Abstract
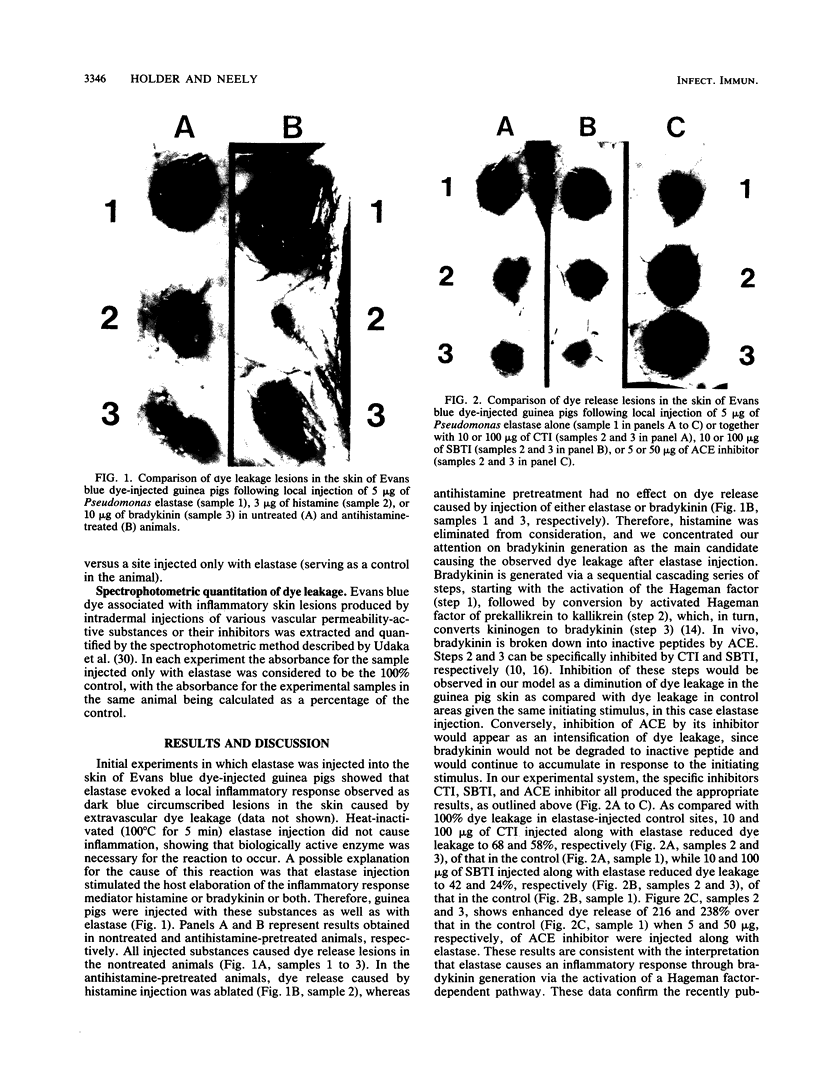
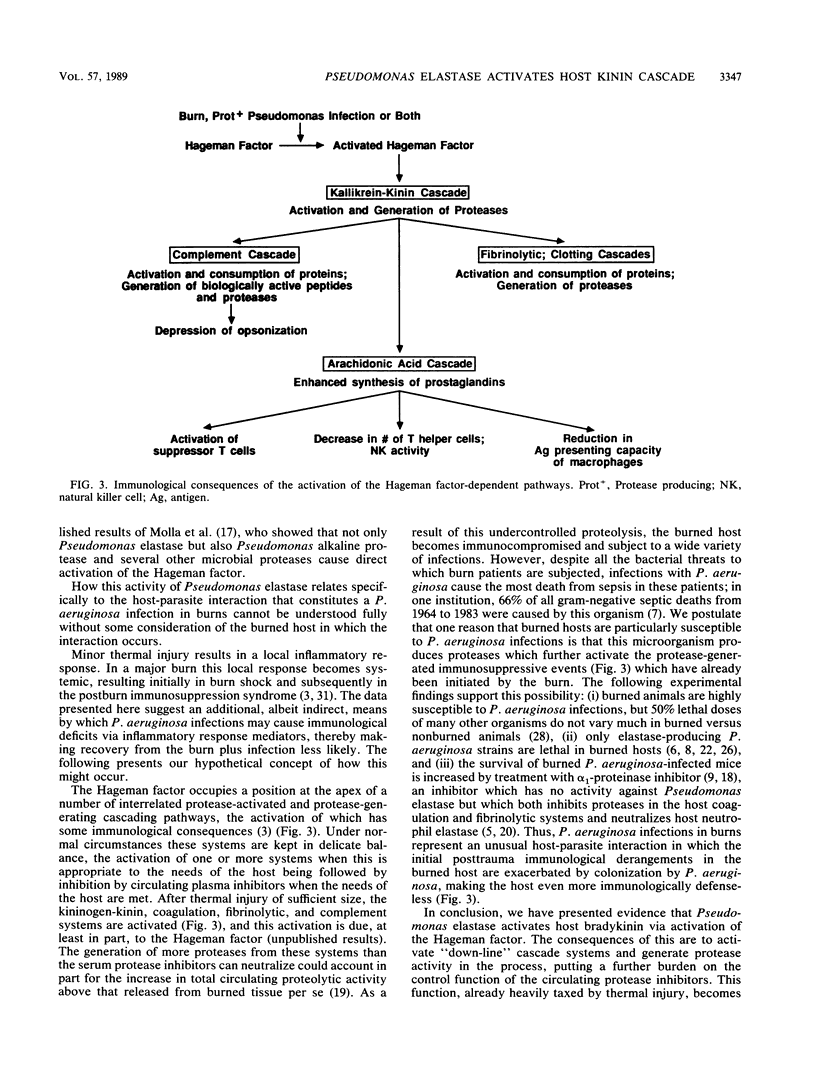
Purified Pseudomonas elastase injected subcutaneously into the skin of an Evans blue dye-injected (intravenously) guinea pig caused dye leakage similar to that observed when histamine or bradykinin was injected in the same animal. The histamine-induced dye leakage was ablated in antihistamine-treated guinea pigs, but elastase- and bradykinin-induced dye leakages were not. Local injections of specific inhibitors of the host Hageman factor-dependent bradykinin-generating pathway given immediately prior to elastase injection reduced dye leakage in a dose-related manner. Elastase-related dye release was enhanced when angiotension-converting enzyme inhibitor, a substance which prevents host enzymes from breaking down bradykinin, was injected prior to elastase injection. We conclude that Pseudomonas elastase generates bradykinin in the infected host via a Hageman factor-dependent pathway.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. L., Stager D., Levin D. L. Pseudomonas aeruginosa corneal infections in seriously ill children. Clin Pediatr (Phila) 1982 Feb;21(2):123–124. doi: 10.1177/000992288202100210. [DOI] [PubMed] [Google Scholar]
- Baker N. R. Role of exotoxin A and proteases of Pseudomonas aeruginosa in respiratory tract infections. Can J Microbiol. 1982 Feb;28(2):248–255. doi: 10.1139/m82-033. [DOI] [PubMed] [Google Scholar]
- Bjornson A. B., Bjornson H. S. Theoretical interrelationships among immunologic and hematologic sequelae of thermal injury. Rev Infect Dis. 1984 Sep-Oct;6(5):704–714. doi: 10.1093/clinids/6.5.704. [DOI] [PubMed] [Google Scholar]
- Bodey G. P., Bolivar R., Fainstein V., Jadeja L. Infections caused by Pseudomonas aeruginosa. Rev Infect Dis. 1983 Mar-Apr;5(2):279–313. doi: 10.1093/clinids/5.2.279. [DOI] [PubMed] [Google Scholar]
- Cohen A. B. Mechanism of action of alpha-1-antitrypsin. J Biol Chem. 1973 Oct 25;248(20):7055–7059. [PubMed] [Google Scholar]
- Hojima Y., Pierce J. V., Pisano J. J. Hageman factor fragment inhibitor in corn seeds: purification and characterization. Thromb Res. 1980 Oct 15;20(2):149–162. doi: 10.1016/0049-3848(80)90381-3. [DOI] [PubMed] [Google Scholar]
- Holder I. A. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: effect of treatment with protease inhibitors. Rev Infect Dis. 1983 Nov-Dec;5 (Suppl 5):S914–S921. doi: 10.1093/clinids/5.supplement_5.s914. [DOI] [PubMed] [Google Scholar]
- Holder I. A., Haidaris C. G. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: extracellular protease and elastase as in vivo virulence factors. Can J Microbiol. 1979 May;25(5):593–599. doi: 10.1139/m79-085. [DOI] [PubMed] [Google Scholar]
- Holder I. A., Neely A. N. Combined host and specific anti-Pseudomonas-directed therapy for Pseudomonas aeruginosa infections in burned mice: experimental results and theoretic considerations. J Burn Care Rehabil. 1989 Mar-Apr;10(2):131–137. doi: 10.1097/00004630-198903000-00006. [DOI] [PubMed] [Google Scholar]
- Howe T. R., Iglewski B. H. Isolation and characterization of alkaline protease-deficient mutants of Pseudomonas aeruginosa in vitro and in a mouse eye model. Infect Immun. 1984 Mar;43(3):1058–1063. doi: 10.1128/iai.43.3.1058-1063.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata R., Yamamoto T., Matsumoto K., Maeda H. A serratial protease causes vascular permeability reaction by activation of the Hageman factor-dependent pathway in guinea pigs. Infect Immun. 1985 Jun;48(3):747–753. doi: 10.1128/iai.48.3.747-753.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. P., Meier H. L., Mandle R., Jr The Hageman factor dependent pathways of coagulation, fibrinolysis, and kinin-generation. Semin Thromb Hemost. 1976 Jul;3(1):1–26. doi: 10.1055/s-0028-1087162. [DOI] [PubMed] [Google Scholar]
- Lei M. G., Reeck G. R. Combined use of trypsin-agarose affinity chromatography and reversed-phase high-performance liquid chromatography for the purification of single-chain protease inhibitor from corn seeds. J Chromatogr. 1986 Aug 29;363(2):315–321. doi: 10.1016/s0021-9673(01)83751-1. [DOI] [PubMed] [Google Scholar]
- Matsumoto K., Yamamoto T., Kamata R., Maeda H. Pathogenesis of serratial infection: activation of the Hageman factor-prekallikrein cascade by serratial protease. J Biochem. 1984 Sep;96(3):739–749. doi: 10.1093/oxfordjournals.jbchem.a134892. [DOI] [PubMed] [Google Scholar]
- Molla A., Yamamoto T., Akaike T., Miyoshi S., Maeda H. Activation of hageman factor and prekallikrein and generation of kinin by various microbial proteinases. J Biol Chem. 1989 Jun 25;264(18):10589–10594. [PubMed] [Google Scholar]
- Neely A. N., Nathan P., Highsmith R. F. Plasma proteolytic activity following burns. J Trauma. 1988 Mar;28(3):362–367. doi: 10.1097/00005373-198803000-00012. [DOI] [PubMed] [Google Scholar]
- Ohlsson K. Alpha1-antitrypsin and alpha2-macroglobulin. Interactions with human neutrophil collagenase and elastase. Ann N Y Acad Sci. 1975 Jun 13;256:409–419. doi: 10.1111/j.1749-6632.1975.tb36067.x. [DOI] [PubMed] [Google Scholar]
- Ohman D. E., Burns R. P., Iglewski B. H. Corneal infections in mice with toxin A and elastase mutants of Pseudomonas aeruginosa. J Infect Dis. 1980 Oct;142(4):547–555. doi: 10.1093/infdis/142.4.547. [DOI] [PubMed] [Google Scholar]
- Pavlovskis O. R., Wretlind B. Assessment of protease (elastase) as a Pseudomonas aeruginosa virulence factor in experimental mouse burn infection. Infect Immun. 1979 Apr;24(1):181–187. doi: 10.1128/iai.24.1.181-187.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington J. E., Reynolds H. Y., Carbone P. P. Pseudomonas pneumonia. A retrospective study of 36 cases. Am J Med. 1973 Aug;55(2):155–160. doi: 10.1016/0002-9343(73)90163-0. [DOI] [PubMed] [Google Scholar]
- Purdue G. Patterns of infection over the past ten years. Infection patterns, 1982-1984. J Burn Care Rehabil. 1987 Jan-Feb;8(1):39–43. [PubMed] [Google Scholar]
- Saelinger C. B., Snell K., Holder I. A. Experimental studies on the pathogenesis of infections due to Pseudomonas aeruginosa: direct evidence for toxin production during Pseudomonas infection of burned skin tissues. J Infect Dis. 1977 Oct;136(4):555–561. doi: 10.1093/infdis/136.4.555. [DOI] [PubMed] [Google Scholar]
- Stevens R. M., Teres D., Skillman J. J., Feingold D. S. Pneumonia in an intensive care unit. A 30-month experience. Arch Intern Med. 1974 Jul;134(1):106–111. [PubMed] [Google Scholar]
- Stieritz D. D., Holder I. A. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: description of a burned mouse model. J Infect Dis. 1975 Jun;131(6):688–691. doi: 10.1093/infdis/131.6.688. [DOI] [PubMed] [Google Scholar]
- Tarr K. H., Constable I. J. Pseudomonas endophthalmitis associated with scleral necrosis. Br J Ophthalmol. 1980 Sep;64(9):676–679. doi: 10.1136/bjo.64.9.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Hwang W. S., Shahrabadi M. S., Que J. U. Alteration of pulmonary structure by Pseudomonas aeruginosa exoenzyme S. J Med Microbiol. 1988 Jun;26(2):133–141. doi: 10.1099/00222615-26-2-133. [DOI] [PubMed] [Google Scholar]