated sequencer (Amersham Pharmacia Biotech), and positive samples were directly sequenced or first cloned using a TOPO‐TA cloning kit (Invitrogen, Merelbeke, Belgium) and subsequently sequenced on the ABI 3130 XL (Applied Biosystems). Our bisulphite sequencing protocol was first essayed on genomic DNA directly isolated from human blood samples by analysis of 102 clones. Blanks were included in each bisulphite sequencing experiment, and none of them showed amplification.
Results
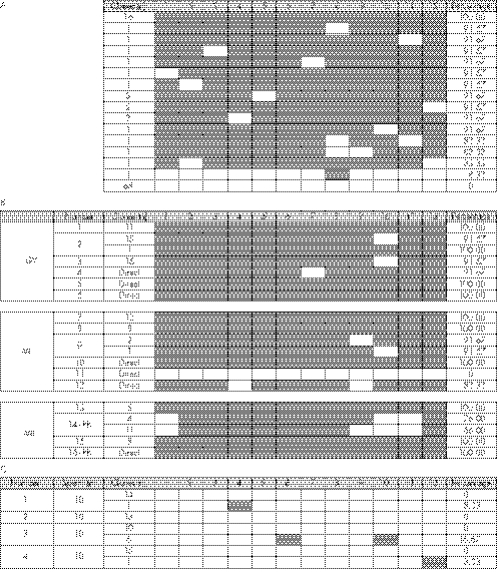
The bisulphite sequencing protocol was optimised for the analysis of single germ cells. In this way, we avoided the pooling of research oocytes from different ART cycles, which would involve loss of patient‐specific information. Validation of the protocol on genomic DNA gave an average methylation pattern of 30.8% (377 methylated CpG sites/1223 analysed CpGs) after several independent bisulphite sequencing experiments. Thirty three of the clones had a methylated pattern and 69 had an unmethylated pattern (fig 1A).
Figure 1 Methylation analysis of the KvDMR1 in (A) genomic DNA isolated from blood, (B) oocytes and (C) sperm cells. The 12 CpG dinucleotides analysed are given in the top row of the graph (1–12). Samples were directly sequenced (direct) after polymerase chain reaction or cloned before sequencing. The total number of clones analysed is mentioned, and clones with the same pattern are grouped. White boxes, unmethylated CpGs; black boxes, methylated CpGs; GV, germinal vesicle; MI, metaphase I; MII, metaphase II.
Oocytes at different stages of nuclear maturity (germinal vesicles stages, metaphase I and metaphase II stage oocytes) were analysed for their methylation status at the KvDMR1. The amplification efficiency of the specifically developed single‐cell protocol was 25% (16 of 64 oocytes amplified). An overall methylated pattern was found in 15 of 16 oocytes analysed (fig 1B). However, an aberrant unmethylated pattern was detected in one of the metaphase I oocytes (number 11).
To verify whether paternal unmethylated patterns were present in mature male germ cells, a methylation analysis was carried out on four pools of ten sperm cells from ejaculates with normal semen parameters. An unmethylated pattern was found in all the sperm samples analysed (fig 1C).
Discussion
In this work, we studied the methylation status of the human KvDMR1 in oocytes at different stages of nuclear maturity and in sperm cells using a bisulphite sequencing technique, which was optimised for analysis of single cells. The use of fresh oocytes from natural cycles was circumvented by using donated oocytes from ART cycles. Therefore, the influence of fertility problems or environmental factors inherent to the ART procedure cannot be excluded.
Validation of the bisulphite sequencing protocol on genomic DNA showed that about one third of the clones are methylated, the other two thirds being unmethylated. These results are in agreement with the paper of Cerrato et al,19 where an overlapping region was studied, and 26–35% of the clones had methylated CpGs. Although similar results were obtained after bisulphite sequencing with distinct PCRs, a more balanced ratio would have been expected and it cannot be ruled out that preferential amplification of the unmethylated molecules occurred during PCR.
Our results of an overall methylated pattern at the KvDMR1 in 15 of the 16 oocyte samples analysed indicate that the maternal methylation imprints have already been established at the germinal vesicle stage. The finding of opposite methylation patterns at the KvDMR1 in male and female gametes indicates that this methylation imprint is a germline imprint. However, one oocyte failed to complete the cycle of imprint resetting. This unmethylated metaphase I oocyte (number 11) and the completely methylated germinal vesicle (number 6) were notably from the same ICSI cycle, the fifth cycle for a couple with male factor infertility. Interestingly, in this ICSI cycle, several immature oocytes (four in the germinal vesicle and two in the metaphase I stages) were retrieved and only four mature metaphase II oocytes were suitable for ICSI. Two embryos were transferred to the 36‐year‐old woman, but no pregnancy followed. A high number of dysmorph, immature oocytes was consistently found in all previous cycles; further details were not available.
Disruptions at several stages in the imprinting cycle could have caused the unmethylated status of the KvDMR1 in this metaphase I oocyte. Firstly, the imprint acquisition could have failed because of a developmental delay in this oocyte that prevented imprint establishment at the right time. Another possibility is that the ovarian hormonal stimulation inherent to the infertility treatment could have interfered with the imprint acquisition.14 Also, imprint maintenance may be disturbed owing to influences of hormonal stimulation or in vitro culture. In mice, in vitro culture systems have been shown to alter the methylation pattern of imprinted genes in the embryo.20,21 In vitro maturation of human oocytes also altered the methylation pattern of the imprinted H19 gene22; however, this is less probable as this metaphase I oocyte was not matured in vitro, but was just kept in culture between the time it was picked up and the time it was used. In light of the reports of patients with BWS born after ART, certain aspects of this procedure (eg hormonal stimulation14 or in vitro culture system20,21,22) or the couple's fertility problem17 itself may have interfered with the process of imprint establishment, but no conclusions can be drawn as it was not possible to analyse oocytes from natural cycles. Fertilisation of an oocyte unmethylated at KvDMR1 through ART may lead to a BWS syndrome patient.7,8
In summary, we showed that KvDMR1 is a germline differentially methlylated region for which the maternal imprints are already established at the germinal vesicle stage. However, one oocyte had an aberrant methylation pattern.
In light of the recent findings about an association between ART and imprinting syndromes, more molecular studies are needed to consider the safety of ART procedures.
Acknowledgements
We thank the staff of the Centre for Reproductive Medicine for providing the research material. We also thank Professor Dr K Sermon for critically reading this manuscript and M Whitburn, Language Education Centre, for proofreading the manuscript.
Abbreviations
ART - assisted reproductive technologies
BWS - Beckwith–Wiedeman syndrome
DMR - differentially methylated region
ICR - imprinting control region
ICSI - intracytoplasmic sperm injection
KCNQ1 - potassium voltage‐gated channel subfamily Q, member 1
PCR - polymerase chain reaction
Footnotes
Funding: This work was supported by the Fund for Scientific Research, Flanders, Belgium, and the University Research Council.
Competing interests: None.
References
- 1.Da Rocha S T, Ferguson‐Smith A C. Genomic imprinting. Curr Biol 200414646–649. [DOI] [PubMed] [Google Scholar]
- 2.Morgan H D, Santos F, Green K, Dean W, Reik W. Epigenetic reprogramming in mammals. Hum Mol Genet 200514R47–R58. [DOI] [PubMed] [Google Scholar]
- 3.Robertson K D. DNA methylation and human disease. Nat Rev Genet 20056597–610. [DOI] [PubMed] [Google Scholar]
- 4.Weksberg R, Shuman C, Smith A C. Beckwith‐Wiedemann syndrome. Am J Med Genet C Semin Med Genet 200513712–23. [DOI] [PubMed] [Google Scholar]
- 5.Bell A C, Felsenfeld G. Methylation of a CTCF‐dependent boundary controls imprinted expression of the Igf2 gene. Nature 2000405482–485. [DOI] [PubMed] [Google Scholar]
- 6.Hark A T, Schoenherr C J, Katz D J, Ingram R S, Levorse J M, Tilghman S M. CTCF mediates methylation‐sensitive enhancer‐blocking activity at the H19/Igf2 locus. Nature 2000405486–489. [DOI] [PubMed] [Google Scholar]
- 7.Smilinich N J, Day C D, Fitzpatrick G V, Caldwell G M, Lossie A C, Cooper P R, Smallwood A C, Joyce J A, Schofield P N, Reik W, Nicholls R D, Weksberg R, Driscoll D J, Maher E R, Shows T B, Higgins M J. A maternally methylated CpG island in KvLQT1 is associated with an antisense paternal transcript and loss of imprinting in Beckwith‐Wiedemann syndrome. Proc Natl Acad Sci USA 1999968064–8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee M P, DeBaun M R, Mitsuya K, Galonek H L, Brandenburg S, Oshimura M, Feinberg A P. Loss of imprinting of a paternally expressed transcript, with antisense orientation to KVLQT1, occurs frequently in Beckwith‐Wiedemann syndrome and is independent of insulin‐like growth factor II imprinting. Proc Natl Acad Sci USA 1999965203–5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitzpatrick G V, Soloway P D, Higgins M J. Regional loss of imprinting and growth deficiency in mice with a targeted deletion of KvDMR1. Nat Genet 200232426–431. [DOI] [PubMed] [Google Scholar]
- 10.DeBaun M R, Niemitz E L, Feinberg A P. Association of in vitro fertilization with Beckwith‐Wiedemann syndrome and epigenetic alterations of LIT1 and H19. Am J Hum Genet 200372156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gicquel C, Gaston V, Mandelbaum J, Siffroi J P, Flahault A, Le Bouc Y. In vitro fertilization may increase the risk of Beckwith‐Wiedemann syndrome related to the abnormal imprinting of the KCN1OT gene. Am J Hum Genet 2003721338–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maher ER Imprinting and assisted reproductive technology Hum Mol Genet. 2005;14:R133–R138. doi: 10.1093/hmg/ddi107. [DOI] [PubMed] [Google Scholar]
- 13.Halliday J, Oke K, Breheny S, Algar E, Amor D J. Beckwith‐Wiedemann syndrome and IVF: a case‐control study. Am J Hum Genet 200475526–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang A S, Moley K H, Wangler M, Feinberg A P, DeBand M R. Association between Beckwith‐Wiedemann syndrome and assisted reproductive technology: a case series of 19 patients. Fertil Steril 200583349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutcliffe A G, Peters C J, Bowdin S, Temple K, Reardon W, Wilson L, Clayton‐Smith J, Brueton L A, Bannister W, Maher E R. Assisted reproductive therapies and imprinting disorders—a preliminary British survey. Hum Reprod 2006211009–1011. [DOI] [PubMed] [Google Scholar]
- 16.De Rycke M, Liebaers I, Van Steirteghem A. Epigenetic risks related to assisted reproductive technologies: risk analysis and epigenetic inheritance. Hum Reprod 2002172487–2494. [DOI] [PubMed] [Google Scholar]
- 17.Ludwig M, Katalinic A, Gross S, Sutcliffe A, Varon R, Horsthemke B. Increased prevalence of imprinting defects in patients with Angelman syndrome born to subfertile couples. J Med Genet 200542289–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geuns E, De Rycke M, Van Steirteghem A, Liebaers I. Methylation imprints of the imprint control region of the SNRPN‐gene in human gametes and preimplantation embryos. Hum Mol Genet 2003122873–2879. [DOI] [PubMed] [Google Scholar]
- 19.Cerrato F, Vernucci M, Pedone P V, Chiariotti L, Sebastio G, Bruni C B, Riccio A. The 5' end of the KCNQ1OT1 gene is hypomethylated in the Beckwith‐Wiedemann syndrome. Hum Genet 2002111105–107. [DOI] [PubMed] [Google Scholar]
- 20.Doherty A S, Mann M R, Tremblay K D, Bartolomei M S, Schultz M R. Differential effects of culture on imprinted H19 expression in the preimplantation mouse embryo. Biol Reprod 2000621526–1535. [DOI] [PubMed] [Google Scholar]
- 21.Khosla S, Dean W, Brown D, Reik W, Feil R. Culture of preimplantation mouse embryos affects fetal development and the expression of imprinted genes. Biol Reprod 200164918–926. [DOI] [PubMed] [Google Scholar]
- 22.Borghol N, Lornage J, Blachere T, Sophie Garret A, Lefevre A. Epigenetic status of the H19 locus in human oocytes following in vitro maturation. Genomics 200687417–426. [DOI] [PubMed] [Google Scholar]