Abstract
4-Fluoroprolines are among the most useful nonnatural amino acids in chemical biology. Here, practical routes are reported for the synthesis of the 2S,4R, 2S,4S, and 2R,4S diastereomers of 4-fluoroproline. Each route starts with (2S,4R)-4-hydroxyproline, which is a prevalent component of collagen and hence readily available, and uses a fluoride salt to install the fluoro group. Hence, the routes provide process-scale access to these useful nonnatural amino acids.
1. Introduction
The ability to incorporate nonnatural amino acids into proteins is altering the landscape in chemical biology and related fields [1]. Perhaps no other nonnatural amino acid has been as useful in enhancing the conformational stability of proteins as have 4-fluoroprolines. In the proper context, the 4R and 4S diastereomers of 4-fluoroproline have been shown to increase dramatically the conformational stability of collagen [2], which is the most abundant protein in animals. In addition, 4-fluoroproline residues can endow conformational stability upon other proteins [3] as well as peptides [4]. This enhanced stability is the result of the increased tendency of 4-fluoroproline residues to adopt a Cγ-exo or Cγ-endo pyrrolidine ring pucker (2S,4R or 2S,4S diastereomer, respectively), or form a trans or cis peptide bond (2S,4R or 2S,4S diastereomer, respectively). These conformational preferences arise from two stereoelectronic effects [5]: a gauche effect that fixes the pyrrolidine ring pucker [6,7], and an nΠπ* interaction that stabilizes the trans peptide bond [6,8]. Because of these desirable attributes, there is a growing need to access 4-fluoroprolines.
The chemical synthesis of a 4-fluoroproline often begins with a 4-hydroxyproline [9]. (2S,4R)-4-Hydroxyproline (HypOH), which is a prevalent component of collagen, is an especially advantageous starting material for the synthesis of 4-fluoroprolines. The prevalence of this amino acid is extraordinary: the abundance of Hyp within animal proteins is <4%, a value calculated from the abundance of collagen amongst animal proteins (1/3) and the prevalence of Hyp within collagen (<38% × 1/3) [10]. Thus, the abundance of Hyp in animals exceeds that of seven “common” amino acids: Cys, Gln, His, Met, Phe, Trp, and Tyr [11].
Typically, the key reaction in synthetic routes to a 4-fluoroproline involves the SN2 displacement of an activated hydroxyl group in a 4-hydroxyproline by a fluoride ion, resulting in the formation of a C-F bond with the inversion of configuration at C-4. (Diethylamino)sulfur trifluoride (DAST [12]) or a congener, such as 4-morpholinosulfur trifluoride (morph-DAST), is typically employed in this strategy [9,13], as such reagents can both activate the hydroxyl group and supply the fluoride ion. DAST and its congeners are, however, both expensive and explosive [14]. Moreover, the analogous precursor of (2S,4R)-4-fluoroproline—(2S,4S)-4-hydroxyproline—is itself a nonnatural amino acid that is expensive.
Here, we report on practical synthetic routes to three 4-flouroproline diasteromers: 2S,4R (1), 2S,4S (2), and 2R,4S (3). The routes all start with HypOH, and none use a DAST-like reagent. All are amenable to a process scale.
2. Results and discussion
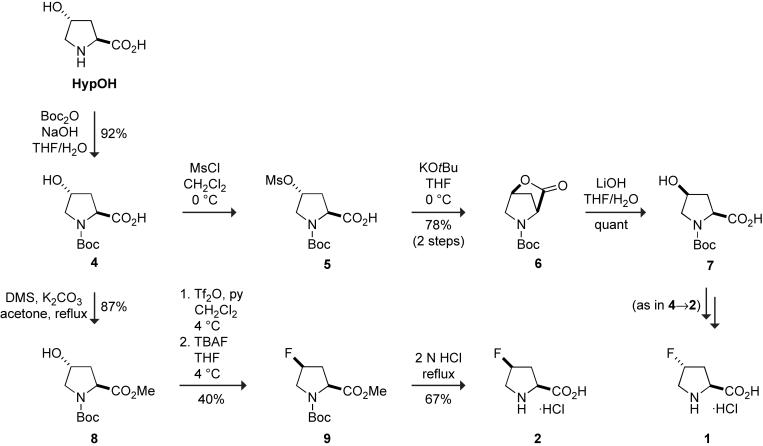
The 2S,4S diastereomer of 4-fluoroproline (2) was synthesized from HypOH by using the SN2 reaction of a fluoride ion to invert the configuration at C-4. Specifically, (2S,4S)-4-fluoroproline (2) was synthesized in four steps from HypOH with an overall yield of 16%. As shown in Scheme 1, the hydroxyl group of compound 4 was activated with trifluoromethane sulfonic anhydride, and the ensuing triflate was displaced with tetra-n-butyl ammonium fluoride (TBAF [15]) to yield protected (2S,4R)-4-fluoroproline; deprotection with acid gave the 2S,4R diastereomer of 4-fluoroproline (2).
Scheme 1.
Likewise, key precursors to the 2S,4R and 2R,4S diastereomers of 4-fluoroproline (1 and 3) were synthesized from HypOH. The routes to these precursors have a notable feature. The route to the 2S,4R diastereomer epimerizes C-4; the route to the 2R,4S diastereomer epimerizes C-2. Each of these epimerizations was accomplished with a distinct lactonization reaction.
N-Boc-(2S,4S)-4-hydroxyproline (7) was synthesized in four steps from HypOH with an overall yield of 72%. As shown in Scheme 1, the amino group of HypOH was protected as a Boc carbamate. Mesylation, followed by the formation of lactone and its hydrolysis, inverted the stereochemistry at C-4. Compound 7 can be taken on to (2S,4R)-4-fluoroproline (1) by the same three steps used to convert compound 4 to (2S,4S)-4-fluoroproline (2) with similar yields (data not shown).
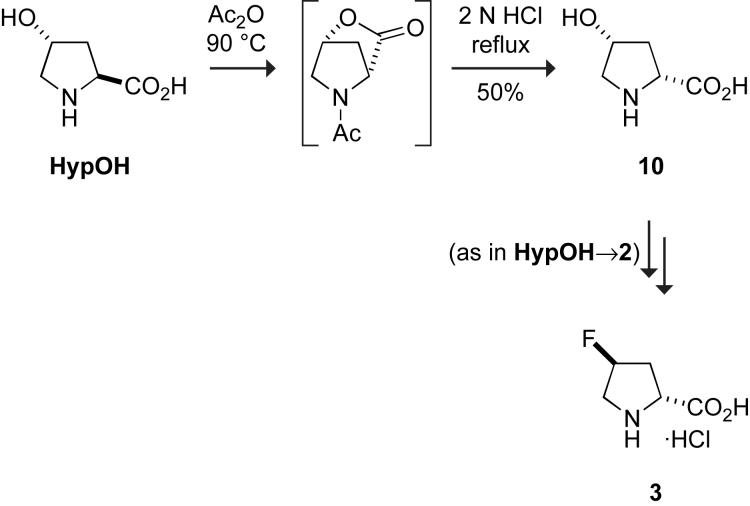
(2R,4R)-4-Hydroxyproline (10) was synthesized from HypOH in one step with a yield of 50%. As shown in Scheme 2, HypOH was treated with acetic anhydride to produce (2R,4R)-4-hydroxyproline lactone. This remarkable transformation has been proposed to proceed via a bicyclic mesoionic intermediate [16], and was used recently in the synthesis of alkaloids from HypOH [17]. The reaction conditions epimerize C-2 of other amino acids, but lead to a single enantiomer here because lactonization traps the 2R epimer. The lactone was hydrolyzed in acid to yield (2R,4R)-4-hydroxyproline. Compound 10 can be taken on to (2R,4S)-4-fluoroproline (3) by the same four steps used to convert HypOH to (2S,4S)-4-fluoroproline (2) with similar yields (data not shown).
Scheme 2.
3. Conclusion
We have described practical routes to the 2S,4R, 2S,4S, and 2R,4R diastereomers of 4-fluoroproline. These routes, which we have used to synthesize 4-fluoroprolines on a kilogram scale, provide ready access to a nonnatural amino acid of growing importance to chemical biology. Finally, we note that these routes accommodate the large-scale synthesis of [F-18]4-fluoroprolines. These probes, along with positron emission tomography, have proven to be useful for monitoring the healing of wounds, permeability of the blood-brain barrier, and other physiological processes [18].
4. Experimental
4.1. Synthesis of N-Boc-(2S,4S)-4-hydroxyproline (7)
4.1.1. N-Boc-(2S,4R)-4-hydroxyproline (4)
HypOH (15.0 g, 0.114 mol) was dissolved in 150 mL of 2:1 THF/H2O. To this solution were added 50 mL of 10% w/v NaOH(aq) and Boc anhydride (30.0 g, 0.137 mol), and the resulting solution was stirred at room temperature overnight. The THF was removed by rotary evaporation under reduced pressure. The residue was dissolved in ethyl acetate, and the pH was adjusted to 2 by the addition of 10% w/v KHSO4(aq). The acidic solution was extracted several times with ethyl acetate. The combined organic extracts were washed with H2O and brine, and then dried over anhydrous Na2SO4(s). The desiccant was removed by filtration, and the solvent was removed by rotary evaporation under reduced pressure to give 4 (24.7 g, 92%) as a syrup.
4.1.2. N-Boc-(2S,4R)-4-mesylproline (5)
Compound 4 (2.00 g, 8.65 mmol) was dissolved in CH2Cl2 at 0 °C. Pyridine (1.40 mL, 17.3 mmol) and mesyl chloride (1.33 mL, 17.3 mmol) were added, and the resulting solution was stirred overnight. The reaction was quenched with water, and the aqueous layer was extracted with CH2Cl2 (3 × 50 mL). The combined extracts were washed with water and brine, dried over anhydrous Na2SO4(s), and evaporated to dryness. The crude product 5 was taken on without purification. (The characterization of compound 5 was as described by Schäfer and corworkers [19].)
4.1.3. N-Boc-(2S,4S)-4-hydroxyproline lactone (6)
Compound 5 (2.80 g, 9.09 mmol) was dissolved in THF containing KOtBu (1.22 g, 10.9 mmol) at 0 °C. The reaction mixture was stirred at room temperature for 12 h. After the reaction was complete (as monitored by TLC), the reaction mixture was quenched with water and extracted with ethyl acetate (2 × 50 mL) to furnish 6 (1.46 g, 78% over two steps) as a syrup. (The characterization of compound 6 was as described by Silverman and coworkers [20].)
4.1.4. N-Boc-(2S,4S)-4-hydroxyproline (7)
Compound 6 (1.00 g, 4.69 mmol) was dissolved in 35 mL of 2:2:3 THF/MeOH/H2O. LiOH (0.33 g, 14.1 mmol) was added, and the resulting solution was stirred overnight. The organic solvent was removed by rotary evaporation under reduced pressure. The residue thus obtained was dissolved in 25 mL of ethyl acetate, and the resulting solution was acidified with a saturated aqueous solution of KHSO4. The aqueous layer was extracted with ethyl acetate (2 × 50 mL), and the combined organic extracts were dried over anhydrous Na2SO4(s) and concentrated under reduced pressure to yield 7 (1.09 g, quant) as a syrup.
4.2. Synthesis of (2S,4S)-4-fluoroproline (2)
4.2.2. N-Boc-(2S,4S)-4-hydroxyproline methylester (8)
Compound 4 (10.0 g, 46.3 mmol) was dissolved in 60 mL of anhydrous acetone. Dimethylsulfate (7.60 mL, 78.7 mmol) and K2CO3 (19.0 g, 138.9 mmol) were added, and the resulting solution was heated at reflux for 6 h. The solution was then cooled to room temperature, filtered through a pad of Celite, and concentrated under reduced pressure. Chromatography (silica gel, 1:1 ethyl acetate/petroleum ether) afforded 8 (9.88 g, 87%) as a solid. (The characterization of compound 8 was as described by Fang and coworkers [21].)
4.2.3. N-Boc-(2S,4S)-4-fluoroproline methylester (9)
Compound 8 (2.98 g, 12.2 mmol) was dissolved in 100 mL of dry methylene chloride. This solution was cooled to 4 °C, and 30 mL of dry pyridine was added, followed by 3.1 mL of trifluoromethane sulfonic anhydride. The solution was allowed to stir for 30 min and then washed with 1 N HCl. The aqueous phase was extracted with methylene chloride (2 × 50 mL), and the combined organic extracts were dried over MgSO4(s) and concentrated under reduced pressure. The reddish syrup thus obtained was dissolved in 100 mL of THF and cooled to 4 °C. To this solution was added a 1.0 M solution of TBAF in THF (1.15 g, 2.5 eq). The resulting mixture was stirred at 4 °C overnight, and then concentrated under reduced pressure. Chromatography (silica gel, 1:1 ethyl acetate/petroleum ether) furnished 9 (1.21 g, 40%) as a solid. (The characterization of compound 9 was as described by Chiba and coworkers [22].)
4.2.4 (2S,4S)-4-fluoroproline (2)
Compound 9 (2.00 g, 8.09 mmol) was dissolved in 10 mL of 2 N HCl. The resulting solution was heated under reflux for 2-4 h. After the reaction was complete (as monitored by TLC), the solution was decolorized with charcoal while still hot, and filtered through a pad of Celite®. Water was removed by rotary evaporation under reduced pressure and as an azeotrope with dry toluene. Crystallization (1:1 ethyl acetate/petroleum ether) afforded the hydrochloride salt of 2 (0.67 g, 49%) as a white solid.
4.3 Synthesis of (2S,4S)-4-hydroxyproline (10)
A stirred mixture of HypOH (6.55 g, 50.0 mmol) in 40 mL of acetic anhydride was heated at 90 °C for 7 h under N2(g). Solvent was removed under reduced pressure. The thick oil thus obtained was dissolved in 25 mL of 2 N HCl, and the resulting solution was heated at reflux for 3 h. The pH of the solution was then adjusted to 6 by the addition of NaOH(aq), water was removed under reduced pressure, and the product was isolated and purified by crystallization (1:1 ethyl acetate/petroleum ether) to yield 10 (3.27 g, 50%) as a solid. (The characterization of compound 10 was as described by Dalla Croce and La Rosa [16].)
Acknowledgements
We are grateful to A. Choudhary and M.D. Shoulders for contributive discussions. This work was supported in part by grant AR044276 (NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1](a).Nilsson BL, Soellner MB, Raines RT. Annu. Rev. Biophys. Biomolec. Struct. 2005;34:91–118. doi: 10.1146/annurev.biophys.34.040204.144700. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang L, Xie J, Schultz PG. Annu. Rev. Biophys. Biomol. Struct. 2006;35:225–249. doi: 10.1146/annurev.biophys.35.101105.121507. [DOI] [PubMed] [Google Scholar]
- [2](a).Holmgren SK, Taylor KM, Bretscher LE, Raines RT. Nature. 1998;392:666–667. doi: 10.1038/33573. [DOI] [PubMed] [Google Scholar]; (b) Holmgren SK, Bretscher LE, Taylor KM, Raines RT. Chem. Biol. 1999;6:63–70. doi: 10.1016/S1074-5521(99)80003-9. [DOI] [PubMed] [Google Scholar]; (c) Hodges JA, Raines RT. J. Am. Chem. Soc. 2003;125:9262–9263. doi: 10.1021/ja035881z. [DOI] [PubMed] [Google Scholar]; (d) Doi M, Nishi Y, Uchlyama S, Nishluchi Y, Nakazawa T, Ohkubo T, Kobayashi Y. J. Am. Chem. Soc. 2003;125:9922–9923. doi: 10.1021/ja035997v. [DOI] [PubMed] [Google Scholar]; (e) Persikov AV, Ramshaw JA, Kirkpatrick A, Brodsky B. J. Am. Chem. Soc. 2003;125:11500–11501. doi: 10.1021/ja036673+. [DOI] [PubMed] [Google Scholar]; (f) Barth D, Milbradt AG, Renner C, Moroder L. ChemBioChem. 2004;5:79–86. doi: 10.1002/cbic.200300702. [DOI] [PubMed] [Google Scholar]; (g) Nishi Y, Uchiyama S, Doi M, Nishiuchi Y, Nakazawa T, Ohkubo T, Kobayashi Y. J. Am. Chem. Soc. 2005;44:6034–6042. doi: 10.1021/bi047887m. [DOI] [PubMed] [Google Scholar]
- [3](a).Renner C, Alefelder S, Bae JH, Budisa N, Huber R, Moroder L. Angew. Chem., Int. Ed. 2001;40:923–925. [PubMed] [Google Scholar]; (b) Kim W, McMillan RA, Snyder JP, Conticello VP. J. Am. Chem. Soc. 2005;127:18121–18132. doi: 10.1021/ja054105j. [DOI] [PubMed] [Google Scholar]; (c) Steiner T, Hess P, Bae JH, Wiltschi B, Moroder L, Budisa N. PLoS One. 2008;3:e1680. doi: 10.1371/journal.pone.0001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4](a).Horng J-C, Raines RT. Protein Sci. 2006;15:74–83. doi: 10.1110/ps.051779806. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Staas DD, Savage KL, Sherman VL, Shimp HL, Lyle TA, Tran LO, Wiscount CM, McMasters DR, Sanderson PE, Williams PD, Lucas BJ, Jr., Krueger JA, Lewis SD, White RB, Yu S, Wong BK, Kochansky CJ, Anari MR, Yan Y, Vacca JP. Bioorg. Med. Chem. 2006;14:6900–6916. doi: 10.1016/j.bmc.2006.06.040. [DOI] [PubMed] [Google Scholar]; (c) Naduthambi D, Zondlo NJ. J. Am. Chem. Soc. 2006;128:12430–12431. doi: 10.1021/ja0648458. [DOI] [PubMed] [Google Scholar]
- [5].Raines RT. Protein Sci. 2006;15:1219–1225. doi: 10.1110/ps.062139406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6](a).Eberhardt ES, Panasik N, Jr., Raines RT. J. Am. Chem. Soc. 1996;118:12261–12266. doi: 10.1021/ja9623119. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Bretscher LE, Jenkins CL, Taylor KM, DeRider ML, Raines RT. J. Am. Chem. Soc. 2001;123:777–778. doi: 10.1021/ja005542v. [DOI] [PubMed] [Google Scholar]; (c) DeRider ML, Wilkens SJ, Waddell MJ, Bretscher LE, Weinhold F, Raines RT, Markley JL. J. Am. Chem. Soc. 2002;124:2497–2505. doi: 10.1021/ja0166904. [DOI] [PubMed] [Google Scholar]
- [7](a).O’Hagan D, Bilton C, Howard JAK, Knight L, Tozer DJ. J. Chem. Soc., Perkin Trans. 2. 2000:605–607. [Google Scholar]; (b) Briggs CRS, O’Hagan D, Howard JAK, Yufit DS. J. Fluorine Chem. 2003;119:9–13. [Google Scholar]
- [8](a).Hinderaker MP, Raines RT. Protein Sci. 2003;12:1188–1194. doi: 10.1110/ps.0241903. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hodges JA, Raines RT. Org. Lett. 2006;8:4695–4697. doi: 10.1021/ol061569t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Doi M, Nishi Y, Kiritoshi N, Iwata T, Nago M, Nakano H, Uchiyama S, Nakazawa T, Wakamiya T, Kobayashi Y. Tetrahedron. 2002;58:8453–8459. [Google Scholar]
- [10].Ramshaw JAM, Shah NK, Brodsky B. J. Struct. Biol. 1998;122:86–91. doi: 10.1006/jsbi.1998.3977. [DOI] [PubMed] [Google Scholar]
- [11].McCaldon P, Argos P. Proteins: Struct. Funct. Genet. 1988;4:99–122. doi: 10.1002/prot.340040204. [DOI] [PubMed] [Google Scholar]
- [12](a).Von Halasz SP, Glemser O. Chem. Ber. 1971;104:1247–1255. [Google Scholar]; (b) Markovskij LN, Pashinnik VE, Kirsanov AV. Synthesis. 1973:787–789. [Google Scholar]; (c) Middleton WJ. J. Org. Chem. 1975;40:574–578. [Google Scholar]
- [13].Thomas KM, Naduthambi D, Tririya G, Zondlo NJ. Org. Lett. 2005;7:2397–2400. doi: 10.1021/ol0506720. [DOI] [PubMed] [Google Scholar]
- [14].Messina PA, Mange KC, Middleton WJ. J. Fluorine Chem. 1989;42:137–143. [Google Scholar]
- [15].Sun H, DiMagno SG. J. Am. Chem. Soc. 2005;127:2050–2051. doi: 10.1021/ja0440497. [DOI] [PubMed] [Google Scholar]
- [16].Dalla Croce P, La Rosa C. Tetrahedron: Asymmetry. 2002;13:197–201. [Google Scholar]
- [17].Ma Z, Hu H, Xiong W, Zhai H. Tetrahedron. 2007;63:7523–7531. [Google Scholar]
- [18](a).Jones HA, Hamacher K, Clark JC, Schofield JB, Krausz T, Haslett C, Boogis AR. Toxicol. Appl. Pharmacol. 2005;207:230–236. doi: 10.1016/j.taap.2005.02.027. [DOI] [PubMed] [Google Scholar]; (b) Langen KJ, Hamacher K, Bauer D, Bröer S, Pauleit D, Herzog H, Floeth F, Zilles K, Coenen HH. J. Cereb. Blood Flow Metab. 2005;25:607–616. doi: 10.1038/sj.jcbfm.9600065. [DOI] [PubMed] [Google Scholar]
- [19].Bernard H, Bülow G, Lange UEW, mack H, Pfeiffer T, Schäfer B, Setiz W, Zierke T. Synthesis. 2004:2367–2375. [Google Scholar]
- [20].Seo J, Martásek P, Roman LJ, Silverman RB. Bioorg. Med. Chem. 2007;15:1928–1938. doi: 10.1016/j.bmc.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liu F-Z, Fang H, Zhu H-W, Wang Q, Yang Y, Xu W-F. Bioorg. Med. Chem. 2008;16:578–585. doi: 10.1016/j.bmc.2007.04.030. [DOI] [PubMed] [Google Scholar]
- [22].Chiba J, Takayama G, Takashi T, Yokoyama M, Nakayama A, Baldwin JJ, McDonald E, Moriarty KJ, Sarko CR, Saionz KW, Swanson R, Hussain Z, Wong A, Machinaga N. Bioorg. Med. Chem. 2006;14:2725–2746. doi: 10.1016/j.bmc.2005.11.058. [DOI] [PubMed] [Google Scholar]