Abstract
The 34-kilodalton (kDa) antigen of Treponema pallidum subsp. pallidum (T. pallidum) is a pathogen-specific integral membrane protein. DNA sequence analysis of the cloned gene revealed an open reading frame encoding a primary product of 204 residues with a molecular mass of 22,087 daltons. Sequences that correspond to a consensus Escherichia coli promoter and a ribosome-binding site were found upstream from the AUG start codon that begins the open reading frame, suggesting that the cloned gene can use its own regulatory sequences for expression. Examination of the deduced amino acid sequence revealed the presence of a typical procaryotic leader peptide 19 amino acids long; processing results in a mature molecule with a molecular mass of 20,123 daltons. Pulse-chase experiments with E. coli minicells confirmed that the 34-kDa antigen is synthesized as a higher-molecular-weight precursor that is processed to a mature form with the electrophoretic mobility that is characteristic for this protein. The presence in the leader peptide of the sequence Phe-Ser-Ala-Cys suggested that the 34-kDa antigen is a proteolipid. Although hydropathy analysis of the deduced amino acid sequence of the mature 34-kDa antigen predicted that the molecule was primarily hydrophilic, both the native and recombinant 34-kDa molecules displayed hydrophobic biochemical behavior by fractionating into the detergent phase after extraction of intact organisms with Triton X-114. Cell fractionation experiments with E. coli showed that the 34-kDa molecule was localized in both the inner and outer membranes of the recombinant host. The combined data demonstrate that the 34-kDa antigen is an integral membrane protein that behaves in a biochemically consistent manner in both T. pallidum and E. coli.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehr W., Gotschlich E. C., Hitchcock P. J. The virulence-associated gonococcal H.8 gene encodes 14 tandemly repeated pentapeptides. Mol Microbiol. 1989 Jan;3(1):49–55. doi: 10.1111/j.1365-2958.1989.tb00103.x. [DOI] [PubMed] [Google Scholar]
- Chamberlain N. R., Brandt M. E., Erwin A. L., Radolf J. D., Norgard M. V. Major integral membrane protein immunogens of Treponema pallidum are proteolipids. Infect Immun. 1989 Sep;57(9):2872–2877. doi: 10.1128/iai.57.9.2872-2877.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain N. R., Radolf J. D., Hsu P. L., Sell S., Norgard M. V. Genetic and physicochemical characterization of the recombinant DNA-derived 47-kilodalton surface immunogen of Treponema pallidum subsp. pallidum. Infect Immun. 1988 Jan;56(1):71–78. doi: 10.1128/iai.56.1.71-78.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Henning U. Nucleotide sequence of the gene for the peptidoglycan-associated lipoprotein of Escherichia coli K12. Eur J Biochem. 1987 Feb 16;163(1):73–77. doi: 10.1111/j.1432-1033.1987.tb10738.x. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Cover W. H., Ryan J. P., Bassford P. J., Jr, Walsh K. A., Bollinger J., Randall L. L. Suppression of a signal sequence mutation by an amino acid substitution in the mature portion of the maltose-binding protein. J Bacteriol. 1987 May;169(5):1794–1800. doi: 10.1128/jb.169.5.1794-1800.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
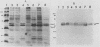
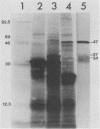
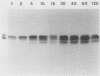
- Cunningham T. M., Walker E. M., Miller J. N., Lovett M. A. Selective release of the Treponema pallidum outer membrane and associated polypeptides with Triton X-114. J Bacteriol. 1988 Dec;170(12):5789–5796. doi: 10.1128/jb.170.12.5789-5796.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas W. S., Ray P. H., Leong J., Benedict C. D., Stamm L. V., Bassford P. J., Jr Identification and purification of a recombinant Treponema pallidum basic membrane protein antigen expressed in Escherichia coli. Infect Immun. 1987 May;55(5):1106–1115. doi: 10.1128/iai.55.5.1106-1115.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehring R., Beyreuther K., Wright J. K., Overath P. In vitro and in vivo products of E. coli lactose permease gene are identical. Nature. 1980 Feb 7;283(5747):537–540. doi: 10.1038/283537a0. [DOI] [PubMed] [Google Scholar]
- Emini E. A., Hughes J. V., Perlow D. S., Boger J. Induction of hepatitis A virus-neutralizing antibody by a virus-specific synthetic peptide. J Virol. 1985 Sep;55(3):836–839. doi: 10.1128/jvi.55.3.836-839.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehniger T. E., Radolf J. D., Walfield A. M., Cunningham T. M., Miller J. N., Lovett M. A. Native surface association of a recombinant 38-kilodalton Treponema pallidum antigen isolated from the Escherichia coli outer membrane. Infect Immun. 1986 May;52(2):586–593. doi: 10.1128/iai.52.2.586-593.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehniger T. E., Walfield A. M., Cunningham T. M., Radolf J. D., Miller J. N., Lovett M. A. Purification and characterization of a cloned protease-resistant Treponema pallidum-specific antigen. Infect Immun. 1984 Nov;46(2):598–607. doi: 10.1128/iai.46.2.598-607.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. B., Pedersen P. E., Schouls L. M., Severin E., van Embden J. D. Genetic characterization and partial sequence determination of a Treponema pallidum operon expressing two immunogenic membrane proteins in Escherichia coli. J Bacteriol. 1985 Jun;162(3):1227–1237. doi: 10.1128/jb.162.3.1227-1237.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley C. B., Reynolds R. P. Analysis of E. coli promoter sequences. Nucleic Acids Res. 1987 Mar 11;15(5):2343–2361. doi: 10.1093/nar/15.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindersson P., Cockayne A., Schouls L. M., van Emden J. D. Immunochemical characterization and purification of Treponema pallidum antigen TpD expressed by Escherichia coli K12. Sex Transm Dis. 1986 Oct-Dec;13(4):237–244. doi: 10.1097/00007435-198610000-00006. [DOI] [PubMed] [Google Scholar]
- Hsu P. L., Chamberlain N. R., Orth K., Moomaw C. R., Zhang L. Q., Slaughter C. A., Radolf J. D., Sell S., Norgard M. V. Sequence analysis of the 47-kilodalton major integral membrane immunogen of Treponema pallidum. Infect Immun. 1989 Jan;57(1):196–203. doi: 10.1128/iai.57.1.196-203.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. A., Marchitto K. S., Miller J. N., Norgard M. V. Monoclonal antibody with hemagglutination, immobilization, and neutralization activities defines an immunodominant, 47,000 mol wt, surface-exposed immunogen of Treponema pallidum (Nichols). J Exp Med. 1984 Nov 1;160(5):1404–1420. doi: 10.1084/jem.160.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
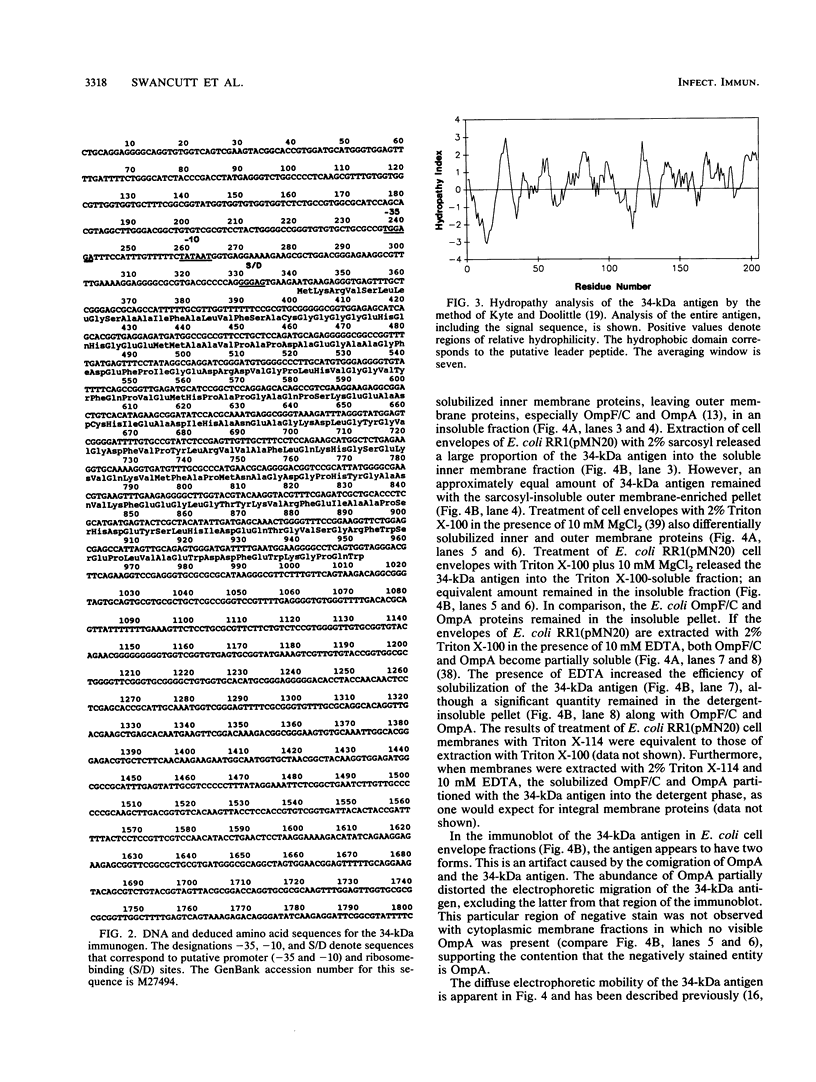
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Maley F., Guarino D. U. Differential binding of sodium dodecyl sulfate to amino acids as evidenced by elution from Sephadex. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1425–1430. doi: 10.1016/s0006-291x(77)80138-1. [DOI] [PubMed] [Google Scholar]
- Marchitto K. S., Jones S. A., Schell R. F., Holmans P. L., Norgard M. V. Monoclonal antibody analysis of specific antigenic similarities among pathogenic Treponema pallidum subspecies. Infect Immun. 1984 Sep;45(3):660–666. doi: 10.1128/iai.45.3.660-666.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgard M. V., Chamberlain N. R., Swancutt M. A., Goldberg M. S. Cloning and expression of the major 47-kilodalton surface immunogen of Treponema pallidum in Escherichia coli. Infect Immun. 1986 Nov;54(2):500–506. doi: 10.1128/iai.54.2.500-506.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Orndorff P. E., Falkow S. Nucleotide sequence of pilA, the gene encoding the structural component of type 1 pili in Escherichia coli. J Bacteriol. 1985 Apr;162(1):454–457. doi: 10.1128/jb.162.1.454-457.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn C. W., Cockayne A., Bailey M. J. The outer membrane of Treponema pallidum: biological significance and biochemical properties. J Gen Microbiol. 1985 Sep;131(9):2349–2357. doi: 10.1099/00221287-131-9-2349. [DOI] [PubMed] [Google Scholar]
- Queen C., Korn L. J. A comprehensive sequence analysis program for the IBM personal computer. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):581–599. doi: 10.1093/nar/12.1part2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf J. D., Chamberlain N. R., Clausell A., Norgard M. V. Identification and localization of integral membrane proteins of virulent Treponema pallidum subsp. pallidum by phase partitioning with the nonionic detergent triton X-114. Infect Immun. 1988 Feb;56(2):490–498. doi: 10.1128/iai.56.2.490-498.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf J. D., Fehniger T. E., Silverblatt F. J., Miller J. N., Lovett M. A. The surface of virulent Treponema pallidum: resistance to antibody binding in the absence of complement and surface association of recombinant antigen 4D. Infect Immun. 1986 May;52(2):579–585. doi: 10.1128/iai.52.2.579-585.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf J. D., Norgard M. V. Pathogen specificity of Treponema pallidum subsp. pallidum integral membrane proteins identified by phase partitioning with Triton X-114. Infect Immun. 1988 Jul;56(7):1825–1828. doi: 10.1128/iai.56.7.1825-1828.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf J. D., Norgard M. V., Schulz W. W. Outer membrane ultrastructure explains the limited antigenicity of virulent Treponema pallidum. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2051–2055. doi: 10.1073/pnas.86.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson S. M., Kettman J. R., Miller J. N., Norgard M. V. Murine monoclonal antibodies specific for virulent Treponema pallidum (Nichols). Infect Immun. 1982 Jun;36(3):1076–1085. doi: 10.1128/iai.36.3.1076-1085.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Effect of ethylenediaminetetraacetic acid, Triton X-100, and lysozyme on the morphology and chemical composition of isolate cell walls of Escherichia coli. J Bacteriol. 1971 Oct;108(1):553–563. doi: 10.1128/jb.108.1.553-563.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol. 1971 Oct;108(1):545–552. doi: 10.1128/jb.108.1.545-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm L. V., Hodinka R. L., Wyrick P. B., Bassford P. J., Jr Changes in the cell surface properties of Treponema pallidum that occur during in vitro incubation of freshly extracted organisms. Infect Immun. 1987 Sep;55(9):2255–2261. doi: 10.1128/iai.55.9.2255-2261.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm L. V., Kerner T. C., Jr, Bankaitis V. A., Bassford P. J., Jr Identification and preliminary characterization of Treponema pallidum protein antigens expressed in Escherichia coli. Infect Immun. 1983 Aug;41(2):709–721. doi: 10.1128/iai.41.2.709-721.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swancutt M. A., Twehous D. A., Norgard M. V. Monoclonal antibody selection and analysis of a recombinant DNA-derived surface immunogen of Treponema pallidum expressed in Escherichia coli. Infect Immun. 1986 Apr;52(1):110–119. doi: 10.1128/iai.52.1.110-119.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walfield A. M., Hanff P. A., Lovett M. A. Expression of Treponema pallidum antigens in Escherichia coli. Science. 1982 Apr 30;216(4545):522–523. doi: 10.1126/science.7041257. [DOI] [PubMed] [Google Scholar]
- Yu F., Inouye S., Inouye M. Lipoprotein-28, a cytoplasmic membrane lipoprotein from Escherichia coli. Cloning, DNA sequence, and expression of its gene. J Biol Chem. 1986 Feb 15;261(5):2284–2288. [PubMed] [Google Scholar]
- van Embden J. D., van der Donk H. J., van Eijk R. V., van der Heide H. G., de Jong J. A., van Olderen M. F., Osterhaus A. B., Schouls L. M. Molecular cloning and expression of Treponema pallidum DNA in Escherichia coli K-12. Infect Immun. 1983 Oct;42(1):187–196. doi: 10.1128/iai.42.1.187-196.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Analysis of the distribution of charged residues in the N-terminal region of signal sequences: implications for protein export in prokaryotic and eukaryotic cells. EMBO J. 1984 Oct;3(10):2315–2318. doi: 10.1002/j.1460-2075.1984.tb02132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]