Abstract
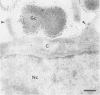
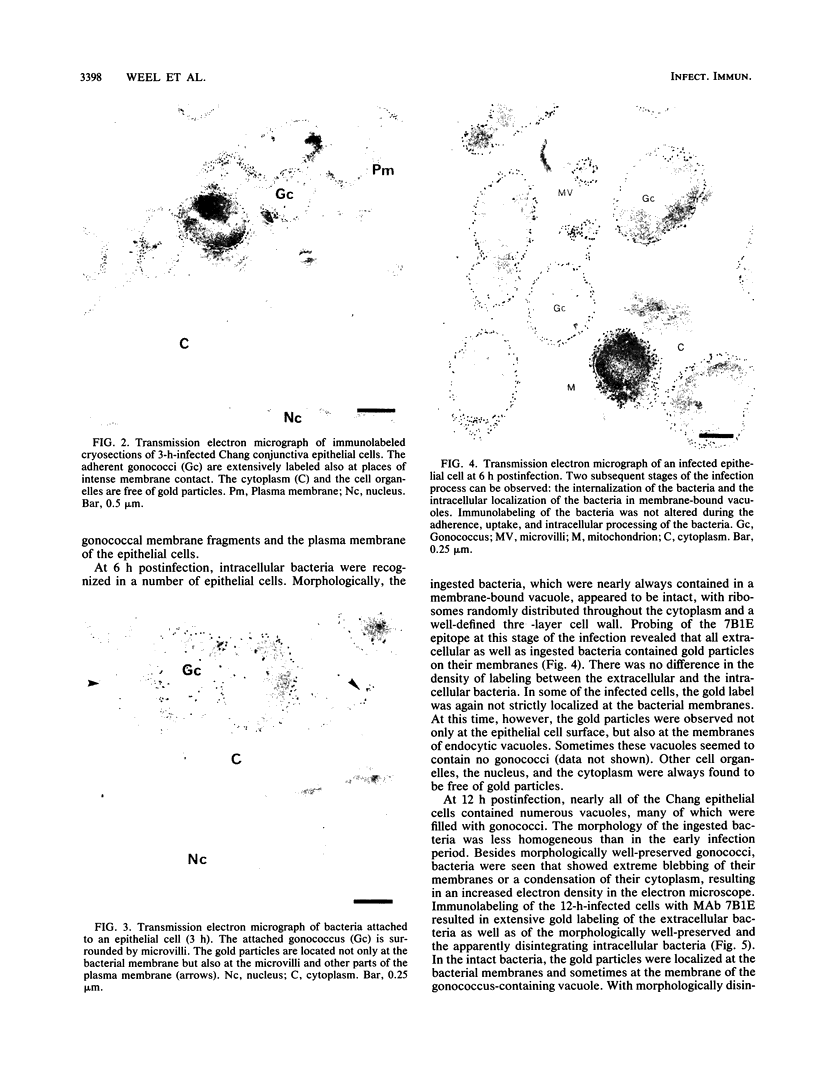
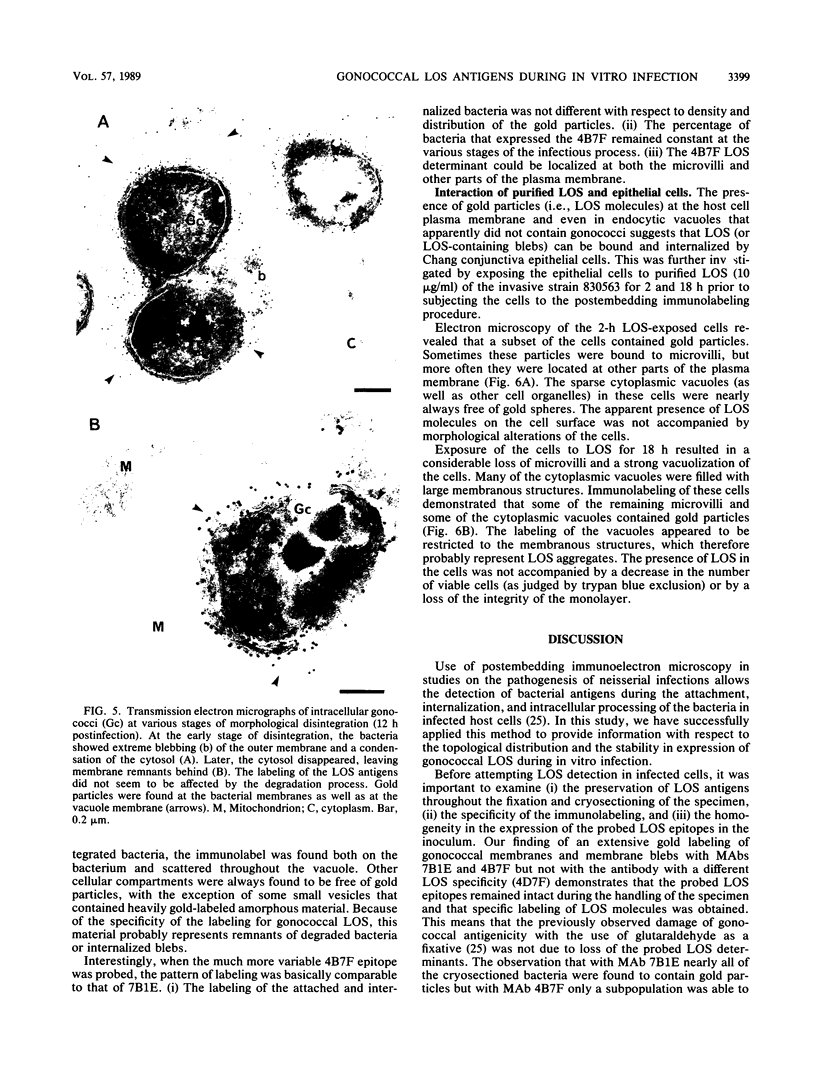
Immunoelectron microscopy enables the detection and localization of bacterial antigens during in vitro infection (J.F.L. Weel and J.P.M. van Putten, Microb. Pathog. 4:213-222, 1988). In this study, we have used this method to get information on the role of lipooligosaccharides (LOS) in the pathogenesis of neisserial infections at the mucosal level. Ultrathin cryosections of Chang conjunctive epithelial cells infected with Neisseria gonorrhoeae (3 to 18 h) were incubated with LOS-specific monoclonal antibodies and gold-labeled protein A and viewed in the electron microscope. Our results demonstrate that the probed LOS determinants are stably expressed during the adherence, internalization, and intracellular processing of the bacteria. There was no indication of an adaptation of the gonococcal LOS expression to the host cell environment or of a degradation of the probed epitopes. The gold particles, representing LOS molecules, were predominantly located at the bacterial membranes, but sometimes the host cell plasma membrane was labeled as well, suggesting that LOS or LOS-containing membrane fragments interacted with the eucaryotic cells. This was confirmed when purified LOS was added to the cells. Two hours after LOS exposure, gold particles were observed at the plasma membrane of a subpopulation of the cells. After 18 h of LOS exposure, gold particles were also found in large vacuoles inside the cells, suggesting that LOS molecules were internalized by the cells. The function of observed LOS binding and endocytosis in the pathogenesis of neisserial infections remains to be defined.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apicella M. A., Shero M., Jarvis G. A., Griffiss J. M., Mandrell R. E., Schneider H. Phenotypic variation in epitope expression of the Neisseria gonorrhoeae lipooligosaccharide. Infect Immun. 1987 Aug;55(8):1755–1761. doi: 10.1128/iai.55.8.1755-1761.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella M. A., Westerink M. A., Morse S. A., Schneider H., Rice P. A., Griffiss J. M. Bactericidal antibody response of normal human serum to the lipooligosaccharide of Neisseria gonorrhoeae. J Infect Dis. 1986 Mar;153(3):520–526. doi: 10.1093/infdis/153.3.520. [DOI] [PubMed] [Google Scholar]
- Connelly M. C., Allen P. Z. Antigenic specificity and heterogeneity of lipopolysaccharides from pyocin-sensitive and -resistant strains of Neisseria gonorrhoeae. Infect Immun. 1983 Sep;41(3):1046–1055. doi: 10.1128/iai.41.3.1046-1055.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. D., McGraw P. A., Melly M. A. Localization of gonococcal lipopolysaccharide and its relationship to toxic damage in human fallopian tube mucosa. Infect Immun. 1986 Feb;51(2):425–430. doi: 10.1128/iai.51.2.425-430.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarco de Hormaeche R., Jessop H., Senior K. Gonococcal variants selected by growth in vivo or in vitro have antigenically different LPS. Microb Pathog. 1988 Apr;4(4):289–297. doi: 10.1016/0882-4010(88)90089-7. [DOI] [PubMed] [Google Scholar]
- Eaton L. J., Rest R. F. In vivo degradation of gonococcal outer membrane proteins within human leukocyte phagolysosomes. Infect Immun. 1983 Dec;42(3):1034–1040. doi: 10.1128/iai.42.3.1034-1040.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans B. A. Ultrastructural study of cervical gonorrhea. J Infect Dis. 1977 Aug;136(2):248–255. doi: 10.1093/infdis/136.2.248. [DOI] [PubMed] [Google Scholar]
- Griffiss J. M., O'Brien J. P., Yamasaki R., Williams G. D., Rice P. A., Schneider H. Physical heterogeneity of neisserial lipooligosaccharides reflects oligosaccharides that differ in apparent molecular weight, chemical composition, and antigenic expression. Infect Immun. 1987 Aug;55(8):1792–1800. doi: 10.1128/iai.55.8.1792-1800.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrell R. E., Griffiss J. M., Macher B. A. Lipooligosaccharides (LOS) of Neisseria gonorrhoeae and Neisseria meningitidis have components that are immunochemically similar to precursors of human blood group antigens. Carbohydrate sequence specificity of the mouse monoclonal antibodies that recognize crossreacting antigens on LOS and human erythrocytes. J Exp Med. 1988 Jul 1;168(1):107–126. doi: 10.1084/jem.168.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Apicella M. A. Isolation of a lipopolysaccharide mutant of Neisseria gonorrhoeae: an analysis of the antigenic and biologic difference. J Infect Dis. 1982 Feb;145(2):206–216. doi: 10.1093/infdis/145.2.206. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Mintz C. S., Sarafian S. K., Bartenstein L., Bertram M., Apicella M. A. Effect of dilution rate on lipopolysaccharide and serum resistance of Neisseria gonorrhoeae grown in continuous culture. Infect Immun. 1983 Jul;41(1):74–82. doi: 10.1128/iai.41.1.74-82.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman J. T., Buchanan T. M. Monoclonal antibody activity against native and denatured forms of gonococcal outer membrane proteins as detected within ultrathin, longitudinal slices of polyacrylamide gels. J Immunol Methods. 1984 Dec 31;75(2):265–274. doi: 10.1016/0022-1759(84)90110-8. [DOI] [PubMed] [Google Scholar]
- Schneider H., Hale T. L., Zollinger W. D., Seid R. C., Jr, Hammack C. A., Griffiss J. M. Heterogeneity of molecular size and antigenic expression within lipooligosaccharides of individual strains of Neisseria gonorrhoeae and Neisseria meningitidis. Infect Immun. 1984 Sep;45(3):544–549. doi: 10.1128/iai.45.3.544-549.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Hammack C. A., Apicella M. A., Griffiss J. M. Instability of expression of lipooligosaccharides and their epitopes in Neisseria gonorrhoeae. Infect Immun. 1988 Apr;56(4):942–946. doi: 10.1128/iai.56.4.942-946.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Joiner K., Guymon L. F., Cohen M. S., Sparling P. F. Serum sensitivity of Neisseria gonorrhoeae: the role of lipopolysaccharide. J Infect Dis. 1984 Feb;149(2):175–183. doi: 10.1093/infdis/149.2.175. [DOI] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J. A new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol. 1985 Jul;38(1):87–93. [PubMed] [Google Scholar]
- Stephens D. S., Whitney A. M., Melly M. A., Hoffman L. H., Farley M. M., Frasch C. E. Analysis of damage to human ciliated nasopharyngeal epithelium by Neisseria meningitidis. Infect Immun. 1986 Feb;51(2):579–585. doi: 10.1128/iai.51.2.579-585.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam M. R., Buchanan T. M., Sandström E. G., Holmes K. K., Knapp J. S., Siadak A. W., Nowinski R. C. Serological classification of Neisseria gonorrhoeae with monoclonal antibodies. Infect Immun. 1982 Jun;36(3):1042–1053. doi: 10.1128/iai.36.3.1042-1053.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E. L., Patel P. V., Parsons N. J., Martin P. M., Smith H. Lipopolysaccharide alteration is associated with induced resistance of Neisseria gonorrhoeae to killing by human serum. J Gen Microbiol. 1986 May;132(5):1407–1413. doi: 10.1099/00221287-132-5-1407. [DOI] [PubMed] [Google Scholar]
- Tjia K. F., van Putten J. P., Pels E., Zanen H. C. The interaction between Neisseria gonorrhoeae and the human cornea in organ culture. An electron microscopic study. Graefes Arch Clin Exp Ophthalmol. 1988;226(4):341–345. doi: 10.1007/BF02172964. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. A study of positive staining of ultrathin frozen sections. J Ultrastruct Res. 1978 Jun;63(3):287–307. doi: 10.1016/s0022-5320(78)80053-7. [DOI] [PubMed] [Google Scholar]
- Virji M., Heckels J. E. The role of common and type-specific pilus antigenic domains in adhesion and virulence of gonococci for human epithelial cells. J Gen Microbiol. 1984 May;130(5):1089–1095. doi: 10.1099/00221287-130-5-1089. [DOI] [PubMed] [Google Scholar]
- Weel J. F., van Putten J. P. Ultrastructural localization of gonococcal antigens in infected epithelial cells as visualized by post-embedding immuno-electronmicroscopy. Microb Pathog. 1988 Mar;4(3):213–222. doi: 10.1016/0882-4010(88)90071-x. [DOI] [PubMed] [Google Scholar]