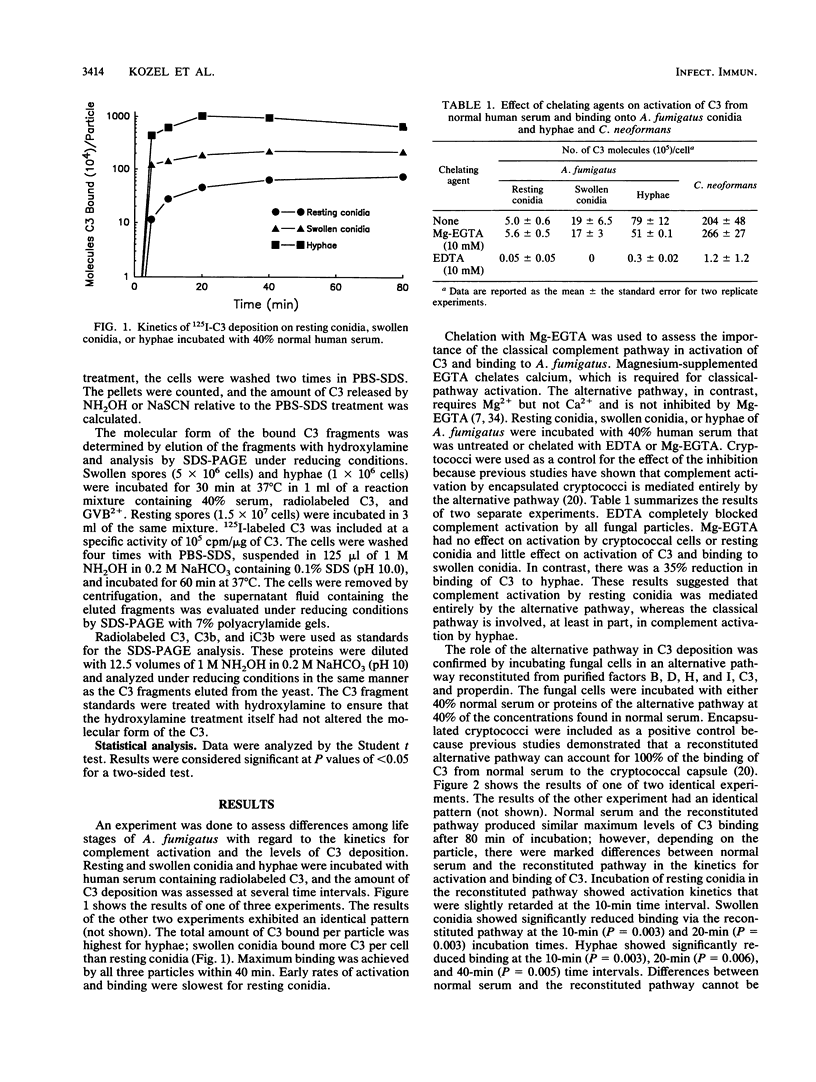
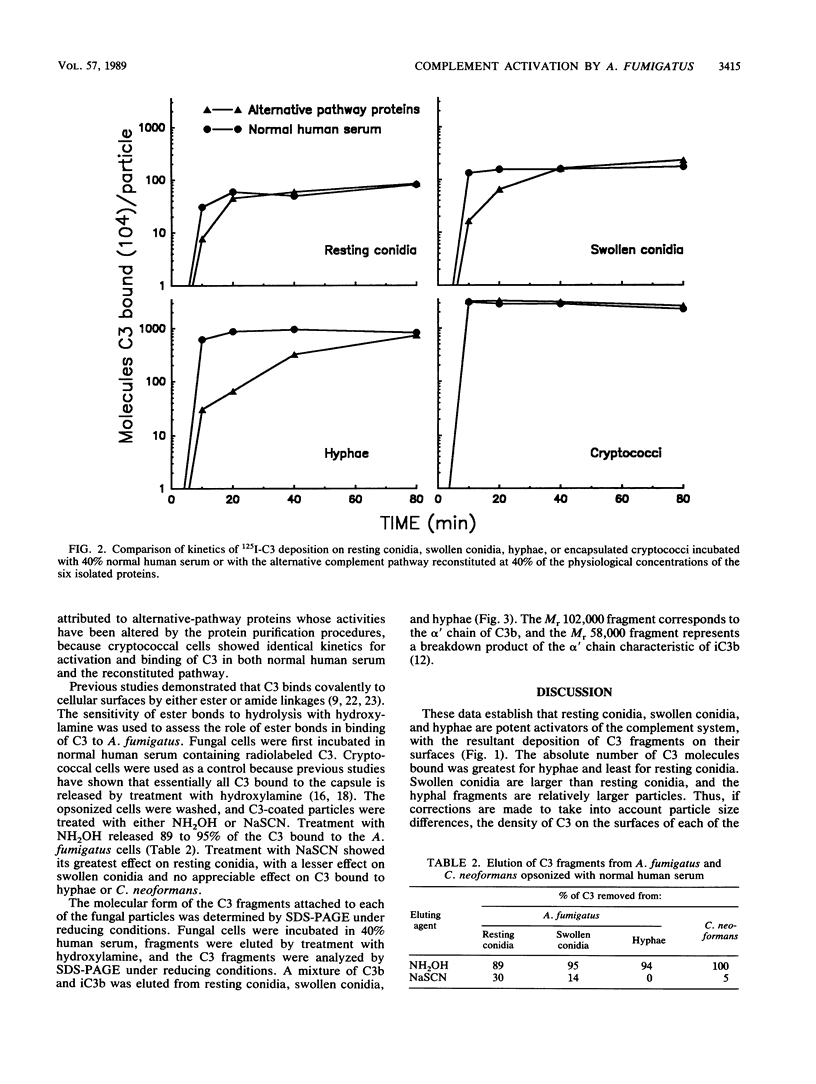
Abstract
Complement activation by Aspergillus fumigatus may play a crucial role in stimulating binding and killing of this organism by phagocytes. We examined the amount and type of C3 deposited on resting conidia, swollen conidia, and hyphae of A. fumigatus after incubation in pooled human serum. All three life forms of A. fumigatus were potent activators of the complement cascade, with deposition on the organisms of similar amounts of C3 per unit of surface area. The rate of deposition was slowest for resting conidia, although maximal deposition was still achieved within 40 min. The roles of the alternative and classical pathways were assessed by use of serum chelated with magnesium EGTA [magnesium ethylene glycol-bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid] and with an alternative pathway reconstituted from the six purified alternative-pathway proteins. Complement activation by resting conidia was mediated by the alternative pathway. In contrast, there was a progressive dependence on the classical pathway as the fungal particles matured into swollen conidia and then hyphae. Treatment with hydroxylamine, which disrupts ester linkages, removed 89 to 95% of the C3 bound to all three forms of A. fumigatus. This released C3 contained a mixture of C3b and iC3b, as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography. These data demonstrate that although all three forms of A. fumigatus are potent activators of the complement system, the transition from resting conidia to swollen conidia to hyphae results in progressive changes in the manner in which the fungal particles interact with the complement system. The lack of participation of the classical pathway in complement activation by resting conidia may have important implications regarding their ability to effectively stimulate phagocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen M. S., Isturiz R. E., Malech H. L., Root R. K., Wilfert C. M., Gutman L., Buckley R. H. Fungal infection in chronic granulomatous disease. The importance of the phagocyte in defense against fungi. Am J Med. 1981 Jul;71(1):59–66. doi: 10.1016/0002-9343(81)90259-x. [DOI] [PubMed] [Google Scholar]
- Davis A. E., 3rd The C3b inactivator of the human complement system: homology with serine proteases. FEBS Lett. 1981 Nov 16;134(2):147–150. doi: 10.1016/0014-5793(81)80588-1. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Clark R. A. Damage to Aspergillus fumigatus and Rhizopus oryzae hyphae by oxidative and nonoxidative microbicidal products of human neutrophils in vitro. Infect Immun. 1982 Nov;38(2):487–495. doi: 10.1128/iai.38.2.487-495.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Krzesicki R., Epstein B., Jao W. Damage to hyphal forms of fungi by human leukocytes in vitro. A possible host defense mechanism in aspergillosis and mucormycosis. Am J Pathol. 1978 May;91(2):313–328. [PMC free article] [PubMed] [Google Scholar]
- Fine D. P., Marney S. R., Jr, Colley D. G., Sergent J. S., Des Prez R. M. C3 shunt activation in human serum chelated with EGTA. J Immunol. 1972 Oct;109(4):807–809. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Gadd K. J., Reid K. B. The binding of complement component C3 to antibody-antigen aggregates after activation of the alternative pathway in human serum. Biochem J. 1981 May 1;195(2):471–480. doi: 10.1042/bj1950471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D. L., Johnson G. M., Hostetter M. K. Ligand-receptor interactions in the phagocytosis of virulent Streptococcus pneumoniae by polymorphonuclear leukocytes. J Infect Dis. 1986 Oct;154(4):619–626. doi: 10.1093/infdis/154.4.619. [DOI] [PubMed] [Google Scholar]
- Hammer C. H., Wirtz G. H., Renfer L., Gresham H. D., Tack B. F. Large scale isolation of functionally active components of the human complement system. J Biol Chem. 1981 Apr 25;256(8):3995–4006. [PubMed] [Google Scholar]
- Harrison R. A., Lachmann P. J. The physiological breakdown of the third component of human complement. Mol Immunol. 1980 Jan;17(1):9–20. doi: 10.1016/0161-5890(80)90119-4. [DOI] [PubMed] [Google Scholar]
- Hess W. M., Stocks D. L. Surface characteristics of Aspergillus conidia. Mycologia. 1969 May-Jun;61(3):560–571. [PubMed] [Google Scholar]
- Kan V. L., Bennett J. E. Lectin-like attachment sites on murine pulmonary alveolar macrophages bind Aspergillus fumigatus conidia. J Infect Dis. 1988 Aug;158(2):407–414. doi: 10.1093/infdis/158.2.407. [DOI] [PubMed] [Google Scholar]
- Kerr M. A. Human factor B. Methods Enzymol. 1981;80(Pt 100):102–112. doi: 10.1016/s0076-6879(81)80010-9. [DOI] [PubMed] [Google Scholar]
- Kozel T. R., Brown R. R., Pfrommer G. S. Activation and binding of C3 by Candida albicans. Infect Immun. 1987 Aug;55(8):1890–1894. doi: 10.1128/iai.55.8.1890-1894.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Mastroianni R. P. Inhibition of phagocytosis by cryptococcal polysaccharide: dissociation of the attachment and ingestion phases of phagocytosis. Infect Immun. 1976 Jul;14(1):62–67. doi: 10.1128/iai.14.1.62-67.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Pfrommer G. S. Activation of the complement system by Cryptococcus neoformans leads to binding of iC3b to the yeast. Infect Immun. 1986 Apr;52(1):1–5. doi: 10.1128/iai.52.1.1-5.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Pfrommer G. S., Guerlain A. S., Highison B. A., Highison G. J. Strain variation in phagocytosis of Cryptococcus neoformans: dissociation of susceptibility to phagocytosis from activation and binding of opsonic fragments of C3. Infect Immun. 1988 Nov;56(11):2794–2800. doi: 10.1128/iai.56.11.2794-2800.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Wilson M. A., Pfrommer G. S., Schlageter A. M. Activation and binding of opsonic fragments of C3 on encapsulated Cryptococcus neoformans by using an alternative complement pathway reconstituted from six isolated proteins. Infect Immun. 1989 Jul;57(7):1922–1927. doi: 10.1128/iai.57.7.1922-1927.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law S. K. C3 receptors on macrophages. J Cell Sci Suppl. 1988;9:67–97. doi: 10.1242/jcs.1988.supplement_9.4. [DOI] [PubMed] [Google Scholar]
- Law S. K., Levine R. P. Interaction between the third complement protein and cell surface macromolecules. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2701–2705. doi: 10.1073/pnas.74.7.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law S. K., Lichtenberg N. A., Levine R. P. Evidence for an ester linkage between the labile binding site of C3b and receptive surfaces. J Immunol. 1979 Sep;123(3):1388–1394. [PubMed] [Google Scholar]
- Lehrer R. I., Jan R. G. Interaction of Aspergillus fumigatus Spores with Human Leukocytes and Serum. Infect Immun. 1970 Apr;1(4):345–350. doi: 10.1128/iai.1.4.345-350.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesavre P. H., Hugli T. E., Esser A. F., Müller-Eberhard H. J. The alternative pathway C3/C5 convertase: chemical basis of factor B activation. J Immunol. 1979 Aug;123(2):529–534. [PubMed] [Google Scholar]
- Levitz S. M. Aspergillosis. Infect Dis Clin North Am. 1989 Mar;3(1):1–18. [PubMed] [Google Scholar]
- Levitz S. M., Diamond R. D. A rapid colorimetric assay of fungal viability with the tetrazolium salt MTT. J Infect Dis. 1985 Nov;152(5):938–945. doi: 10.1093/infdis/152.5.938. [DOI] [PubMed] [Google Scholar]
- Levitz S. M., Diamond R. D. Mechanisms of resistance of Aspergillus fumigatus Conidia to killing by neutrophils in vitro. J Infect Dis. 1985 Jul;152(1):33–42. doi: 10.1093/infdis/152.1.33. [DOI] [PubMed] [Google Scholar]
- Levitz S. M., Selsted M. E., Ganz T., Lehrer R. I., Diamond R. D. In vitro killing of spores and hyphae of Aspergillus fumigatus and Rhizopus oryzae by rabbit neutrophil cationic peptides and bronchoalveolar macrophages. J Infect Dis. 1986 Sep;154(3):483–489. doi: 10.1093/infdis/154.3.483. [DOI] [PubMed] [Google Scholar]
- Medicus R. G., Esser A. F., Fernandez H. N., Müller-Eberhard H. J. Native and activated properdin: interconvertibility and identity of amino- and carboxy-terminal sequences. J Immunol. 1980 Feb;124(2):602–606. [PubMed] [Google Scholar]
- Nagasawa S., Ichihara C., Stroud R. M. Cleavage of C4b by C3b inactivator: production of a nicked form of C4b, C4b', as an intermediate cleavage product of C4b by C3b inactivator. J Immunol. 1980 Aug;125(2):578–582. [PubMed] [Google Scholar]
- Pangburn M. K., Morrison D. C., Schreiber R. D., Müller-Eberhard H. J. Activation of the alternative complement pathway: recognition of surface structures on activators by bound C3b. J Immunol. 1980 Feb;124(2):977–982. [PubMed] [Google Scholar]
- Platts-Mills T. A., Ishizaka K. Activation of the alternate pathway of human complements by rabbit cells. J Immunol. 1974 Jul;113(1):348–358. [PubMed] [Google Scholar]
- Reynolds H. Y., Newball H. H. Analysis of proteins and respiratory cells obtained from human lungs by bronchial lavage. J Lab Clin Med. 1974 Oct;84(4):559–573. [PubMed] [Google Scholar]
- Ross G. D., Medof M. E. Membrane complement receptors specific for bound fragments of C3. Adv Immunol. 1985;37:217–267. doi: 10.1016/s0065-2776(08)60341-7. [DOI] [PubMed] [Google Scholar]
- Schaffner A., Douglas H., Braude A. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J Clin Invest. 1982 Mar;69(3):617–631. doi: 10.1172/JCI110489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim R. B., DiScipio R. G. Purification and structural studies on the complement-system control protein beta 1H (Factor H). Biochem J. 1982 Aug 1;205(2):285–293. doi: 10.1042/bj2050285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Pangburn M. K., Vogel C. W., Müller-Eberhard H. J. Molecular architecture of human properdin, a positive regulator of the alternative pathway of complement. J Biol Chem. 1984 Apr 10;259(7):4582–4588. [PubMed] [Google Scholar]
- Waldorf A. R., Levitz S. M., Diamond R. D. In vivo bronchoalveolar macrophage defense against Rhizopus oryzae and Aspergillus fumigatus. J Infect Dis. 1984 Nov;150(5):752–760. doi: 10.1093/infdis/150.5.752. [DOI] [PubMed] [Google Scholar]
- Washburn R. G., Hammer C. H., Bennett J. E. Inhibition of complement by culture supernatants of Aspergillus fumigatus. J Infect Dis. 1986 Dec;154(6):944–951. doi: 10.1093/infdis/154.6.944. [DOI] [PubMed] [Google Scholar]