Abstract
Background
Mendelian susceptibility to mycobacterial disease (MSMD) is associated with infection caused by weakly virulent mycobacteria in otherwise healthy people. Causal germline mutations in five autosomal genes (IFNGR1, IFNGR2, STAT1, IL12RB1, IL12B) and one X‐linked (NEMO) gene have been described. The gene products are physiologically related, as they are involved in interleukin 12/23‐dependent, interferon γ‐mediated immunity. However, no genetic aetiology has yet been identified for about half the patients with MSMD.
Methods
A large kindred was studied, including four male maternal relatives with recurrent mycobacterial disease, suggesting X‐linked recessive inheritance. Three patients had recurrent disease caused by the bacille Calmette–Guérin vaccine, and the fourth had recurrent tuberculosis. The infections showed tropism for the peripheral lymph nodes.
Results
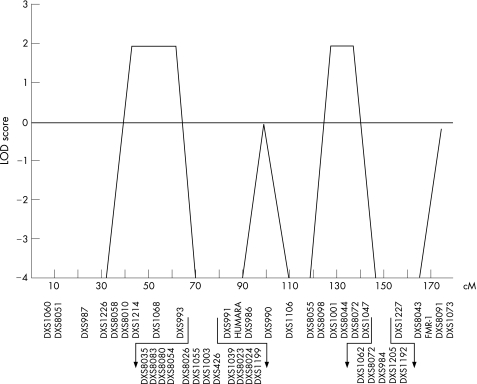
Known autosomal and X‐linked genetic aetiologies of MSMD were excluded through genetic and immunological investigations. Genetic linkage analysis of the X‐chromosome identified two candidate regions, on Xp11.4–Xp21.2 and Xq25–Xq26.3, with a maximum LOD score of 2.
Conclusion
A new X‐linked recessive form of MSMD is reported, paving the way for the identification of a new MSMD‐causing gene.
Mendelian susceptibility to mycobacterial disease (MSMD, MIM 209950) is a rare syndrome1,2 involving predisposition to clinical disease caused by poorly virulent mycobacterial species, such as bacille Calmette–Guérin (BCG) vaccines3,4 and non‐tuberculous, environmental mycobacteria.5 The patients are also vulnerable to the more virulent Mycobacterium tuberculosis.6,7,8,9,10,11 Typically, patients are not particularly prone to other infections, except salmonellosis, which affects less than half the cases. MSMD is clinically heterogeneous, and outcome is correlated with the type of histological lesions present.12 It was initially believed that MSMD was inherited as an autosomal recessive trait as a rule,3,4,5 until X‐linked recessive inheritance patterns were reported in one multiplex kindred.13,14
Key points
Mendelian susceptibility to mycobacterial disease (MSMD) is characterised by clinical disorders caused by poorly virulent mycobacteria in otherwise healthy people.
Mutations in NEMO leucine zipper domain are associated with X‐linked recessive MSMD.
We have reported a novel form of X‐linked recessive‐MSMD.
Five disease‐causing autosomal genes (IFNGR1, IFNGR2, STAT1, IL12RB1 and IL12B) have been found.2,15IFNGR1 and IFNGR2 encode the interferon (IFN) γR1 and IFN γR2 chains of the receptor for IFN γ, a pleiotropic cytokine secreted by natural killer and T lymphocyte cells. STAT1 encodes signal transducer and activator of transcription 1 (Stat 1), an essential molecule in the IFN γR signalling pathway. IL12B encodes the p40 subunit of interleukin (IL) 12 and IL23, two cytokines secreted by macrophages and dendritic cells. Finally, IL12RB1 encodes the β1 chain shared by the receptors for IL12 and IL23, expressed in natural killer and T cells. Mutations in IFNGR1, IFNGR2 and STAT1 impair cellular responses to IFN γ, and mutations in IL12B and IL12RB1 impair the production of IFN γ. The five MSMD‐causing autosomal genes are thus immunologically related. A high degree of allelic heterogeneity at these five loci accounts for the existence of at least 12 known distinct genetic disorders including autosomal dominant IFNGR1 deficiency.2,15,16,17,18,19
Familial X‐linked recessive MSMD was clinically described in 1994.13,21 Four males in two generations of a non‐consanguineous family developed disseminated mycobaterial complex infection.20,21 The patients' monocytes showed impaired IL12 production on phytohaemagglutinin (PHA) activation, even though their T cells were intrinsically able to produce IFN γ on stimulation by control monocytes.13 Together with S M Holland, we recently identified the molecular genetic basis of XR‐MSMD in this American kindred and in two other unrelated families from France and Germany.14 Surprisingly, specific mutations affecting the leucine zipper domain (LZD) of nuclear factor‐κB essential modulator (NEMO)22,26 were found in the three kindreds. We describe here a large French kindred with a new X‐linked recessive form of MSMD (XR‐MSMD).
Case reports and family data
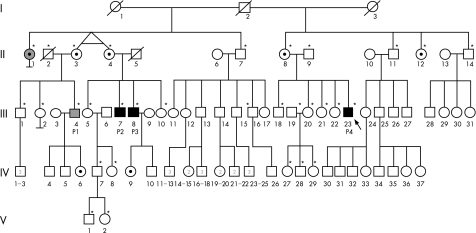
Figure 1 shows the pedigree. All members of the kindred live in France and are of French descent. Informed consent was obtained from all the family members (fig 1A).
Figure 1 A kindred with X‐linked recessive predisposition to mycobacterial diseases. (A) Pedigree of the family with X‐linked recessive‐Mendelian susceptibility to mycobacterial disease (XR‐MSMD‐2). Each generation is designated by a Roman numeral (I, II, III, IV, V), and each individual is represented by an Arabic numeral. Patients with bacille Calmette–Guérin (BCG) disease are represented by black symbols and patients with tuberculosis by grey symbols. The proband is indicated by an arrow. The seven obligate carriers are indicated. Individuals selected for the X‐chromosome scan are indicated by asterisks.
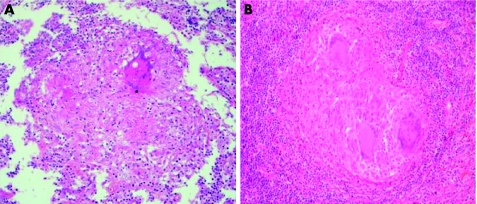
Patient 1 (P1, III‐4) was born in 1953 and was not vaccinated with BCG in infancy. He remained healthy until the age of 10 years, at which time he presented with symptomatic primary tuberculosis of the lungs, with a positive tuberculosis skin test (Mantoux skin test) indicating delayed‐type hypersensitivity to tuberculous purified protein derivative. He was treated with isoniazid for 12 months and recovered. At 34 years of age, he was hospitalised with fever, peripheral lymphoadenopathy, hepatomegaly, fatigue and anorexia. A computed tomography scan showed multiple mediastinal lymph nodes. He showed strong delayed‐type hypersensitivity to tuberculous purified protein derivative. Histological examination of a clavicular lymph node showed granulomas with eosinophil, necrosis with caseum, surrounded by rare epithelioid and giant cells and a lack of visible acid‐fast rods (fig 2A). Cultures of all tissue specimens and biological fluids (blood and urine) tested negative for mycobacteria. Isoniazid, rifampicin, ethambutol and pyrazinamide were given for 23 months and the patient recovered. No other unusual infections since then have been recorded. He is now 52 years of age and is well with no treatment.
Figure 2 Granulomas in lymph node. Biopsy specimens showing epithelioid and giant cells with caseous necrosis. Haematoxylin and eosin staining (×40). Specimens from P1 (A) and the proband (B).
Patient 2 (P2, III‐7) was born in 1950 and was inoculated with M bovis BCG at birth. Regional axillary adenitis developed in the next few months, resolving spontaneously with skin fistulisation. Twelve years later, local ulceration with fistulising lymphadenopathy occurred at the same site, and was treated by surgical excision. At 21 years of age, P2 developed ophthalmic zoster. At 29 years of age, he presented with a second episode of cervical lymph node enlargement. M bovis was cultured from lymph node aspirate. P2 was treated with a combination of isoniazid, rifampicin and ethionamide for 18 months, leading to full recovery. Since then he has had no other unusual diseases. He is now 55 years old and is well with no treatment.
Patient 3 (P3, III‐8) was born in 1955 and vaccinated with BCG at birth. He presented with regional axillary adenitis a few months later. He received no antibiotic treatment and the surgical excision of regional lymph nodes was required. The patient recovered, but no more detailed information is available. He presented with abdominal zoster infection at 17 years of age. At about the same age, he also presented an episode of self‐healing warts on the hands. Serological tests for mycobacteria were negative (<100 U anti‐A60 IgG/ml) when he was 45 years of age. He has had no other unusual infections and remained clinically well with no treatment until the last follow‐up visit at 50 years of age.
Patient 4 (P4, III‐23), born in 1974, is the proband. He was immunised with BCG at 2 years of age. He had regional axillary lymphoadenopathy with skin fistulisation and recovered after surgical excision. Multiple lymph node biopsies showed leucocyte infiltration, epithelioid cell granulomas and multinucleated giant cells. At 21 years of age, P4 developed an intestinal occlusion; surgery showed multiple enlarged abdominal lymph nodes causing volvulus, but none was excised. At 24 years of age, he developed left supraclavicular lymphoadenopathy. The lymph node was excised and the biopsy specimen showed a tuberculoid granuloma. The patient was treated with isoniazid, rifampicin and ethambutol for 12 months, with a partial response. Subcutaneous IFN γ treatment, at a dose of 100 μg three times per week, was initiated, but was stopped after 1 month because of side effects. No other unusual infections were documented. At 27 years of age, P4 presented with cervical adenitis, and surgical excision was performed. Histological examination showed a lymph node with conserved architecture, with well‐circumscribed granulomas containing epithelioid and giant cells surrounding small areas of central caseous necrosis, without visible acid‐fast rods (fig 2B). M bovis BCG was isolated by culture.
The patient had normal numbers of erythrocytes, platelets, monocytes, polymorphonuclear cells and lymphocytes (table 1). Lymphocyte and monocyte subsets were also normally distributed. No defect in proliferation was detected when peripheral blood mononuclear cells (PBMCs) were stimulated with mitogens (PHA, phorbol 12‐miristate 13‐acetate (PMA)–ionomycin, anti‐CD3 antibody), microorganisms (live BCG, heat‐killed Staphylococcus aureus), and antigens (purified protein derivative; data not shown). Serum IgG, A, M and E isotypes, and IgG subclasses were detected in normal amounts. Antibody responses to protein and carbohydrate vaccine antigens were normal. The reduction of nitroblue tetrazolium and chemiluminescence of polymorphonuclear cells were normal. The expression of CD154/CD40L on PMA–ionomycin‐stimulated T cell blasts was normal, as shown by flow cytometry with a CD40L‐specific antibody and a soluble CD40 molecule. Normal serum levels of complement components C3, C4 and normal CH50 activity were found. High‐resolution lymphocyte karyotype was normal. Serological tests for mycobacteria were highly positive (450 IU/l, IgG against A60). No serum antibodies against Salmonella and cytomegalovirus were detected, but IgG antibodies against viral capsid antigen, early antigen and Epstein–Barr virus (EBV) nuclear antigen, EBV antigens and varicella zoster virus antigens were detected. The patient is currently clinically well, with no treatment at 32 years of age.
Table 1 Immunological parameters of the proband (P4).
| Lymphocytes | 1340 | (1000–4000) |
| T cells (%) (normal values)* | ||
| CD3 | 71 | (66–79) |
| CD3/CD4 | 40 | (37–53) |
| CD3/CD8 | 29 | (19–34) |
| TCR αβ/CD3 | 68 | (83–97) |
| TCR γδ/CD3 | 3 | (2–15) |
| NK cells (normal values)* | ||
| CD3−/CD16+CD56+ | 7 | (10–19) |
| B cells (%) (normal values)* | ||
| CD19 | 17 | (11–16) |
| CD5+ | 33.8 | (14–32) |
| CD27− | 89.7 | (51–77) |
| CD27+ | 10.3 | (23–49) |
| Serum immunoglobulin concentration (g/l) (normal values)* | ||
| IgA | 4.31 | (0.70–3.12) |
| IgM | 1.27 | (0.56–3.52) |
| IgG | 16.10 | (6.39–13.4) |
| IgG subclasses | ||
| IgG1 | 8.48 | (4–10.98) |
| IgG2 | 6.85 | (1.23–5.49) |
| IgG3 | 0.345 | (0.211–1.142) |
| IgG4 | 0.186 | (0.084–0.888) |
| IgE (IU/l) | 58 | (<100) |
| C3 (g/l) | 0.94 | (0.75–1.50) |
| C4 (g/l) | 0.25 | (0.16–0.38) |
| NBT (%) | 90 | (>60%) |
| Chemoluminescence | 12.106 | (3–12.106 cpm) |
Ig, immunoglobulin; NBT, nitro blue tetrazolium; NK, natural killer; TCR, T cell receptor.
*Values in brackets are ranges.
Percentages (%) express the fraction of lymphocytes.
Family history
A maternal aunt of all four patients (II‐1), born in 1920, developed tuberculous salpingitis and pulmonary tuberculosis at 29 years of age. Histological examination of the endometrium showed epithelioid and giant cell granulomas. No information is available for mycobacterial culture results. This aunt was treated with streptomycin and isoniazid. A chest x ray taken 6 months after treatment showed lung excavation. She received continuous treatment (streptomycin and isoniazid) for 2 years and recovered, but remained sterile. She later developed intercostal zoster infection. She was not vaccinated with BCG and is currently well at 85 years of age. The mother of patients 2 and 3 (II‐4), born in 1922, had asymptomatic primary pulmonary tuberculosis in adolescence. She had not been vaccinated with BCG, and the previous primary tuberculous infection was diagnosed from a routine chest x ray in adulthood that disclosed calcified mediastinal lymph nodes. This subject has remained clinically well with no treatment. The patients' uncles (II‐7, II‐11, II‐14) and another maternal aunt (II‐12) are healthy, and only one uncle (II‐11) had received BCG vaccination; the mother of patient 4 was not vaccinated with BCG. Other members of the family were immunised with BCG with no adverse effects. None of the family members presented developmental signs of ectodermal dysplasia (EDA)—not even mild signs such as conical teeth or hypodontia. I‐2 was not vaccinated with BCG and did not have tuberculosis. Thus, three male maternal relatives (III‐7, III‐8 and III‐23) presented with MSMD (BCG disease), another male maternal relative (III‐4) had recurrent tuberculosis and one woman (II‐1), the sister of an obligate carrier, had developed a severe form of tuberculosis.
This pedigree strongly suggests that predisposition to mycobacterial disease was inherited as an X‐linked recessive trait with four affected males. The disease‐causing X‐linked gene was transmitted from I‐2, who was not vaccinated with BCG and did not have tuberculosis, possibly owing to a lack of exposure to M tuberculosis, to his five daughters, born to his two subsequent wives (I‐1 and I‐3). Three daughters (II‐3, II‐4, II‐8) transmitted the X‐linked MSMD‐causing gene to at least one affected boy (P1, P2, P3, P4). The other two daughters (II‐1 and II‐12) did not have children; one of them (II‐1) had severe tuberculosis, suggesting that the X‐linked causative allele may be dominant of low penetrance as the other six obligate carriers (II‐3, II‐4, II‐8, II‐12, IV‐6 and IV‐9) did not have mycobacterial disease. Alternatively, patient II‐1 may have had tuberculosis because of skewed inactivation of wild‐type X chromosome. Finally, tuberculosis may have been unrelated to the X‐linked mycobacterial susceptibility gene segregating in their kindred, as its clinical course was clearly distinct from the activities in patient III‐4.
Methods
Diagnosis of disorders of the IL12/IL23–IFN γ pathways
Peripheral blood samples were diluted in the ratio 1:2 in RPMI 1640 and were infected with live BCG (M bovis BCG, Pasteur substrain, at multiplicity of infection 20:1), BCG and IFN γ (5000 IU/ml; Imukin, Boehringer Ingelheim, France) and BCG and IL12p70 (20 ng/ml R&D Systems, Minneapolis, Minnesota, USA).28 Cytokine concentrations were determined by ELISA (human Quantikine IL12p70 kit (R&D Systems) and the Pelipair IFN γ kit (Sanquin, Amsterdam)) according to the manufacturer's instructions. Absorbance was determined with an automated MR5000 ELISA reader (Thermolab Systems, Cergy Pontoise, France). Final results were standardised per million PBMC (pg/ml).
Diagnosis of NEMO‐associated XR‐MSMD
Two populations were obtained from the peripheral PBMC.14 Monocytes were isolated using the Human Monocyte Isolation Kit II (Miltenyi‐Biotec, Cologne, Germany), and 1×105/ml were plated in 1 ml of RPMI supplemented with 10% FCS in 24‐well plates. They were then washed in phosphate‐buffered saline to remove unattached cells, and the remaining adherent cells were incubated overnight in RPMI at 37°C, in an atmosphere containing 5% CO2. The next day, PBMC were plated in 150 mm2 tissue culture plates for 2 h, and T lymphocytes were isolated from the non‐adherent cells. T lymphocytes were isolated by PanT‐Isolation Kit II human cells (Miltenyi‐Biotec), and mixed with the monocytes and activated by incubation with PHA (diluted 1/700, Bacto‐Becton Dickinson, Sparks, Maryland, USA) for 48 h; supernatants were collected after 18 and 48 h. We detected IFN γ and IL12 production with ELISA kits (IFNγ‐Sanquin and IL12p70 R&D Systems). NEMO was detected on western blots of total protein extract from EBV‐transformed B cells, carried out in 1% Nonidet P‐40 buffer. For western blotting, proteins were first separated by electrophoresis in 10% polyacrylamide gels containing sodium dodecyl sulphate. The proteins were then transferred to membranes, which were probed with goat anti‐human IKKγ (M‐18, sc‐8256 Santa Cruz Biotechnology, Santa Cruz, California, USA) and mouse anti‐human STAT2 (A‐7, sc‐1668 Santa Cruz) antibodies. Flow cytometry showed that the EBV‐B cells displayed a pattern of staining similar to that reported in a previous study.14 Cells were incubated with anti‐NEMO antibody (611306‐BD Pharmingen, California, USA) and the corresponding IgG1 isotype (BD Pharmingen), and primary antibody binding was detected with Alexa Fluor 488‐labelled anti‐mouse antibodies (Molecular Probes, Invitrogen, Eugene, Oregon, USA).
Genotyping and linkage analysis
Human genomic DNA was isolated from the blood samples of 30 people from the family as described previously.8 The primary genetic scan was carried out using a panel of 18 polymorphic microsatellites spanning the X chromosome, which is available on request (ABI Prism Linkage Mapping Set 2, V.2.5, Applied Biosystems). To refine the interesting regions, additional microsatellites on the X chromosome were genotyped by polymerase chain reaction using standard techniques (fig 5). Amplified products were analysed with an ABI 3100 Prism‐Genetic analyser (Applied Biosystems). Linkage analysis was carried out assuming X‐linked recessive inheritance with complete penetrance for male carriers and a frequency of the deleterious at 0.0001. The four male patients, including three patients with bona fide MSMD and one patient with tuberculosis were considered to be affected. Two‐point and multipoint (logarithm of odds) LOD score were computed using the xgh program of GENEHUNTER software (http://www‐genome.wi.mit.edu/ftp/distribution/software/genehunter).28 Fine mapping was conducted in regions with a LOD score >1.
Figure 5 X chromosome scan. (A) Multipoint linkage analysis of the X chromosome. The position of the polymorphic markers on the genetic map of the X chromosome is indicated in centimorgans (cM) based on female recombination fractions of the genetic map (Marshfield). The x axis represents the position of the markers used along chromosome X, the y axis depicts the LOD score. The candidate regions comprised cytogenetic regions Xp11.4–Xp21.2 and Xq25–Xq26.3.
Results
Investigation of the IL12/IL23–IFN γ circuit
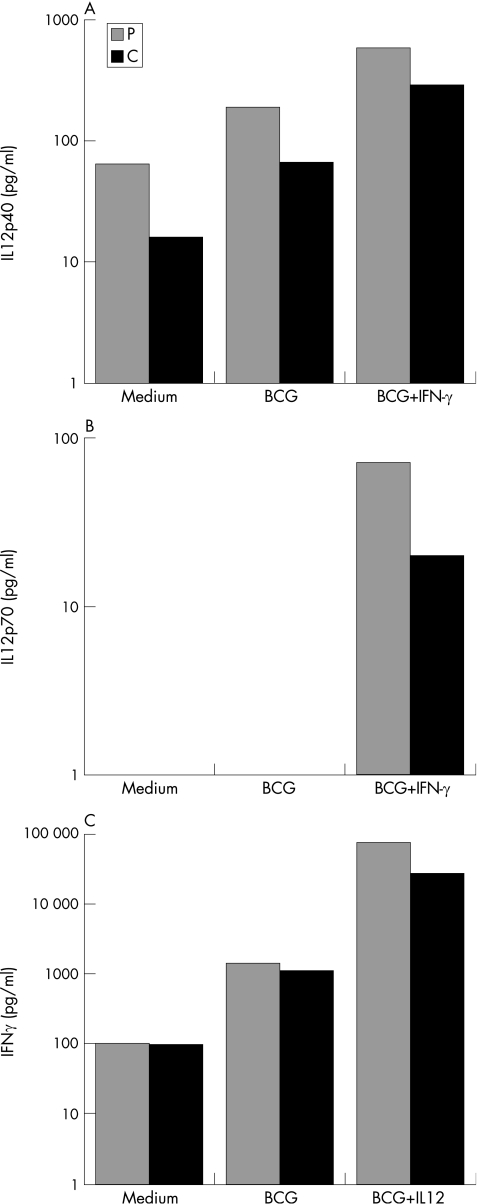
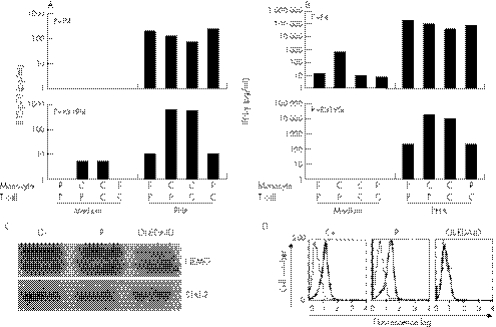
We investigated the IL12/IL23–IFN γ circuit, because germline mutations in five autosomal genes (IFNGR1, IFNGR2, STAT1, IL12B and IL12RB1) and one X‐linked gene (NEMO) are associated with impaired IL‐12/23‐dependent IFN γ‐mediated immunity and MSMD.2,14 We stimulated whole blood cells with live BCG, alone or with IL12 or IFN γ, and measured IFN γ and IL12 levels in the supernatant.27 The secretion of IL12p40 and IL12p70 after 18 h of stimulation with BCG, and BCG and IFN γ, as shown by ELISA, was normal in the proband, taking several healthy controls as the reference group (fig 3A,B).27 We incubated whole blood from the patient with BCG alone, or with BCG and recombinant IL‐12, and detected normal levels of IFN γ in the supernatant (fig 3C). We amplified and sequenced the coding regions of these five disease‐causing genes from genomic DNA and cDNA. No mutations were found, and all cDNAs had a normal structure. These experiments excluded all 12 known forms of autosomal recessive and dominant disorders of the IL12/IL23–IFN γ circuit.17,18,27
Figure 3 Investigation of the interleukin (IL12)–interferon (IFNγ) circuit. (A) Production of IL12p40 by bacille Calmette–Guérin (BCG) (20:1) and BCG‐ and IFNγ (5000 IU/ml)‐stimulated whole‐blood cells from a healthy positive control (C) and the proband (P). Supernatants were harvested after 18 h of activation for cytokine quantification by ELISA. (B) Production of IL12p70 by whole‐blood cells stimulated with BCG alone or with BCG and IFN γ, after 18 h of activation. (C) Production of IFN γ by whole‐blood cells, either unstimulated (medium) or stimulated with BCG (20:1) alone or with BCG and IL12p70 (20 ng/ml). The supernatants were harvested after 72 h of activation for cytokine quantification by ELISA. The data from one of two experiments are shown.
In‐depth investigation of NEMO‐associated XR‐MSMD
X‐linked recessive inheritance of MSMD was recently shown to be associated with specific mutations affecting the LZD of NEMO in three unrelated families.14 These patients had abnormal CD40‐dependent IL12 production by monocytes in the presence of autologous and heterologous PHA‐activated T cells13,14 (fig 4A). In contrast, T cells from patients with NEMO mutations produced normal amounts of IFN γ in response to activation by PHA in the presence of autologous or heterologous monocytes (fig 4B). In the two patients (P1 and P4) studied here, IL12 production by monocytes was strictly normal in response to costimulation with PHA and T cells, whether autologous or heterologous (fig 4A). The patients' T cells also showed normal IFN γ production in this coculture assay. The cDNA structure of NEMO was intact and no mutations were found (data not shown). The level of expression of NEMO in an EBV‐B cell line and fresh blood cells was normal, as shown by western blotting23 (fig 4C). NEMO was also normally expressed in all leucocyte subsets, as shown by flow cytometry, excluding the possibility of a subtle regulatory mutation with a cell‐specific effect (fig 4D). The immunological, genetic and biochemical data therefore unambiguously excluded NEMO as the MSMD‐causing gene in this kindred.
Figure 4 Investigation of the nuclear factor‐κB essential modulator (NEMO)–interleukin (IL)12 circuit. (A) IL12p70 production was measured by ELISA after 18 h of coculture of monocytes and T lymphocytes, from the patient (P) or a control (C) (upper panel), and NEMO R319Q or a (C) with or without PHA (1/700). (B) Interferon γ production in the same conditions was measured after 48 h by ELISA. This experiment is representative of two independent experiments. (C) Expression of NEMO and Stat 2 in Epstein–Barr virus (EBV)‐B cells from a healthy positive control (C+), the proband (P4) and a patient with NEMO‐OL‐ectodermal dysplasia with immunodeficiency (EDA‐ID). The data from one of two experiments are shown. (D) Expression of NEMO in EBV‐B cells from the control, the patient and a NEMO‐OL‐EDA‐ID patient, as shown by flow cytometry; antibodies are specifically directed against the C‐terminal part of NEMO.
X chromosome linkage analysis
We finally genotyped 18 microsatellite markers scattered at 5–10 cM intervals along the X chromosome, in 30 informative family members (fig 5). The four male patients were considered to be affected. Results of the primary screen showed that two regions, flanked by markers DXS1226 and DXS991, and DXS1001 and DXS1062, provided LOD scores >1, and additional microsatellites; 14 and 7, respectively, were genotyped in both regions. A negative multipoint LOD score (−0.22 at DXS1073) was observed in the region surrounding NEMO (fig 5). In contrast, multipoint LOD scores of +1.93 were obtained in the two previously detected regions Xp11.4–Xp21.2 and Xq25–Xq26.3. This value of 1.93 corresponds to the maximum LOD score that can be expected from this pedigree. The first region spans from DXS8010 to DXS DXS1003, and covers 18.6 Mb, including 129 known genes. The second region ranges from DXS1001 to DXS8072, and corresponds to 17.4 Mb, including 70 known genes. None of the confirmed and putative genes in the two regions has been directly involved in IL12/IL23 and IFN γ‐mediated immunity.
Discussion
We report a large kindred with a new form of XR‐MSMD. We excluded mutations in the NEMO gene as a cause of XR‐MSMD‐1 using a combination of clinical (lack of even mild signs of ectodermal dysplasia), immunological (normal IL12 production by monocytes), genetic (normal sequence of the NEMO coding region, normal cDNA structure), biochemical (normal NEMO protein production) and linkage (NEMO‐encompassing region excluded by X scan) grounds. This novel condition, designated XR‐MSMD‐2, was characterised by recurrent mycobacterial disease in four male maternal relatives (P1, P2, P3 and P4). The four patients, now 55, 52, 50 and 32 years old, have otherwise remained healthy, with the possible exception of varicella zoster, which was diagnosed in two patients and which might share an immunological aetiology with mycobacterial disease. Three patients with MSMD had clinical disease caused by the weakly virulent BCG vaccine, and the fourth had tuberculosis. Recurrent adenitis caused by BCG is a well‐known clinical presentation of MSMD.8,29 Tuberculosis as the sole clinical manifestation has been reported recently in patients with genetic defects typically associated with MSMD, such as IL12Rβ1 deficiency.7,9,10,11
When the gene responsible for XR‐MSMD‐2 is identified, it will thus be important to test selected patients with tuberculosis for pathogenic Mendelian mutations in this gene.30,31 Moreover, several lines of epidemiological and experimental evidence suggest that tuberculosis in the general population may also reflect a complex genetic predisposition including the X chromosome.2,11,32,33,34,35 The first genomewide scan carried out for tuberculosis was based on a series of affected families with two or more siblings from Gambia and South Africa. Two regions, on chromosomes 15q and Xq, seemed to be involved.36 The maximum LOD score for the X chromosome was obtained for Xq26 (marker DXS 1047), a region included in the Xq region linked to XR‐MSMD‐2. The XR‐MSMD‐2 gene will therefore be a good candidate gene for studies of the genetic predisposition to tuberculosis, from both Mendelian and complex genetic perspectives.11
The recent publication of the complete genomic sequence of the X chromosome should also facilitate the identification of this new gene.37 There are 129 known genes in one of the candidate intervals and 70 in the other. The identification of other families with XR‐MSMD‐2 would also facilitate our search for the genetic basis of this condition, making it possible to refine the X‐chromosome scan and to narrow down the candidate intervals. It is unknown whether the gene associated with XR‐MSMD‐2 is connected with the IL12/IL23–IFN γ axis or with the CD40‐triggered, NEMO‐dependent NF‐κB‐mediated induction of IL12, two interconnected immunological circuits. In any event, elucidation of the genetic basis of this condition should be of great benefit to patients affected with MSMD and their families worldwide, facilitating diagnosis and treatment. It should also pave the way for the study of X‐linked susceptibility to tuberculosis, by providing a new candidate gene for Mendelian and complex genetic studies.
Electronic database information
The accession numbers and URLs for the data presented in this article are as follows: UCSC Human Genome Project Working Draft, http://www.genome.ucsc.edu. Marshfield and http://www.marshfieldclinic.org/research/genetics
Acknowledgements
We thank the family members for their continued interest and cooperation. We also thank all members of the laboratory of Human Genetics of Infectious Diseases for helpful discussions. We thank F Geissmann (Hôpital Necker, Paris, France) for phenotyping monocytes and dendritic cells, C Elbim (Hôpital Bichat, Paris, France) for the evaluation of granulocyte function and F Le Deist (Hôpital Sainte Justine, Montréal, Canada) for CD40L evaluation. We would also like to thank C Bidalled, M Courat and T Leclerc for excellent secretarial and technical assistance.
Abbreviations
BCG - bacille Calmette–Guérin
EBV - Epstein–Barr virus
IFN - interferon
LOD - logarithm of odds
LZD - leucine zipper domain
MSMD - Mendelian susceptibility to mycobacterial disease
NEMO - nuclear factor‐κB essential modulator
PBMC - peripheral blood mononuclear cells
Stat 1 - signal transducer and activator of transcription 1
XR‐MSMD - X‐linked recessive MSMD
Footnotes
CP and CF contributed equally to this paper. J‐LC is an international scholar of the Howard Hughes Medical Institute.
Funding: JB was supported by Fondation Schlumberger and INSERM. This work was supported by Fondation BNP‐Paribas, Foundation Schlumberger, Institut Universitaire de France and EU grant QLK2‐CT‐2002‐00846.
Competing interests: None.
References
- 1.Hamosh A, Scott A F, Amberger J S, Bocchini C A, McKusick V A. Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res 200533D514–D517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casanova J L, Abel L. Genetic dissection of immunity to mycobacteria: the human model. Annu Rev Immunol 200220581–620. [DOI] [PubMed] [Google Scholar]
- 3.Casanova J L, Jouanguy E, Lamhamedi S, Blanche S, Fischer A. Immunological conditions of children with BCG disseminated infection. Lancet 1995346581. [DOI] [PubMed] [Google Scholar]
- 4.Casanova J L, Blanche S, Emile J F, Jouanguy E, Lamhamedi S, Stephan J L, Bernaudin F, Bordigoni P, Turck D, Lachaux A, Albertini M, Bourrillon A, Dommergues J P, Pocidalo M A, Le Deist F, Gaillard J L, Griscelli C, Fischer A. Idiopathic disseminated bacillus Calmette‐Guerin infection: a French national retrospective study. Pediatrics 199698(Pt 1)774–778. [PubMed] [Google Scholar]
- 5.Levin M, Newport M J, D'Souza S, Kalabalikis P, Brown I N, Lenicker H M, Agius P V, Davies E G, Thrasher A, Klein N, Blackwell J M. Familial disseminated atypical mycobacterial infection in childhood: a human mycobacterial susceptibility gene? Lancet 199534579–83. [DOI] [PubMed] [Google Scholar]
- 6.Jouanguy E, Lamhamedi‐Cherradi S, Altare F, Fondaneche M C, Tuerlinckx D, Blanche S, Emile J F, Gaillard J L, Schreiber R, Levin M, Fischer A, Hivroz C, Casanova J L. Partial interferon‐gamma receptor 1 deficiency in a child with tuberculoid bacillus Calmette‐Guerin infection and a sibling with clinical tuberculosis. J Clin Invest 19971002658–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altare F, Ensser A, Breiman A, Reichenbach J, Baghdadi J E, Fischer A, Emile J F, Gaillard J L, Meinl E, Casanova J L. Interleukin‐12 receptor beta1 deficiency in a patient with abdominal tuberculosis. J Infect Dis 2001184231–236. [DOI] [PubMed] [Google Scholar]
- 8.Picard C, Fieschi C, Altare F, Al‐Jumaah S, Al‐Hajjar S, Feinberg J, Dupuis S, Soudais C, Al‐Mohsen I Z, Genin E, Lammas D, Kumararatne D S, Leclerc T, Rafii A, Frayha H, Murugasu B, Wah L B, Sinniah R, Loubser M, Okamoto E, Al‐Ghonaium A, Tufenkeji H, Abel L, Casanova J L. Inherited interleukin‐12 deficiency: IL12B genotype and clinical phenotype of 13 patients from six kindreds. Am J Hum Genet 200270336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caragol I, Raspall M, Fieschi C, Feinberg J, Larrosa M N, Hernandez M, Figueras C, Bertran J M, Casanova J L, Espanol T. Clinical tuberculosis in 2 of 3 siblings with interleukin‐12 receptor beta1 deficiency. Clin Infect Dis 200337302–306. [DOI] [PubMed] [Google Scholar]
- 10.Ozbek N, Fieschi C, Yilmaz B T, de Beaucoudrey L, Demirhan B, Feinberg J, Bikmaz Y E, Casanova J L. Interleukin‐12 receptor beta 1 chain deficiency in a child with disseminated tuberculosis. Clin Infect Dis 200540e55–e58. [DOI] [PubMed] [Google Scholar]
- 11.Alcais A, Fieschi C, Abel L, Casanova J L. Tuberculosis in children and adults: two distinct genetic diseases. J Exp Med 20052021617–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emile J F, Patey N, Altare F, Lamhamedi S, Jouanguy E, Boman F, Quillard J, Lecomte‐Houcke M, Verola O, Mousnier J F, Dijoud F, Blanche S, Fischer A, Brousse N, Casanova J L. Correlation of granuloma structure with clinical outcome defines two types of idiopathic disseminated BCG infection. J Pathol 199718125–30. [DOI] [PubMed] [Google Scholar]
- 13.Frucht D M, Holland S M. Defective monocyte costimulation for IFN‐gamma production in familial disseminated Mycobacterium avium complex infection: abnormal IL‐12 regulation. J Immunol 1996157411–416. [PubMed] [Google Scholar]
- 14.Filipe Santos O, Bustamante J, Haverkamp M, Vinolo E, Ku C L, Puel A, Frucht D, Christel K, von Bernuth H, Jouanguy E, Feinberg J, Durandy A, Senechal B, Chapgier A, Vogt G, de Beaucoudrey L, Fieschi C, Picard C, Garfa M, Chemli J, Bejaoui M, Tsolia M, Kutukculer N, Plebani A, Notarangelo L, Bodemer C, Geissmann F, Israël A, Véron M, Knackstedt M, Barbouche R, Abel L, Magdorf K, Gendrel D, Agou F, Holland S M, Casanova J L. X‐linked susceptibility to mycobacteria is caused by mutations in the NEMO leucin zipper domain that impair CD40‐dependent IL‐12 production. J Exp Med 20062031745–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dorman S E, Holland S M. Interferon‐gamma and interleukin‐12 pathway defects and human disease. Cytokine Growth Factor Rev 200011321–333. [DOI] [PubMed] [Google Scholar]
- 16.Rosenzweig S D, Holland S M. Defects in the interferon‐gamma and interleukin‐12 pathways. Immunol Rev 200520338–47. [DOI] [PubMed] [Google Scholar]
- 17.Vogt G, Chapgier A, Yang K, Chuzhanova N, Feinberg J, Fieschi C, Boisson‐Dupuis S, Alcais A, Filipe‐Santos O, Bustamante J, de Beaucoudrey L, Al‐Mohsen I, Al‐Hajjar S, Al‐Ghonaium A, Adimi P, Mirsaeidi M, Khalilzadeh S, Rosenzweig S, de la Calle Martin O, Bauer T R, Puck J M, Ochs H D, Furthner D, Engelhorn C, Belohradsky B, Mansouri D, Holland S M, Schreiber R D, Abel L, Cooper D N, Soudais C, Casanova J L. Gains of glycosylation comprise an unexpectedly large group of pathogenic mutations. Nat Genet 200537692–700. [DOI] [PubMed] [Google Scholar]
- 18.Chapgier A, Boisson‐Dupuis S, Jouanguy E, Vogt G, Feinberg J, Prochnicka‐Chalufour A, Casrouge A, Yang K, Soudais C, Fieschi C, Filipe Santos O, Bustamante J, Picard C, de Beaucoudrey L, Emile J F, Arkwright P, Schreiber R D, Rolinck‐Werninghaus C, Rösen‐Wolff A, Magdorf K, Roesler J, Casanova J L. Dominant and recessive phenotypes conferred by STAT1 alleles. PloS Genet. 2006;2:e131
- 19.Rosenzweig S D, Dorman S E, Uzel G, Shaw S, Scurlock A, Brown M R, Buckley R H, Holland S M. A novel mutation in IFN‐gamma receptor 2 with dominant negative activity: biological consequences of homozygous and heterozygous states. J Immunol 20041734000–4008. [DOI] [PubMed] [Google Scholar]
- 20.Holland S M, Eisenstein E M, Kuhns D B, Turner M L, Fleisher T A, Strober W, Gallin J I. Treatment of refractory disseminated nontuberculous mycobacterial infection with interferon gamma. A preliminary report. N Engl J Med 19943301348–1355. [DOI] [PubMed] [Google Scholar]
- 21.Nedorost S T, Elewski B, Tomford J W, Camisa C. Rosacea‐like lesions due to familial Mycobacterium avium‐intracellulare infection. Int J Dermatol 199130491–497. [DOI] [PubMed] [Google Scholar]
- 22.Zonana J, Elder M E, Schneider L C, Orlow S J, Moss C, Golabi M, Shapira S K, Farndon P A, Wara D W, Emmal S A, Ferguson B M. A novel X‐linked disorder of immune deficiency and hypohidrotic ectodermal dysplasia is allelic to incontinentia pigmenti and due to mutations in IKK‐gamma (NEMO). Am J Hum Genet 2000671555–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doffinger R, Smahi A, Bessia C, Geissmann F, Feinberg J, Durandy A, Bodemer C, Kenwrick S, Dupuis‐Girod S, Blanche S, Wood P, Rabia S H, Headon D J, Overbeek P A, Le Deist F, Holland S M, Belani K, Kumararatne D S, Fischer A, Shapiro R, Conley M E, Reimund E, Kalhoff H, Abinum M, Munnich A, Israel A, Courtois G, Casanova J L. X‐linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF‐kappaB signaling. Nat Genet 200127277–285. [DOI] [PubMed] [Google Scholar]
- 24.Jain A, Ma C A, Liu S, Brown M, Cohen J, Strober W. Specific missense mutations in NEMO result in hyper‐IgM syndrome with hypohydrotic ectodermal dysplasia. Nat Immunol 20012223–228. [DOI] [PubMed] [Google Scholar]
- 25.Puel A, Picard C, Ku C L, Smahi A, Casanova J L. Inherited disorders of NF‐kappaB‐mediated immunity in man. Curr Opin Immunol 20041634–41. [DOI] [PubMed] [Google Scholar]
- 26.Ku C L, Yang K, Bustamante J, Puel A, von Bernuth H, Dos Santos O, Lawrence T, Chang H H, Al‐Mousa H, Picard C, Casanova J L. Inherited disorders of human Toll‐like receptor signalling: immunological implications. Immunol Rev 200520310–20. [DOI] [PubMed] [Google Scholar]
- 27.Feinberg J, Fieschi C, Doffinger R, Feinberg M, Leclerc T, Boisson‐Dupuis S, Picard C, Bustamante J, Chapgier A, Filipe‐Santos O, Ku C L, de Beaucoudrey L, Reichenbach J, Antoni G, Balde R, Alcais A, Casanova J L. Bacillus Calmette Guerin triggers the IL12/IFN‐gamma axis by an IRAK‐4‐ and NEMO‐dependent, non‐cognate interaction between monocytes, NK, and T lymphocytes. Eur J Immunol 2004343276–3284. [DOI] [PubMed] [Google Scholar]
- 28.Kruglyak L, Daly M J, Reeve‐Daly M P, Lander E S. Parametric and nonparametric linkage analysis: a unified multipoint approach. Am J Hum Genet 1996581347–1363. [PMC free article] [PubMed] [Google Scholar]
- 29.Fieschi C, Dupuis S, Catherinot E, Feinberg J, Bustamante J, Breiman A, Altare F, Baretto R, Le Deist F, Kayal S, Koch H, Richter D, Brezina M, Aksu G, Wood P, Al‐Jumaah S, Raspall M, Da Silva Duarte A J, Tuerlinckx D, Virelizier J L, Fischer A, Enright A, Bernhoft J, Cleary A M, Vermylen C, Rodriguez‐Gallego C, Davies G, Blutters‐Sawatzki R, Siegrist C A, Ehlayel M S, Novelli V, Haas W H, Levy J, Freihorst J, Al‐Hajjar S, Nadal D, De Moraes Vasconcelos D, Jeppsson O, Kutukculer N, Frecerova K, Caragol I, Lammas D, Kumararatne D S, Abel L, Casanova J L. Low penetrance, broad resistance, and favorable outcome of interleukin 12 receptor beta1 deficiency: medical and immunological implications. J Exp Med 2003197527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casanova J L, Abel L. Inborn errors of immunity to infection: the rule rather than the exception. J Exp Med 2005202197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casanova J L, Fieschi C, Bustamante J, Reichenbach J, Remus N, von Bernuth H, Picard C. From idiopathic infectious diseases to novel primary immunodeficiencies. J Allergy Clin Immunol 2005116426–430. [DOI] [PubMed] [Google Scholar]
- 32.Abel L, Casanova J L. Genetic predisposition to clinical tuberculosis: bridging the gap between simple and complex inheritance. Am J Hum Genet 200067274–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Remus N, El Baghdadi J, Fieschi C, Feinberg J, Quintin T, Chentoufi M, Schurr E, Benslimane A, Casanova J L, Abel L. Association of IL12RB1 polymorphisms with pulmonary tuberculosis in adults in Morocco. J Infect Dis 2004190580–587. [DOI] [PubMed] [Google Scholar]
- 34.Akahoshi M, Nakashima H, Miyake K, Inoue Y, Shimizu S, Tanaka Y, Okada K, Otsuka T, Harada M. Influence of interleukin‐12 receptor beta1 polymorphisms on tuberculosis. Hum Genet 2003112237–243. [DOI] [PubMed] [Google Scholar]
- 35.Baghdali J E, Orlova M, Alter A, Ranque B, Chantoufi M, Lazrak F, Archane M I, Casanova J L, Benslimane A, Schurr E, Abel A. An autosomal dominant major gene confers predisposition to pulmonary tuberculosis in adults. J Exp Med 20062031679–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bellamy R, Beyers N, McAdam K P, Ruwende C, Gie R, Samaai P, Bester D, Meyer M, Corrat T, Collin T, Camidge D R, Wilkinson D, Hoal‐van Helden E, Whittle H C, Amos W, van Helden P, Hill A V. Genetic susceptibility to tuberculosis in Africans: a genome-wide scan. Proc Natl Acad Sci U S A 2000978005–8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ross M T, Grafham D V, Coffey A J, Scherer S, McLay K, Muzny D, Platzer M, Howell G R, Burrows C, Bird C P, Frankish A, Lovell F L, Howe K L, Ashurst J L, Fulton R S, Sudbrak R, Wen G, Jones M C, Hurles M E, Andrews T D, Scott C E, Searle S, Ramser J, Whittaker A, Deadman R, Carter N P, Hunt S E, Chen R, Cree A, Gunaratne P, Havlak P, Hodgson A, Metzker M L, Richards S, Scott G, Steffen D, Sodergren E, Wheeler D A, Worley K C, Ainscough R, Ambrose K D, Ansari‐Lari M A, Aradhya S, Ashwell R I, Babbage A K, Bagguley C L, Ballabio A, Banerjee R, Barker G E, Barlow K F, Barrett I P, Bates K N, Beare D M, Beasley H, Beasley O, Beck A, Bethel G, Blechschmidt K, Brady N, Bray‐Allen S, Bridgeman A M, Brown A J, Brown M J, Bonnin D, Bruford E A, Buhay C, Burch P, Burford D, Burgess J, Burrill W, Burton J, Bye J M, Carder C, Carrel L, Chako J, Chapman J C, Chavez D, Chen E, Chen G, Chen Y, Chen Z, Chinault C, Ciccodicola A, Clark S Y, Clarke G, Clee C M, Clegg S, Clerc‐Blankenburg K, Clifford K, Cobley V, Cole C G, Conquer J S, Corby N, Connor R E, David R, Davies J, Davis C, Davis J, Delgado O, Deshazo D, Dhami P, Ding Y, Dinh H, Dodsworth S, Draper H, Dugan‐Rocha S, Dunham A, Dunn M, Durbin K J, Dutta I, Eades T, Ellwood M, Emery‐Cohen A, Errington H, Evans K L, Faulkner L, Francis F, Frankland J, Fraser A E, Galgoczy P, Gilbert J, Gill R, Glockner G, Gregory S G, Gribble S, Griffiths C, Grocock R, Gu Y, Gwilliam R, Hamilton C, Hart E A, Hawes A, Heath P D, Heitmann K, Hennig S, Hernandez J, Hinzmann B, Ho S, Hoffs M, Howden P J, Huckle E J, Hume J, Hunt P J, Hunt A R, Isherwood J, Jacob L, Johnson D, Jones S, de Jong P J, Joseph S S, Keenan S, Kelly S, Kershaw J K, Khan Z, Kioschis P, Klages S, Knights A J, Kosiura A, Kovar‐Smith C, Laird G K, Langford C, Lawlor S, Leversha M, Lewis L, Liu W, Lloyd C, Lloyd D M, Loulseged H, Loveland J E, Lovell J D, Lozado R, Lu J, Lyne R, Ma J, Maheshwari M, Matthews L H, McDowall J, McLaren S, McMurray A, Meidl P, Meitinger T, Milne S, Miner G, Mistry S L, Morgan M, Morris S, Muller I, Mullikin J C, Nguyen N, Nordsiek G, Nyakatura G, O'Dell C N, Okwuonu G, Palmer S, Pandian R, Parker D, Parrish J, Pasternak S, Patel D, Pearce A V, Pearson D M, Pelan S E, Perez L, Porter K M, Ramsey Y, Reichwald K, Rhodes S, Ridler K A, Schlessinger D, Schueler M G, Sehra H K, Shaw‐Smith C, Shen H, Sheridan E M, Shownkeen R, Skuce C D, Smith M L, Sotheran E C, Steingruber H E, Steward C A, Storey R, Swann R M, Swarbreck D, Tabor P E, Taudien S, Taylor T, Teague B, Thomas K, Thorpe A, Timms K, Tracey A, Trevanion S, Tromans A C, d'Urso M, Verduzco D, Villasana D, Waldron L, Wall M, Wang Q, Warren J, Warry G L, Wei X, West A, Whitehead S L, Whiteley M N, Wilkinson J E, Willey D L, Williams G, Williams L, Williamson A, Williamson H, Wilming L, Woodmansey R L, Wray P W, Yen J, Zhang J, Zhou J, Zoghbi H, Zorilla S, Buck D, Reinhardt R, Poustka A, Rosenthal A, Lehrach H, Meindl A, Minx P J, Hillier L W, Willard H F, Wilson R K, Waterston R H, Rice C M, Vaudin M, Coulson A, Nelson D L, Weinstock G, Sulston J E, Durbin R, Hubbard T, Gibbs R A, Beck S, Rogers J, Bentley D R. The DNA sequence of the human X chromosome. Nature 2005434325–337. [DOI] [PMC free article] [PubMed] [Google Scholar]