Abstract
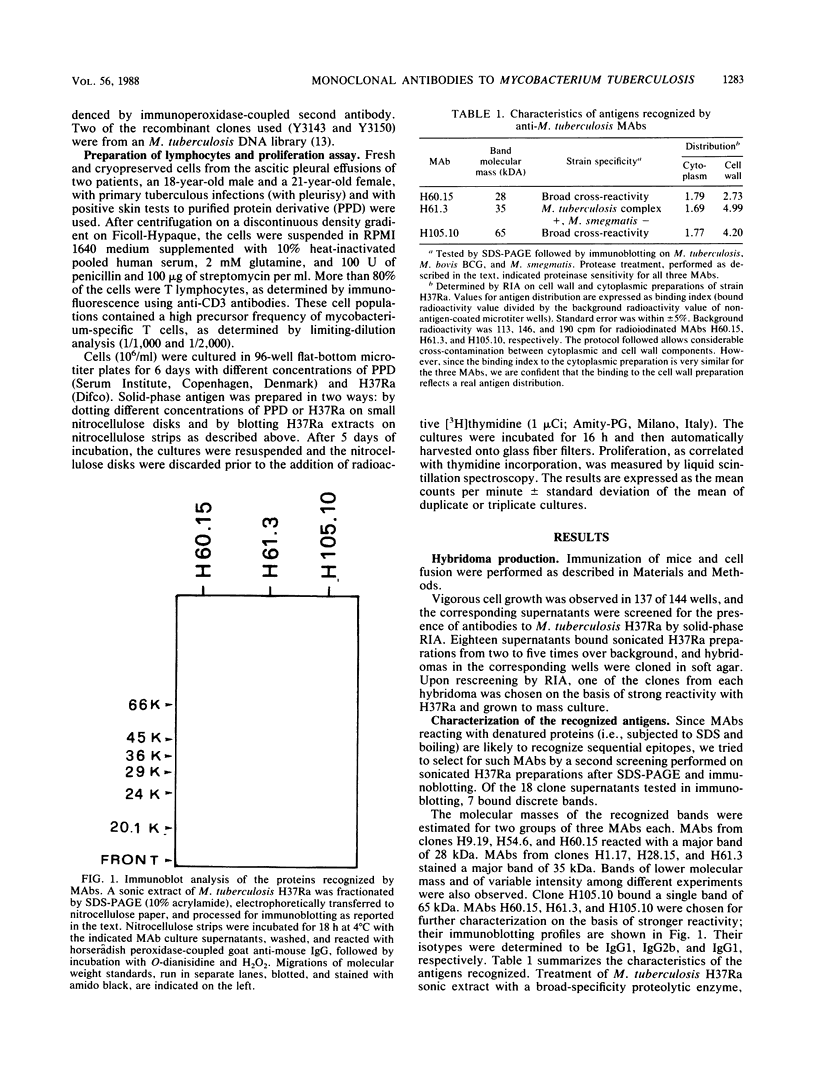
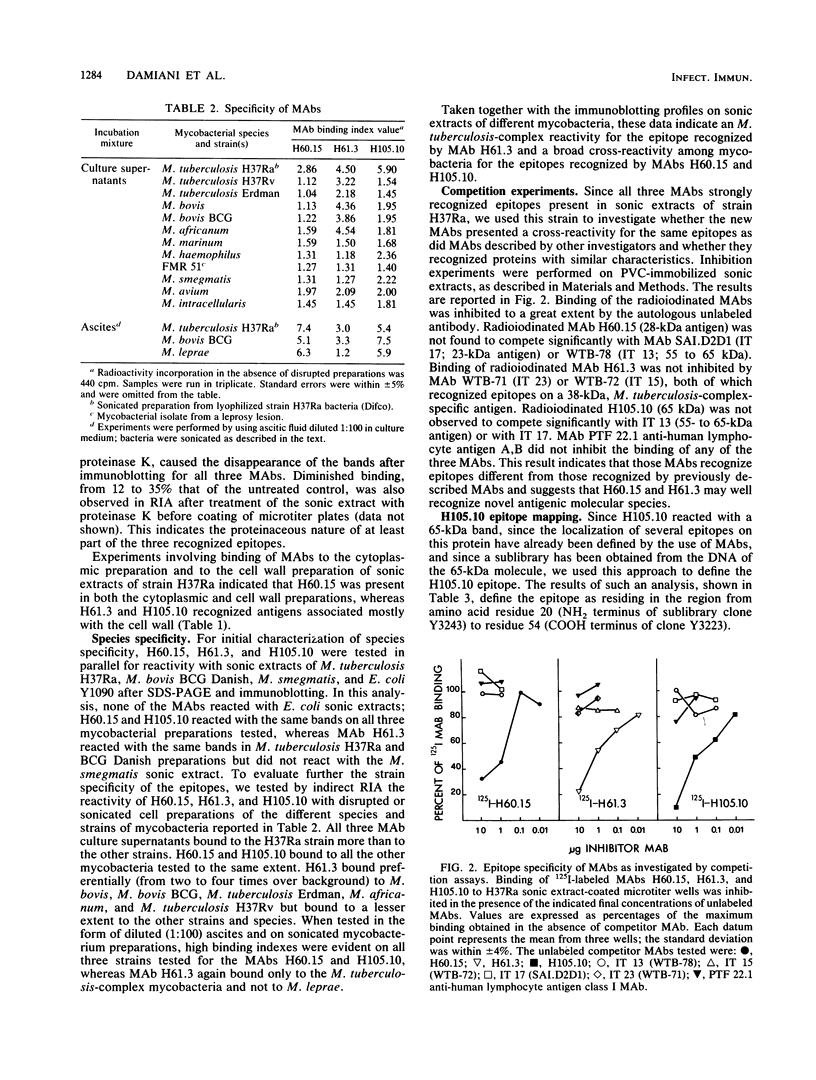
Three monoclonal antibodies (H60.15, H61.3, and H105.10) directed to protein antigens of Mycobacterium tuberculosis were obtained and characterized. H60.15 recognizes a protein with a molecular mass of 28 kilodaltons (kDa) with broad cross-reactivity on a panel of 12 species and strains of mycobacteria. H61.3 reacts with a 35-kDa protein present in M. tuberculosis, Mycobacterium bovis BCG, and M. africanum. On the basis of the antigen molecular masses and competition experiments with other monoclonal antibodies, H60.15 and H61.3 seem to be the first described monoclonal antibodies to these M. tuberculosis proteins. H105.10 binds to the cross-reactive 65-kDa protein present in mycobacteria. Epitope mapping of H105.10 was performed by using the M. leprae DNA sublibrary available in bacteriophage lambda gt11 for this antigen and revealed that its epitope resides in the region from amino acids 20 to 54. The 28-, 35-, and 65-kDa antigens isolated by immunoblotting and presented on nitrocellulose to pleural effusion T cells from tuberculosis patients induced a proliferative response, indicating the presence of T-cell epitopes. These observations indicate that two protein antigens should be added to the list of antigens detectable in M. tuberculosis by monoclonal antibodies. The common feature of such proteins, the elicitation of an immune response of limited or broad cross-reactivity for mycobacteria, encourages the search for their role in the pathogenesis of mycobacterioses.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRODIE A. F., GRAY C. T. Bacterial particles in oxidative phosphorylation. Science. 1957 Mar 22;125(3247):534–537. doi: 10.1126/science.125.3247.534. [DOI] [PubMed] [Google Scholar]
- Buchanan T. M., Nomaguchi H., Anderson D. C., Young R. A., Gillis T. P., Britton W. J., Ivanyi J., Kolk A. H., Closs O., Bloom R. Characterization of mycobacterial species specificity of 14 separate epitopes which reacted with monoclonal antibodies to the 65,000 molecular weight protein molecule of Mycobacterium leprae. Lepr Rev. 1986 Dec;57 (Suppl 2):63–66. doi: 10.5935/0305-7518.19860055. [DOI] [PubMed] [Google Scholar]
- Colizzi V. In vivo and in vitro administration of interleukin 2-containing preparation reverses T-cell unresponsiveness in Mycobacterium bovis BCG-infected mice. Infect Immun. 1984 Jul;45(1):25–28. doi: 10.1128/iai.45.1.25-28.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiani G., Frascio M., Benatti U., Morelli A., Zocchi E., Fabbi M., Bargellesi A., Pontremoli S., De Flora A. Monoclonal antibodies to human erythrocyte glucose 6-phosphate dehydrogenase. FEBS Lett. 1980 Sep 22;119(1):169–173. doi: 10.1016/0014-5793(80)81023-4. [DOI] [PubMed] [Google Scholar]
- Damiani G., Zocchi E., Fabbi M., Bargellesi A., Patrone F. A monoclonal antibody to platelet glycoproteins IIb and IIIa complex: its use in purifying human megakaryocytes from sternal bone marrow aspirates for immunofluorescence studies of Ia-like antigens. Exp Hematol. 1983 Mar;11(3):169–177. [PubMed] [Google Scholar]
- Damiani G., Zocchi E., Martinoli C., Querzola F., Zaccheo D. Establishment and characterization of a new human cloned myelomonocytic cell line (ZC-1.6). Exp Hematol. 1985 Oct;13(9):861–868. [PubMed] [Google Scholar]
- Daniel T. M., Anderson P. A. The isolation by immunoabsorbent affinity chromatography and physicochemical characterization of Mycobacterium tuberculosis antigen 5. Am Rev Respir Dis. 1978 Mar;117(3):533–539. doi: 10.1164/arrd.1978.117.3.533. [DOI] [PubMed] [Google Scholar]
- De Bruyn J., Bosmans R., Turneer M., Weckx M., Nyabenda J., Van Vooren J. P., Falmagne P., Wiker H. G., Harboe M. Purification, partial characterization, and identification of a skin-reactive protein antigen of Mycobacterium bovis BCG. Infect Immun. 1987 Jan;55(1):245–252. doi: 10.1128/iai.55.1.245-252.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr A. G., Nakane P. K. Immunohistochemistry with enzyme labeled antibodies: a brief review. J Immunol Methods. 1981;47(2):129–144. doi: 10.1016/0022-1759(81)90114-9. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt J., Coates A. R., Mitchison D. A., Ivanyi J. The use of murine monoclonal antibodies without purification of antigen in the serodiagnosis of tuberculosis. J Immunol Methods. 1982 Dec 17;55(2):205–211. doi: 10.1016/0022-1759(82)90032-1. [DOI] [PubMed] [Google Scholar]
- Husson R. N., Young R. A. Genes for the major protein antigens of Mycobacterium tuberculosis: the etiologic agents of tuberculosis and leprosy share an immunodominant antigen. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1679–1683. doi: 10.1073/pnas.84.6.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanyi J., Krambovitis E., Keen M. Evaluation of a monoclonal antibody (TB72) based serological test for tuberculosis. Clin Exp Immunol. 1983 Nov;54(2):337–345. [PMC free article] [PubMed] [Google Scholar]
- Lamb J. R., Ivanyi J., Rees A. D., Rothbard J. B., Howland K., Young R. A., Young D. B. Mapping of T cell epitopes using recombinant antigens and synthetic peptides. EMBO J. 1987 May;6(5):1245–1249. doi: 10.1002/j.1460-2075.1987.tb02360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra V., Sweetser D., Young R. A. Efficient mapping of protein antigenic determinants. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7013–7017. doi: 10.1073/pnas.83.18.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oftung F., Mustafa A. S., Husson R., Young R. A., Godal T. Human T cell clones recognize two abundant Mycobacterium tuberculosis protein antigens expressed in Escherichia coli. J Immunol. 1987 Feb 1;138(3):927–931. [PubMed] [Google Scholar]
- Patarroyo M. E., Parra C. A., Pinilla C., del Portillo P., Torres M. L., Clavijo P., Salazar L. M., Jimenez C. Immunogenic synthetic peptides against mycobacteria of potential immunodiagnostic and immunoprophylactic value. Lepr Rev. 1986 Dec;57 (Suppl 2):163–168. doi: 10.5935/0305-7518.19860068. [DOI] [PubMed] [Google Scholar]
- Thole J. E., Dauwerse H. G., Das P. K., Groothuis D. G., Schouls L. M., van Embden J. D. Cloning of Mycobacterium bovis BCG DNA and expression of antigens in Escherichia coli. Infect Immun. 1985 Dec;50(3):800–806. doi: 10.1128/iai.50.3.800-806.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. B., Lamb J. R. T lymphocytes respond to solid-phase antigen: a novel approach to the molecular analysis of cellular immunity. Immunology. 1986 Oct;59(2):167–171. [PMC free article] [PubMed] [Google Scholar]
- Young D., Kent L., Rees A., Lamb J., Ivanyi J. Immunological activity of a 38-kilodalton protein purified from Mycobacterium tuberculosis. Infect Immun. 1986 Oct;54(1):177–183. doi: 10.1128/iai.54.1.177-183.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Bloom B. R., Grosskinsky C. M., Ivanyi J., Thomas D., Davis R. W. Dissection of Mycobacterium tuberculosis antigens using recombinant DNA. Proc Natl Acad Sci U S A. 1985 May;82(9):2583–2587. doi: 10.1073/pnas.82.9.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]




