Abstract
Background
Schimke immuno‐osseous dysplasia (SIOD) is a fatal autosomal recessive disorder caused by loss‐of‐function mutations in swi/snf‐related matrix‐associated actin‐dependent regulator of chromatin, subfamily a‐like 1 (SMARCAL1).
Methods
Analysis of detailed autopsies to correlate clinical and pathological findings in two men severely affected with SIOD.
Results
As predicted by the clinical course, T cell deficiency in peripheral lymphoid organs, defective chondrogenesis, focal segmental glomerulosclerosis, cerebral ischaemic lesions and premature atherosclerosis were identified. Clinically unexpected findings included a paucity of B cells in the peripheral lymphoid organs, emperipolesis‐like (penetration of one cell by another) abnormalities in the adenohypophysis, fatty infiltration of the cardiac right ventricular wall, pulmonary emphysema, testicular hypoplasia with atrophy and azospermia, and clustering of small cerebral vessels.
Conclusions
A regulatory role for the SMARCAL1 protein in the proliferation of chondrocytes, lymphocytes and spermatozoa, as well as in the development or maintenance of cardiomyocytes and in vascular homoeostasis, is suggested. Additional clinical management guidelines are recommended as this study has shown that patients with SIOD may be at risk of pulmonary hypertension, combined immunodeficiency, subcortical ischaemic dementia and cardiac dysfunction.
The osteochondrodysplasias are a heterogeneous group of inherited disorders of skeletal growth causing disproportionate short stature. Of the 230 distinct osteochondrodysplasias,2 several have been associated with nephrotic syndrome or immunodeficiency.2 Schimke immuno‐osseous dysplasia (SIOD), an autosomal recessive panethnic multisystem osteochondrodysplasia, is characterised by dysmorphism,3,4 spondyloepiphysial dysplasia,5,6 T cell immunodeficiency3,5 and nephrotic syndrome.3,5,7,8,9,10,11 Less penetrant features include hypothyroidism,3 migraine‐like headaches,12 cerebral ischaemia7,13 and enteropathy.14,15,16,17 Stroke, severe opportunist infections, bone marrow failure, complications of renal failure or an undefined pulmonary disease,3 result in premature mortality in most patients during childhood to early adolescence; however, a few patients remain alive in their third and fourth decades of life.18
SIOD is caused by biallelic loss‐of‐function mutations in swi/snf‐related matrix‐associated actin‐dependent regulator of chromatin, subfamily a‐like 1 (SMARCAL1).19 Previous studies have shown that SMARCAL1 encodes a protein homologous to the SNF2 family of chromatin remodelling proteins.20,21 However, the mechanism by which SMARCAL1 mutations cause SIOD remains unknown. Hypotheses on the pathophysiology have considered SIOD to be an autoimmune disorder,5,14 a connective tissue disorder,16,22,23 a vascular endothelial disorder,7,8,24 a metabolic disorder affecting chondrocyte and T cell differentiation,5,16 or a cellular proliferation disorder.3,19
Murine Smarcal1 is expressed in tissues equivalent to those implicated as affected by clinical symptoms.25 From this observation and clinical data, we have hypothesised that SIOD arises autonomously in each cell type. To determine whether disease occurs in each of these tissues as hypothesised and to elucidate further the pathophysiology of SIOD and potential functions for SMARCAL1, we undertook a detailed analysis of autopsy samples from two patients with SIOD and correlated the findings with their clinical course. Although secondary effects may confound interpretation of some findings, we find that the loss of functional SMARCAL1 has tissue‐specific effects on cellular proliferation, development and maintenance, and uncovered previously unknown pathological features relevant for management of patients with SIOD.
Clinical reports
Patient 1, SD600
The propositus was a 13.7‐year‐old boy, who died 12 days after cardiopulmonary arrest during a lymph node biopsy. He was the second child of healthy non‐consanguineous parents. The parents, northern and eastern European descendants, had a healthy older daughter. The boy was born at 35 weeks of gestation by emergency Caesarian section for oligohydramnios and growth retardation. Because of poor postnatal growth, hip pain and tooth root malformation, he had an extensive evaluation at 7 years of age when he was found to have the key physical features of SIOD including proteinuria (table 1). His renal disease progressed to end‐stage renal failure, requiring dialysis and later transplantation.
Table 1 Summary of the patients' clinical signs and symptoms.
| Clinical characteristic | Patient | |
|---|---|---|
| SD600 | SD840 | |
| Sex | Male | Male |
| Dysmorphism | ||
| Broad low nasal bridge | Yes | Yes |
| Bulbous nasal tip | Yes | Yes |
| Microdontia | Yes | Yes |
| Hyperpigmented macules | Yes | Yes |
| Lumbar lordosis | Yes | Yes |
| Protuberant abdomen | Yes | Yes |
| Skeleton and growth | ||
| Poor growth | Yes | Yes |
| Disproportionate short stature | Yes | Yes |
| Spondyloepiphysial dysplasia | Yes | Yes |
| Degenerative hip disease | Yes | Yes |
| Development | ||
| Normal cognitive, motor, language, social development | Yes | Yes |
| Normal school performance | Yes | Yes |
| Endocrinology | ||
| Raised level of thyroid‐stimulating hormone | No | No |
| Haematology | ||
| Lymphopenia | Yes | Yes |
| Neutropenia | Yes | No |
| Anaemia | Yes | No |
| Thrombocytopenia | Yes | No |
| Immunology | ||
| Recurrent infections | No | Yes |
| Circulating T cell deficiency | Yes | Yes |
| Circulating B cell deficiency | No | NC |
| Hypogammaglobulnaemia | Yes | No |
| Nephrology | ||
| FSGS | Yes | Yes |
| Progressive renal failure | Yes | Yes |
| Renal transplant | Yes | Yes |
| Donor age (years) | 46 | 15 |
| Hypertension before transplant | Yes | Yes |
| Hypertension after transplant | Yes | Yes |
| Neurology | ||
| TIAs | Yes | Yes |
| Strokes | Yes | No |
| Migraines | No | Yes |
| Pulmonary | ||
| Pulmonary hypertension | Yes | Yes |
| Dyspnoea | No | Yes |
| Genetics | ||
| Normal karyotype | Yes | NC |
| SMARCAL1 mutations | ||
| Paternal allele | 2542G→T E848X | 1248‐9insC T417fs427X |
| Maternal allele | 2542G→T E848X | 2104T→G F702V |
FSGS, focal segmental glomerulosclerosis; NC, not countable; SMARCAL1, swi/snf‐related matrix‐associated actin‐dependent regulator of chromatin, subfamily a‐like 1; TIA, transient ischaemic attack.
At 7¾ years, he had his first transient ischaemic attack (TIA) requiring treatment with cyproheptadine and aspirin. Brain magnetic resonance imaging at 12 years of age showed non‐enhancing multifocal deep white matter lesions correlating with clinically detectable neurological deficits.
By 9 years of age, he had developed lymphopenia (CD4+ T cell deficiency) and hypogammaglobulinaemia. In the last year of life, he progressively developed neutropenia, anaemia and thrombocytopenia.
Patient 2, SD840
The propositus was a 23‐year‐old man who died of pulmonary hypertension and right cardiac failure. He was the only child of healthy non‐consanguineous German parents. Table 1 summarises his clinical history, which was previously reported by Lücke et al.24
Materials and methods
Human subjects
The parents gave informed consent approved by the Institutional Review Board of Baylor College of Medicine (H‐9669; Houston, Texas, USA). Postmortem tissue samples were obtained from the referring pathologist. The clinical data were collected from questionnaires completed by the referring primary care physician, and from medical records and summaries provided by that physician.
Autopsy
The autopsies were performed within 24 h after death. The autopsy of SD600 was performed at the University of Minnesota (Minneapolis, Minnesota, USA), and that of SD840 was performed at Hannover Medical School (Hannover, Germany).
Histopathology and immunohistopathology
After formalin fixation, paraffin‐wax‐embedded tissues were sectioned at 5 μm. Histochemical and immunohistochemical examinations were performed according to standard procedures. Stains included haematoxylin and eosin (H&E), luxol fast blue/H&E, Verhoeff Van Giesson, Masson trichrome and periodic acid‐Schiff. Immunohistochemical antibodies included: glial fibrillary acidic protein (1:100, Dako, Carpinteria, California, USA) and smooth muscle actin (1:200, Dako). These were used after antigen retrieval with citrate at pH 6.0. Other antibodies included adrenocorticotropic hormone (1:200, Dako Carpinteria, California, USA), luteinising hormone (1:10 000, Dako), human gonadotrophic hormone (1:2000, Bimedia), human growth hormone (1:2000, Dako), Ki67 (1:500, Dako), CD4 (1:200, Dako), CD8 (1:50, Dako), CD20 (1:800, Dako) and CD3 (1:300, Cell Marque, Hot Springs, Arizona, USA).
Mutation identification
We identified mutations in the SMARCAL1 gene as described previously.19
Results
Table 2 summarises the gross pathology findings for both patients. The following histopathology paragraphs are organised according to the frequency of reported clinical symptoms among patients with SIOD.
Table 2 Summary of gross pathology and organ weights.
| Feature | Measure | Gross pathology and comments | |||
|---|---|---|---|---|---|
| Patient | Ref¶ | Patient | |||
| SD600 | SD840 | SD600 | SD840 | ||
| Age (years) | 13.7 | 23 | Small, well nourished | Small, well nourished | |
| Corpse | |||||
| Weight (kg) | 22.2* | 22† | |||
| Length (cm) | 106.5‡ | 111§ | |||
| Organ | |||||
| Bone | NA | NA | Dysplastic | NA | |
| Immune system | |||||
| Thymus (g) | 2.1 | NA | 25 | Normal | NA |
| Lymph node (g) | Normal | Normal | |||
| Spleen (g) | 79.3 | 25 | 62 | Normal | Small, congested |
| Native kidney (g, left/right) | 47/48 | 2 | 120 | Multiple cysts | Shrunken |
| Transplant kidney (g) | 116 | 80 | Normal | Normal | |
| Thyroid (g) | 4.9 | NA | 7 | ||
| Bone marrow | Normal | Normal | |||
| Vasculature | NA | NA | Diffuse fatty streaks in the aorta and pulmonary arteries | Diffuse fatty streaks in the aorta and pulmonary arteries | |
| Brain (g) | 1100 | 1020 | 1300 | Normal | 1 small old cortical infarction, 2 fresh haemorrhagic cortical infarctions |
| Lungs (g) | 305 | 420 | 1035 | Normal anatomy | Congested |
| Heart (g) | 185 | 120 | 100 | Mild ventricular hypertrophy | Mild ventricular hypertrophy |
| Genitalia | Normal Tanner stage 1 | Normal Tanner stage 5 | |||
| Testes (g, left/right) | 0.94/1.01 | NA | 1.67 | Small | |
| Pituitary | Normal anatomy | Normal anatomy | |||
NA, not available.
*50th centile for a 7.5‐year‐old boy.
†50th centile for a 6.5‐year‐old boy.
‡50th centile for a 4.25‐year‐old boy.
§50th centile for a 5.5‐year‐old boy.
¶Reference weights for a 6‐year‐old boy.
Histopathology of tissues usually affected by SIOD
Skeletal
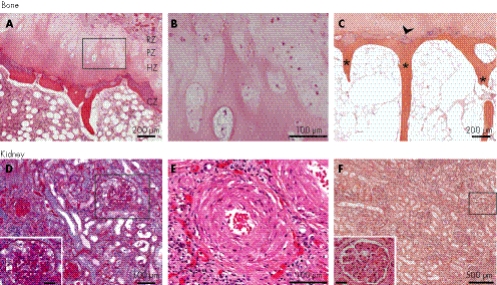
Nearly all individuals with SIOD have vertebral abnormalities and small laterally displaced proximal femoral epiphyses.3,5,9,11 In SD600, all zones of the right femoral growth plate were hypocellular and the growth plate was markedly narrowed, consistent with premature closure. Within the proliferative zone, the chondrocytes were small, irregularly shaped and widely spaced in poorly defined columns (fig 1A,B). Within the hypertrophic zone, there was little ballooning of chondrocytes and only a few small lacunae containing chondrocytes (fig 1C). The zone of calcifying cartilage was abnormally thin, and the trabeculae were fine and delicate (fig 1C). Consistent with the clinical history of hip pain and radiographic findings of degenerative joint disease, the articular cartilage of the proximal femur was eroded, focally lifted and frayed (data not shown).
Figure 1 Bone and renal histopathology. Bone: (A–C) haematoxylin and eosin (H&E) staining of the proximal femoral growth plate of SD600. (A) The growth plate is thin and disorganised, with chondrocyte hypoplasia and poorly formed columns. CZ, calcification zone; HZ, hypertrophic zone; PZ, proliferative zone; RZ, resting zone. (B) Poorly formed columns of chondrocytes in the PZ and HZ. This is a high‐power view of the region outlined in panel A. (C) The calcification zone has few chondrocytes and small lacunae (arrowhead). The ossified bone has thin trabeculae (*). Kidney: (D) Renal cortex from SD600 showing the glomerulosclerosis (inset, bar = 40 μm; periodic acid‐Schiff stain). (E) Renal cortex exhibiting onion skinning of vascular smooth muscle (SD840, H&E stain). (F) Renal cortex from the allograft of SD600 showing normal glomeruli (inset, bar = 40 μm; H&E stain).
In the limited sections of lumbar vertebra available from SD840, the growth plate was closed, and the ossified bone appeared normal. There was no evidence of degenerative joint disease.
Kidney
Nearly all individuals with SIOD develop renal dysfunction. The renal disease begins as proteinuria, evolves into steroid‐resistant nephrotic syndrome and progresses to end‐stage renal failure requiring dialysis or renal transplantation.3,9,11,23 Generally, the pathologist's interpretation of the renal biopsy is FSGS.3,9
The renal tissue from SD600 showed mesangial proliferation with increased periodic acid‐Schiff‐positive mesangial matrix, focal segmental lesions in many glomeruli, tubular atrophy and readily identifiable globally sclerotic glomeruli (fig 1D). The kidney of SD840 exhibited generalised glomerulosclerosis and thickened blood vessel walls with mural onion‐skin formation (fig 1E). These findings and the clinical features of SIOD are probably the consequence of a cell‐autonomous deficiency of SMARCAL1, as the renal allografts had no findings suggestive of recurrent FSGS (fig 1F).
Immune system
Approximately 80% of patients with SIOD have lymphopenia, which is predominantly due to a deficiency of CD4+ T cells, whereas B cell counts and immunoglobin levels are usually normal.3,5 Half of these patients develop recurrent opportunistic infections.3
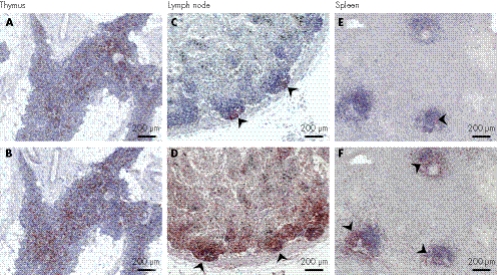
The thymus of SD600 had an age‐appropriate architecture and cellularity (fig 2A,B), but the lymph nodes, spleen and Peyer's patches were strikingly abnormal. As represented by a hilar lymph node (fig 2C,D), the follicles and paracortices were poorly defined and organised, and contained few B and T cells. The splenic B and T cells were sparse and poorly organised within the few remaining follicles (fig 2E,F). The jejunal Peyer's patches had reduced B cells but a normal number of T cells. The ratio of CD4+ to CD8+ T cells was decreased in the thymus (fig 2A,B) and Peyer's patches, but not in the lymph node.
Figure 2 Peripheral lymphoid organ immunohistochemistry. (A,B) The thymus from SD600 has fewer CD4+ (A, anti‐OPD4) than CD8+ cells (B, anti‐CD8). (C,D) A hilar lymph node from SD600 showing depletion of follicular B (C, arrows, anti‐CD20) and T cells and aberrant T cell localisation (D, arrows, anti‐CD3). (E,F) Decreased number of B (E, arrow, anti‐CD20) and T cells (F, arrow, anti‐CD3) in splenic tissue from SD600.
In contrast with SD600, the quantity and distribution of B cells and T cells in the splenic follicles of SD840 were normal. However, the number of T cells in the red pulp and the ratio of CD4+ to CD8+ cells were decreased.
Histopathology of tissues variably affected by SIOD
In addition to spondyloepiphysial dysplasia, renal failure and T cell deficiency, patients with SIOD often manifest hypothyroidism, deficiency of other blood cell lineages, fine hair, tooth abnormalities, corneal opacities and cerebrovascular events.3 The penetrance and expressivity of these features differs even among affected siblings with the same mutations.17
Thyroid
A quarter of patients with SIOD have serological hypothyroidism,3 but neither SD600 nor SD840 had detectable thyroid hormone abnormalities. Consistent with this history, the thyroid from SD600 had normal structure, colloid appearance and variability of follicular size. We were unable to obtain thyroid tissue from SD840.
Haematopoietic system
A third of the patients with SIOD have neutropenia, half have anaemia, and one in ten develop bone marrow failure, often with fatal outcome.3 Despite neutropenia, anaemia and thrombocytopenia, the bone marrow of SD600 was only mildly hypocellular (30–40% cellularity), and showed an appropriate ratio of erythroid to myeloid cells (3:1), a normal percentage of megakaryocytes, and a normal distribution of immature and mature cells in each lineage. The lumbar vertebral bone marrow of SD840 also had normal cellularity (40–50%), lineage ratio, and distribution of immature and mature cells.
Arterial vasculature
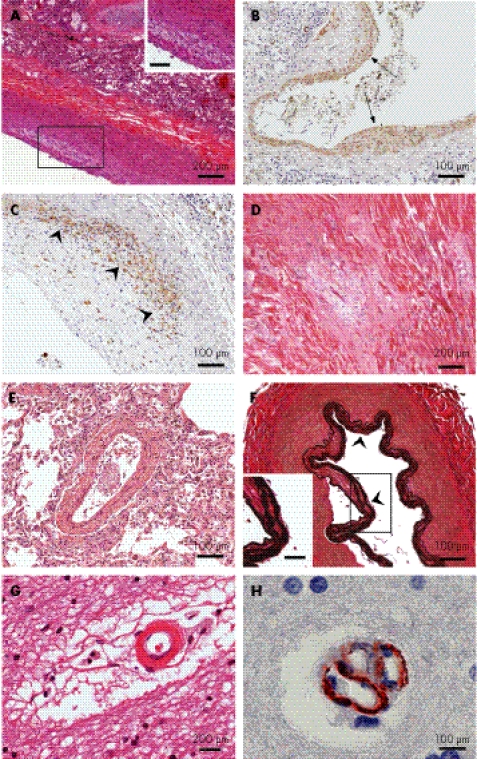
Half of the patients with SIOD experience strokes and TIAs,3,5,7,24 and the clinical signs and neuroimaging studies are suggestive of a mechanism involving atherosclerosis. Both of our patients had many large arteries with atherosclerotic features much in advance of their chronological ages. These features included focal lipid deposition, focal myointimal proliferation, macrophage invasion, foam cells, fibrous transformation and calcium deposits (fig 3A–C). In addition, SD840 had a subclinical inferior left ventricular cardiac infarct (fig 3D).
Figure 3 Histopathology and immunohistochemistry of the arterial vasculature. (A) Pulmonary artery from SD840 showing foam cells in the intima (inset, bar = 100 μm; haematoxylin and eosin (H&E) stain). (B) Focal myointimal proliferation (arrows) within a pulmonary artery wall (SD840, anti‐smooth muscle actin). (C) Macrophage invasion of a pulmonary artery atherosclerotic plaque (SD840, anti‐KP‐1). The region of maximal macrophage infiltration is shown by the arrowheads. (D) Myocardial infarction of the left cardiac ventricular tissue (SD840, H&E stain). (E) Muscular hyperplasia in the wall of a small pulmonary artery (SD840, H&E stain). (F) Diffuse thickening, splitting and fraying of the internal elastic layer (arrowheads) of the basilar artery (SD600, Verhoeff Van Giesson stain. Inset, bar = 100 μm). (G) Cerebral white matter showing an arteriole with a thickened wall, hyaline deposits and surrounding filamentous connective tissue (SD600, H&E stain). (H) Clustering of small intracerebral blood vessels (SD840, anti‐smooth muscle actin).
The pulmonary and systemic arteries from our two patients also exhibited changes consistent with pulmonary and systemic hypertension. The large and mid‐sized pulmonary arteries had a hyperplastic lamina muscularis (fig 3E), and many of the mid‐sized and small pulmonary arteries showed focal thickening, splitting and fraying of the internal elastic layer. The abdominal aorta had increased elastic fibres throughout the intima and media, and a flattened endothelium. The coronary arteries showed intimal hyperplasia. Cerebral arteries exhibited focal intimal hyperplasia with thickening and splitting of the internal elastic layer (fig 3F). In both brains, the smaller vessels throughout the white matter had focally thick walls with hyaline deposits. They were surrounded by a filamentous connective tissue in the Virchow–Robin spaces (fig 3G).
SD840 had clusters of small intracerebral vessels (fig 3H), distinct from recanalisation of thrombi. They may reflect either ectasia and folding of the vessel walls or an aberrant angiogenic response. Moreover, this pathological feature may account for the moyamoya phenomenon reported in some patients with SIOD.22
Brain
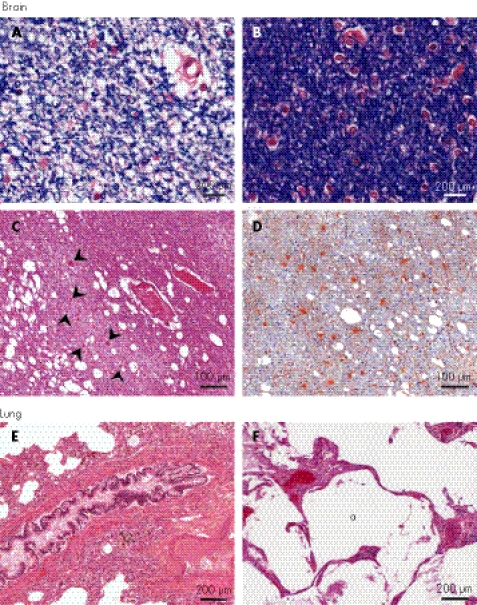
As half of the patients with SIOD develop signs and symptoms of cerebral ischaemia,3,7,13,22 we examined both brains for evidence of acute and remote hypoxic ischaemic injury. To identify brain lesions representative of SIOD rather than sequelae of the terminal cardiac arrest, we focused our analyses on the temporally remote lesions. The cerebral and cerebellar white matter in SD600 had many small areas of poor myelin staining containing rare swollen axons and some gliosis (fig 4A,B). In the older patient SD840, the white matter changes had evolved to an obvious decrease in myelin interspersed with lacunar infarcts and extensive gliosis (fig 4C,D). In addition, SD840 had moderate gliosis and neuronal loss in the cerebral and insular cortex and in the basal ganglia (data not shown). Both of these subcortical and cortical changes are associated with microcirculatory disease and accentuated by hypertension. Also indicative of larger vessel disease and varying degrees of ischaemic insult,26 we identified lesions of selective neuronal death in the anterior nucleus of the thalamus of SD600 and of focal pancortical necrosis in SD840 (data not shown).
Figure 4 Histopathology and immunohistochemistry of the brain and lung. Brain: Focal paucity of myelin in the cerebral white matter from SD600, as shown by reduced (A) and normal (B) luxol fast blue staining. (C) Lacunar infarction with swollen axons (arrowheads) in the white matter (SD840, haematoxylin and eosin (H&E) stain). (D) Gliosis in the infarcted white matter (SD840, anti‐glial fibrillary acidic protein). Lungs: (E) Bronchial smooth muscle hyperplasia (SD600, H&E stain). (F) Dilated airspaces (a) in the lung (SD840, H&E stain).
Lungs
A few patients with SIOD have died from pulmonary disease including pulmonary hypertension.24 SD600 had diffuse bronchial smooth muscle hypertrophy (fig 4E), extensive vascular congestion, intra‐alveolar haemosiderin‐laden macrophages and a few dilated airspaces. SD840 had moderate oedema, panlobular emphysema, and fibrin and hyaline membranes lining the emphysematous airways and remaining alveoli (fig 4F). These primary lung abnormalities including emphysematous changes are the first documented among patients with SIOD. They may require more directive evaluation during life and earlier therapeutic intervention.
Histopathology of tissues reportedly unaffected by SIOD
Our studies in mice showed that the Smarcal1 protein is expressed in several tissues hitherto not reported as clinically affected. These tissues include peripheral and central neurones, the anterior pituitary secretory cells, cardiac and skeletal myocytes, exocrine pancreatic cells, oocytes, and spermatocytes.25 If human SMARCAL1 expression is similar and if it is necessary for the development and maintenance of these cells, then we hypothesise that these cells will manifest pathology in patients with SIOD. Consistent with this hypothesis, we identified changes in postmortem tissue from the testes, heart and anterior pituitary.
Testes
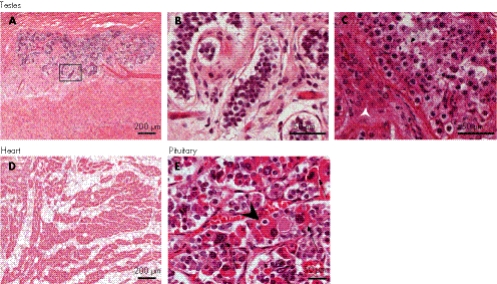
Most patients with SIOD die before sexual maturity,3 and those who have survived into adulthood have not reproduced. However, they do develop secondary sexual characteristics including a menstrual cycle in females (CFB, unpublished). Microscopically, the testes of SD600 had significant interstitial fibrosis, with only a few rudimentary seminiferous tubules and no identifiable spermatogonia, spermatocytes, spermatids or spermatozoa (fig 5A,B). Although less severely affected, the testes of SD840 had diffuse interstitial fibrosis and hypoplasia of cells in all stages of spermatogenesis. Interestingly, SD840 also had Leydig cell hyperplasia (fig 5C, arrowhead), suggesting a possible defect in androgen responsiveness despite normal secondary sexual characteristics.
Figure 5 Histopathology of the testes, heart and pituitary. Testes: (A) interstitial fibrosis and immature seminiferous tubules (SD600, haematoxylin and eosin (H&E) stain). (B) Absence of all stages of spermatogenesis in the seminiferous tubules of SD600. This is a higher magnification of the area outlined in panel A. (C) Paucity of spermatids (arrow) and interstitial Leydig cell hyperplasia (arrowhead; SD840, H&E stain). Heart: (D) right cardiac ventricular tissue from SD600 showing adipose tissue interspersed diffusely between the cardiac myocytes (H&E stain). Pituitary: (E) H&E staining of tissue from the anterior pituitary of SD600. Note the cells with hyperplastic distorted nuclei (big arrows) and structures suggestive of emperipolesis (arrowhead). One cell also appears necrotic (small arrow).
Heart
The ventricular walls of SD840 and SD600 were mildly thickened. The myocytes were mildly to moderately hypertrophic. The free right ventricular wall of SD600 also contained extensive adipose tissue interspersed diffusely between the cardiac myocytes (fig 5D). This intramural adipose tissue was continuous with the external adipose tissue and extended to the subendothelial layer, a finding observed in arrhythmogenic right ventricular dysplasia.27 This suggests that SIOD may cause a defect in right ventricular heart wall development and in cardiomyocyte differentiation. It is consistent with Smarcal1 expression during murine embryonic heart development.25
Pituitary
With the exception of hypothyroidism, endocrine abnormalities are rare in SIOD.3 A few patients are reported to have abnormal growth hormonal responses and circulating cortisol levels. The gross architecture of the anterior and posterior pituitary in each of our patients was normal. Except for mildly reduced human gonadotrophic hormone immunoreactivity in SD600, both pituitaries showed normal patterns of immunoreactivity for prolactin, adrenocorticotropic hormone, growth hormone, human gonadotrophic hormone and luteinising hormone. Both had scattered somatotrophs, lactotrophs, corticotrophs and gonadotrophs with hyperplastic distorted nuclei and others with phagocytosed debris reminiscent of emperipolesis (fig 5E). A few cells appeared necrotic. Hence, despite the normal morphological features in the anterior pituitary, findings in both patients point to an abnormality at the level of the individual cells, adversely affecting the size and biological function.
Discussion
Mutations in SMARCAL1, which encodes a putative chromatin remodelling enzyme, cause SIOD.19 However, the histopathological features associated with this molecular defect have remained undefined until the report of these results. The pathogenesis of SIOD is largely unknown, the various hypotheses offered previously on the subject notwithstanding. We report histopathological findings in support of each one of the pathophysiological mechanisms proposed, except the one on autoimmunity. The disorganised and fragmented arterial internal elastic lamina and the myointimal hyperplasia and premature atherosclerosis are consistent with a connective tissue disorder16,22,23 and a vascular endothelial disorder,7,8,24 respectively. Observations in support of defective cellular differentiation5,16 and proliferation3,19 include the azospermia and the paucity of growth plate chondrocytes and peripheral lymphocytes.
Disease of a cell can arise from within—that is, by a cell‐autonomous mechanism—or be imposed from outside—that is, by a cell‐non‐autonomous mechanism. Glomerular disease did not recur in the renal allograft of SD600 or SD840, supporting a cell‐autonomous mechanism. Also, Lücke et al24 recently showed that atherosclerosis did not recur within the renal allograft during 18 years after transplantation.
The pleiotropic manifestations of SIOD likely reflect both loss of cell‐autonomous SMARCAL1 function and cell‐non‐autonomous consequences. For example, as most patients with SIOD have a normal circulating B cell number, normal in vitro proliferation of B cells, and normal plasma IgA and IgM levels,3 those patients with SIOD with reduced circulating B cells or poor B cell immune responses were generally considered to have a cell‐non‐autonomous deficiency arising from T cell‐dependent B cell activation. However, murine B cells express Smarcal1 and we have shown that SD600 had a B cell deficiency more severe than that observed with absent or diminished T cell‐dependent B cell responses.28 Thus, as SD600 was not treated with high‐dose steroids, we hypothesise that these findings are a manifestation of an intrinsic B cell defect as well as of impaired T cell activation. This hypothesis would explain why some patients with SIOD have recurrent encapsulated bacterial infections, reduced responses to B cell specific antigens, and low plasma IgG levels.3 Also, these observations are the first evidence for combined immunodeficiency in SIOD, and these patients should be evaluated and treated accordingly.
Functional and structural cardiac defects have not been reported for SIOD even though murine Smarcal1 is highly expressed in the developing heart.25 However, SD600 had extensive fatty infiltration resembling that of arrhythmogenic right ventricular cardiomyopathy (ARVCM).27 The fatty infiltration in ARVCM involves the outer two thirds of the myocardium, and spares the right ventricular trabeculations, the ventricular septum and the left ventricular free wall. ARVCM fatty infiltration has been attributed to cardiomyocyte developmental defects and to adipocyte replacement of degenerating myocytes after an inflammatory insult.29 However, we did not observe inflammatory changes in SD600. Lesser degrees of fatty infiltration are also observed with aeging,30 but we consider premature aeging an unlikely aetiology, as we did not see histopathological signs of senescence in this and other tissues. Therefore, as murine Smarcal1 is exclusively expressed in developing murine cardiomyocytes, we propose that this fatty infiltration reflects the loss of SMARCAL1 function in the differentiation of right ventricular cardiomyocytes.
The brains of both of our patients had cortical and subcortical ischaemic lesions similar to those observed with vascular cognitive impairment.31 The pathology was most pronounced in the subcortical white matter, and closely resembled aspects of cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy (CADASIL) and Binswanger's disease.31 CADASIL is characterised by migraines with aura and recurrent strokes causing psychiatric symptoms, progressive cognitive decline, dementia and death32; these symptoms have been observed in some patients with SIOD (CFB, unpublished).12 NOTCH3, the mutant gene associated with CADASIL,33 encodes a transmembrane receptor regulating the differentiation of vascular smooth muscle cells. As SMARCAL1 is also expressed in vascular endothelial and smooth muscle cells, it might also regulate vascular differentiation and integrity.
SMARCAL1 encodes a putative SNF2 chromatin‐remodelling factor.20,21 SNF2 chromatin remodelling factors participate in the replication, recombination, repair, methylation and transcription of DNA.34 Patients with SIOD do not exhibit hypersensitivity to ultraviolet radiation, genomic instability, increased cancer incidence or defective DNA repair after exposure to γ radiation.3 These results suggest that SMARCAL1 is not a regulator of DNA replication, repair or recombination. Rather, to explain the defects of cellular proliferation and differentiation observed in this and previous studies of SIOD, we hypothesise that SMARCAL1 regulates gene expression.
In this model, the loss of SMARCAL1 could cause azospermia and Leydig cell hyperplasia by impairing the transcriptional response to androgens.35,36 Moreover, as transcription programmes underlie cellular proliferation as well as development and ageing, changes in transcription arising from loss of SMARCAL1 could account for the defective pituitary cell biology, the errant differentiation of mesenchymal cells to adipocytes rather than cardiomyocytes, the development of atherosclerosis and impaired proliferation of lymphocytes and chondrocytes.
SMARCAL1 regulation of transcription would also explain the intrafamilial and interfamilial variabilities in expressivity of SIOD. By regulation of a quantitative cellular function such as transcription, phenotypic expression occurs only when the environment, stochastics, and genetic or epigenetic background further impair this function beyond a tissue‐specific or a cell‐specific threshold.37 However, identification of a role for SMARCAL1 in modulating such a quantitative trait requires further investigation.
In summary, this study shows that loss of SMARCAL1 affects both cellular proliferation and differentiation and promotes vascular homoeostasis. These results suggest that SIOD will be a useful model for studying atherosclerosis, stroke, a new form of combined immunodeficiency, and right ventricular dysplasia cardiomyopathy. Our findings also highlight that some patients with SIOD may be at risk of pulmonary hypertension, combined immunodeficiency, subcortical ischaemic dementia and cardiac dysfunction. Further evaluation and treatment of these problems may be warranted.
Acknowledgements
We thank Howard Meyerson, Jennifer L Northrop and Millan Patel for their critical review of this manuscript. This work was supported in part by grants from the National Institute of Diabetes, Digestive and Kidney Diseases, NIH (CFB, DLA), the March of Dimes (CFB), the Gillson Longenbaugh Foundation (CFB), the Dana Foundation (CFB) and the New Development Award, Microscopy, and Administrative Cores of the Mental Retardation and Developmental Disabilities Research Center at Baylor College of Medicine (CFB, DLA).
Abbreviations
ARVCM - arrhythmogenic right ventricular cardiomyopathy
CADASIL - cerebral autosomal dominant arteriopathy with subcortical infarcts and leucoencephalopathy
FSGS - focal segmental glomerulosclerosis
H&E - haematoxylin and eosin
SIOD - Schimke immuno‐osseous dysplasia
SMARCAL1 - swi/snf‐related matrix‐associated actin‐dependent regulator of chromatin, subfamily a‐like 1
TIA - transient ischaemic attack
Footnotes
Published Online First 13 July 2006
References
- 1.Hall C M. International nosology and classification of constitutional disorders of bone (2001). Am J Med Genet 200211365–77. [DOI] [PubMed] [Google Scholar]
- 2.Ming J E, Stiehm E R, Graham J M., Jr Syndromes associated with immunodeficiency. Adv Pediatr 199946271–351. [PubMed] [Google Scholar]
- 3.Boerkoel C F, O'Neill S, Andre J L, Benke P J, Bogdanovic R, Bulla M, Burguet A, Cockfield S, Cordeiro I, Ehrich J H, Frund S, Geary D F, Ieshima A, Illies F, Joseph M W, Kaitila I, Lama G, Leheup B, Ludman M D, McLeod D R, Medeira A, Milford D V, Ormala T, Rener‐Primec Z, Santava A, Santos H G, Schmidt B, Smith G C, Spranger J, Zupancic N, Weksberg R. Manifestations and treatment of Schimke immuno‐osseous dysplasia: 14 new cases and a review of the literature. Eur J Pediatr 20001591–7. [DOI] [PubMed] [Google Scholar]
- 4.Saraiva J M, Dinis A, Resende C, Faria E, Gomes C, Correia A J, Gil J, da Fonseca N. Schimke immuno‐osseous dysplasia: case report and review of 25 patients. J Med Genet 199936786–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spranger J, Hinkel G K, Stoss H, Thoenes W, Wargowski D, Zepp F. Schimke immuno‐osseous dysplasia: a newly recognized multisystem disease. J Pediatr 1991119(Part 1)64–72. [DOI] [PubMed] [Google Scholar]
- 6.Lücke T, Franke D, Clewing J M, Boerkoel C F, Ehrich J H H, Das A M, Živičnjak M. Schimke versus non‐Schimke chronic kidney disease: an anthropometric approach. Pediatrics 2006118(2)e400–e407. [DOI] [PubMed] [Google Scholar]
- 7.Ehrich J H, Burchert W, Schirg E, Krull F, Offner G, Hoyer P F, Brodehl J. Steroid resistant nephrotic syndrome associated with spondyloepiphyseal dysplasia, transient ischemic attacks and lymphopenia. Clin Nephrol 19954389–95. [PubMed] [Google Scholar]
- 8.Ehrich J H, Filler G. A child with nephrotic syndrome and with focal and segmental glomerulosclerosis: do we have to care about associated malformations? Nephrol Dial Transplant 1996112096–2098. [DOI] [PubMed] [Google Scholar]
- 9.Ehrich J H, Offner G, Schirg E, Hoyer P F, Helmchen U, Brodehl J. Association of spondylo‐epiphyseal dysplasia with nephrotic syndrome. Pediatr Nephrol 19904117–121. [DOI] [PubMed] [Google Scholar]
- 10.Ehrich J H H, Offner G, Schirg E, Helmchen U, Brodehl J. Minderwuchssyndrom mit skelettdyplasien und focal segmental sklerosierender glomerulonephritis. Monatsschr Kinderheilkd 19881366 [Google Scholar]
- 11.Schimke R N, Horton W A, King C R. Chondroitin‐6‐sulphaturia, defective cellular immunity, and nephrotic syndrome. Lancet 197121088–1089. [DOI] [PubMed] [Google Scholar]
- 12.Kilic S S, Donmez O, Sloan E A, Elizondo L I, Huang C, Andre J L, Bogdanovic R, Cockfield S, Cordeiro I, Deschenes G, Frund S, Kaitila I, Lama G, Lamfers P, Lucke T, Milford D V, Najera L, Rodrigo F, Saraiva J M, Schmidt B, Smith G C, Stajic N, Stein A, Taha D, Wand D, Armstrong D, Boerkoel C F. Association of migraine‐like headaches with Schimke immuno‐osseous dysplasia. Am J Med Genet A 2005135206–210. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt B, Christen H J, Herkenrath P, Benz‐Bohm G, Muller‐Berghaus J, Querfeld U. Cerebral complications in Schimke immuno‐osseous dysplasia. Eur J Pediatr 1997156789–791. [DOI] [PubMed] [Google Scholar]
- 14.Kaitila I, Savilahti E, Ormala T. Autoimmune enteropathy in Schimke immunoosseous dysplasia. Am J Med Genet 199877427–430. [PubMed] [Google Scholar]
- 15.Hashimoto K, Takeuchi A, Ieshima A, Takada M, Kasagi M. Juvenile variant of Schimke immunoosseous dysplasia. Am J Med Genet 199449266–269. [DOI] [PubMed] [Google Scholar]
- 16.Lama G, Marrone N, Majorana M, Cirillo F, Salsano M E, Rinaldi M M. Spondyloepiphyseal dysplasia tarda and nephrotic syndrome in three siblings. Pediatr Nephrol 1995919–23. [DOI] [PubMed] [Google Scholar]
- 17.Lücke T, Billing H, Sloan E A, Boerkoel C F, Franke D, Zimmering M, Ehrich J H, Das A M. Schimke‐immuno‐osseous dysplasia: new mutation with weak genotype‐phenotype correlation in siblings. Am J Med Genet A 2005135202–205. [DOI] [PubMed] [Google Scholar]
- 18.Lou S, Lamfers P, McGuire N, Boerkoel C F. Longevity in Schimke immuno‐osseous dysplasia. J Med Genet 200239922–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boerkoel C F, Takashima H, John J, Yan J, Stankiewicz P, Rosenbarker L, Andre J L, Bogdanovic R, Burguet A, Cockfield S, Cordeiro I, Frund S, Illies F, Joseph M, Kaitila I, Lama G, Loirat C, McLeod D R, Milford D V, Petty E M, Rodrigo F, Saraiva J M, Schmidt B, Smith G C, Spranger J, Stein A, Thiele H, Tizard J, Weksberg R, Lupski J R, Stockton D W. Mutant chromatin remodeling protein SMARCAL1 causes Schimke immuno‐osseous dysplasia. Nat Genet 200230215–220. [DOI] [PubMed] [Google Scholar]
- 20.Coleman M A, Eisen J A, Mohrenweiser H W. Cloning and characterization of HARP/SMARCAL1: a prokaryotic HepA‐related SNF2 helicase protein from human and mouse. Genomics 200065274–282. [DOI] [PubMed] [Google Scholar]
- 21.Muthuswami R, Truman P A, Mesner L D, Hockensmith J W. A eukaryotic SWI2/SNF2 domain, an exquisite detector of double‐stranded to single‐stranded DNA transition elements. J Biol Chem 20002757648–7655. [DOI] [PubMed] [Google Scholar]
- 22.Boerkoel C F, Nowaczyk M J, Blaser S I, Meschino W S, Weksberg R. Schimke immunoosseous dysplasia complicated by moyamoya phenomenon. Am J Med Genet 199878118–122. [DOI] [PubMed] [Google Scholar]
- 23.Ludman M D, Cole D E, Crocker J F, Cohen M M., Jr Schimke immuno‐osseous dysplasia: case report and review. Am J Med Genet 199347793–796. [DOI] [PubMed] [Google Scholar]
- 24.Lücke T, Marwedel K M, Kanzelmeyer N K, Hori A, Offner G, Kreipe H H, Ehrich J H, Das A M. Generalized atherosclerosis sparing the transplanted kidney in Schimke disease. Pediatr Nephrol 200419672–675. [DOI] [PubMed] [Google Scholar]
- 25.Elizondo L I, Huang C, Northrop J L, Deguchi K, Clewing J M, Armstrong D L, Boerkoel C F. Schimke immuno‐osseous dysplasia: a cell autonomous disorder? Am J Med Genet A 2006140340–348. [DOI] [PubMed] [Google Scholar]
- 26.Auer R N, Sutherland G R. Hypoxia and related conditions. In: Graham DI, Lantos PL, eds. Greenfield's neuropathology. 7th edn. Vol 1. London: Hodder Arnold, 2002233–280.
- 27.Fornes P, Ratel S, Lecomte D. Pathology of arrhythmogenic right ventricular cardiomyopathy/dysplasia—an autopsy study of 20 forensic cases. J Forensic Sci 199843777–783. [PubMed] [Google Scholar]
- 28.Lougaris V, Badolato R, Ferrari S, Plebani A. Hyper immunoglobulin M syndrome due to CD40 deficiency: clinical, molecular, and immunological features. Immunol Rev 200520348–66. [DOI] [PubMed] [Google Scholar]
- 29.Gemayel C, Pelliccia A, Thompson P D. Arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol 2001381773–1781. [DOI] [PubMed] [Google Scholar]
- 30.Burke A P, Farb A, Tashko G, Virmani R. Arrhythmogenic right ventricular cardiomyopathy and fatty replacement of the right ventricular myocardium: are they different diseases? Circulation 1998971571–1580. [DOI] [PubMed] [Google Scholar]
- 31.Jellinger K A. Pathology and pathophysiology of vascular cognitive impairment. A critical update. Panminerva Med 200446217–226. [PubMed] [Google Scholar]
- 32.Kalimo H, Ruchoux M M, Viitanen M, Kalaria R N. CADASIL: a common form of hereditary arteriopathy causing brain infarcts and dementia. Brain Pathol 200212371–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, Alamowitch S, Domenga V, Cecillion M, Marechal E, Maciazek J, Vayssiere C, Cruaud C, Cabanis E A, Ruchoux M M, Weissenbach J, Bach J F, Bousser M G, Tournier‐Lasserve E. Notch3 mutations in CADASIL, a hereditary adult‐onset condition causing stroke and dementia. Nature 1996383707–710. [DOI] [PubMed] [Google Scholar]
- 34.Cho K S, Elizondo L I, Boerkoel C F. Advances in chromatin remodeling and human disease. Curr Opin Genet Dev 200414308–315. [DOI] [PubMed] [Google Scholar]
- 35.Kotula‐Balak M, Bablok L, Fracki S, Jankowska A, Bilinska B. Immunoexpression of androgen receptors and aromatase in testes of patient with Klinefelter's syndrome. Folia Histochem Cytobiol 200442215–220. [PubMed] [Google Scholar]
- 36.Singh R, Shastry P K, Rasalkar A A, Singh L, Thangaraj K. A novel androgen receptor mutation resulting in complete androgen insensitivity syndrome and bilateral Leydig cell hyperplasia. J Androl 200627785–789. [DOI] [PubMed] [Google Scholar]
- 37.Rutherford S L, Henikoff S. Quantitative epigenetics. Nat Genet 2003336–8. [DOI] [PubMed] [Google Scholar]