Abstract
Sequential exposure of a zinc-organometallic intermediate, generated through a zinc carbenoid-mediated chain extension reaction of a β-keto carbonyl, to trimethylsilylchloride and iodine provided regioselective formation of an α-iodomethyl-γ-keto carbonyl. The iodomethyl functionality can be further manipulated to provide side chains that are potential mimics of α-amino acid side chains.
Keywords: Zinc, Carbenoid, Chain Extension, Homoenolate
1. Introduction
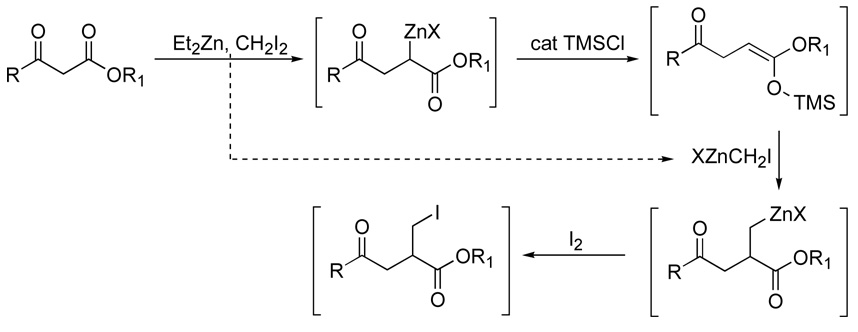
Selective functionalization of carbon skeletons is essential for the development of useful synthetic methodology. A zinc carbenoid-mediated chain extension reaction1 has been developed in our research group in which β-keto esters are converted to γ-keto esters through the intermediacy of a zinc-organometallic intermediate that is localized on the ester’s α-carbon. This organometallic character is reminiscent of the zinc-containing intermediate formed in a classic Reformatsky reaction2 and, as such, we have used this organometallic intermediate in the development of various tandem reaction processes. Treatment of the organometallic intermediate with aldehydes completes a tandem chain extension-aldol process that has been used in peptide isostere formation and natural product synthesis.3 Treatment of the organometallic intermediate with iodine, followed by treatment with a base, provides facile access to α,β-unsaturated-γ-keto esters, which have been used in a number of natural product syntheses.4 Methylation at the α-position has also been accomplished through the activation of the organometallic intermediate by treatment with trimethylsilylchloride (TMSCl) in the presence of excess carbenoid.5 These α-functionalization methods have been complemented recently by a method that incorporates a β–substituent when a substituted carbenoid is used in the chain extension reaction.6
Related to our interest in the further development of this chain extension methodology as a tool for peptide isostere formation, we were interested in identifying methods for the further diversification of the α-substituents. Alkylation of the chain extension’s organometallic intermediate would be very useful, although all efforts to induce alkylation have been unsuccessful. This is consistent with the observation that ethyl iodide, which is a byproduct produced during carbenoid generation,7 is not incorporated into the γ-keto ester by nucleophilic displacement of the iodide. Reports from the literature suggest that alkylations of the zinc Reformatsky intermediate can only occur with activated alkylating agents, under elevated temperatures and/or with polar solvents,8 neither of which are compatible with the zinc carbenoid-mediated chain extension reaction.
As mentioned above, we had developed a limited tandem chain extension-alkylation variation4 that facilitated incorporation of an α-methyl substituent. The proposed mechanism of this reaction involved formation of a putative, transient trimethylsilylketene acetal by treatment of the organometallic intermediate with TMSCl, which then reacts with excess carbenoid to provide a proposed zinc-homoenolate. The β-anionic character inherent to a homo-enolate was demonstrated through deuterium quenching of the reaction mixture. While use of the zinc-homoenolate for additional carbon-carbon formation may be possible, we were interested in trapping the homoenolate with an electrophile that would facilitate a wide variety of addition functionalization strategies. We were particularly interested in the eventual formation of α-side chains that would mimic the α-side chains of amino acids.3b The variability found within amino acid side chains requires incorporation of functionality with sufficient flexibility for the formation of a wide variety of side chains. We report, herein, a tandem chain extension-methylation-iodination reaction (Scheme 1) of β-dicarbonyls that provides a γ-keto carbonyl product in which the α-side chain is appropriately functionalized for additional manipulation.
Scheme 1.
2. Results and Discussion
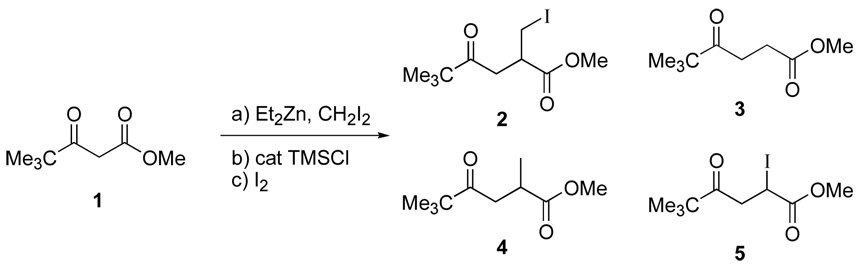
The treatment of methyl pivaloylacetate 1 for ten minutes with the Furukawa reagent, generated from diiodomethane and diethylzinc, provided an intermediate that was treated sequentially with catalytic TMSCl (20 mol %) and iodine (2 equivalents). The reaction can also be perfomed by treatment of the β-dicarbonyl with diethylzinc to induce enolate formation, followed by addition of the diiodomethane, which reacts with the excess diethyl zinc to generate the Furukawa reagent. The corresponding α-iodomethyl-γ-keto ester 2 was isolated after chromatographic purification (Scheme 2). Iodine was chosen as the electrophile due to iodide’s anticipated lability in further manipulations, as well as the recognition that excess iodide is already present in the carbenoid mixture. Efforts to utilize bromine as an electrophile in tandem chain extension reactions have been complicated by iodide’s ability to substitute for the bromide.9
Scheme 2.
While isolation of the desired α-iodomethyl-γ-keto ester was possible, chromatography was necessary to separate compound 2 from a number of side products. Analysis of the crude reaction mixture by 1H NMR revealed the presence of the known compounds γ-keto ester 3,10 α-methylated 4, and α-iodo-γ-keto ester 5.11 The presence of 3 and 5 suggested that the α-methylation step of this three-step process was not proceeding efficiently, while the formation of 4 suggested that insufficient iodine was present to capture the homoenolate. An increase in the reaction time after addition of the TMSCl from 30 min to 60 min inhibited the formation of 3 and 5, while the use of five equivalents of iodine ensured that no 4 was formed.
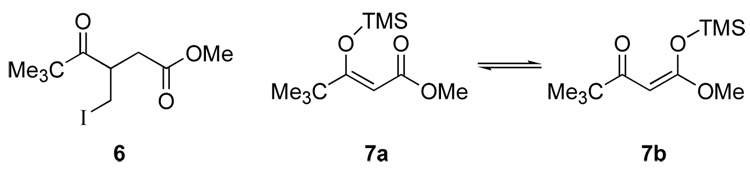
One other by-product was observed in the crude reaction mixtures and proved difficult to separate from the desired product. This compound was believed to be the isomeric iodomethyl-substituted γ-keto ester 6 (Figure 1). The development of nucleophilic character at the β-carbon of the γ-keto ester is inconsistent with our proposed chain extension mechanism; however, a report by Saigo12 suggested that the unanticipated regioselectivity could be explained by cyclopropanation of 7b, followed by ring opening and capture of the excess carbenoid by the ketone enolate. According to Saigo’s hypothesis, compound 7b could be generated in the reaction mixture by zinc-catalyzed isomerization of the TMS group. Formation of 7a and 7b would be possible if TMSCl were incorporated prior to completion of the chain extension reaction. In order to test this hypothesis, the addition of the TMSCl was delayed while the chain extension reaction was allowed to proceed for 30 minutes. This modification facilitated consumption of the starting material prior to addition of the TMSCl, with the result being that the by-product, presumably β-iodomethyl-γ-keto ester 6, was not formed.
Figure 1.
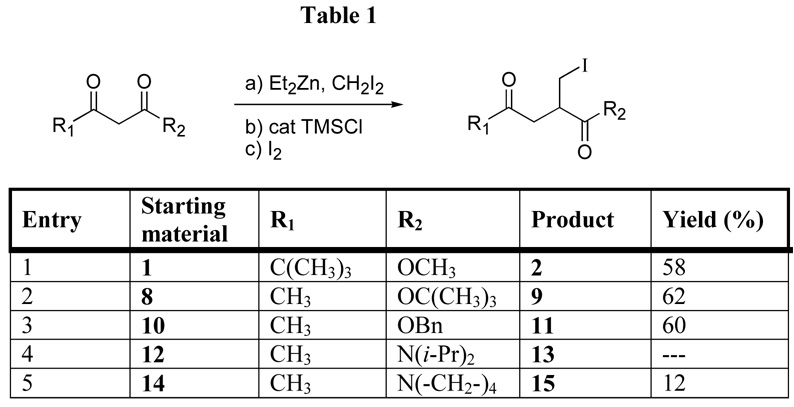
With optimized conditions now available, the formation of α-iodomethyl-γ-keto products from β-keto ester and amide starting materials were studied. The ester starting materials were consistent performers in this reaction, producing isolated yields ranging from 58–62 % (Table 1). Since secondary β-keto amides were inefficient substrates for the chain extension-methylation reaction5 and the modestly acidic NH of a secondary amide would be predicted to quench both the Reformatsky-like organometallic intermediate and the zinc-homoenolate, the decision was made to focus on the reactivity of tertiary β-keto amides. Unfortunately, the tertiary amide starting materials were poor substrates for this tandem reaction. N, N-Diisopropyl 3-oxo-butyramide provided none of the desired α-iodomethylated material. The pyrrolidine amide 14 provided the desired α-iodomethyl-γ-keto amide 15 in only 12% yield. The activation of the Reformatsky-like organometallic intermediate by TMSCl appears to be very inefficient with these substrates, even though Hilgenkamp had reported5 that chain extension-methylation of an N,N-dimethyl β-keto amide proceeded in 53%. The additional steric bulk of tertiary amides 12 and 14 may be responsible for inhibiting formation of the TMS-enol ether.
Scheme 3.
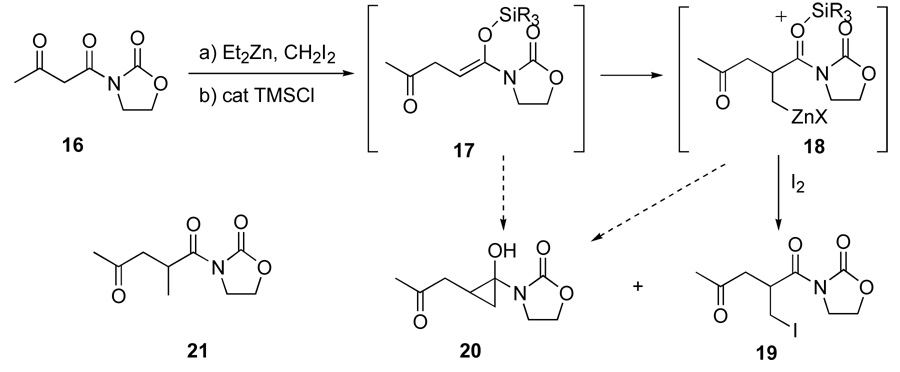
In an effort to broaden the scope of the chain extension-iodomethylation reaction, we investigated the reactivity of β-keto imide starting materials (Scheme 4). The achiral starting material 1613 was converted to a mixture of the α-iodomethylated compound 19 and cyclopropane 20, which was believed to result from either zinc carbenoid cyclopropanation of the TMS-enol ether 17 or cyclization of the homoenolate 18. No cyclopropane-containing products had been observed in α-methylation studies performed with β-keto ester or amide starting materials, which suggests that direct cyclopropanation of the TMS-enol ether is unlikely. In an effort to understand the formation of this unusual product, the reaction was investigated by addition of TMSCl alone to the chain extended Reformatsky-like intermediate (removal of the iodination step). Both an increase in the amount of TMSCl and longer reaction times increased the cyclopropane:α-methylation (20:21) product ratio, which suggests that the homoenolate intermediate 18 participates as a precursor to the cyclopropanation event. Based upon the guidance from equivalency and time studies, the formation of the iodomethylated 19 was effected in 31%, a modest yield consistent with the reactions involving tertiary amides described above.
Scheme 4.
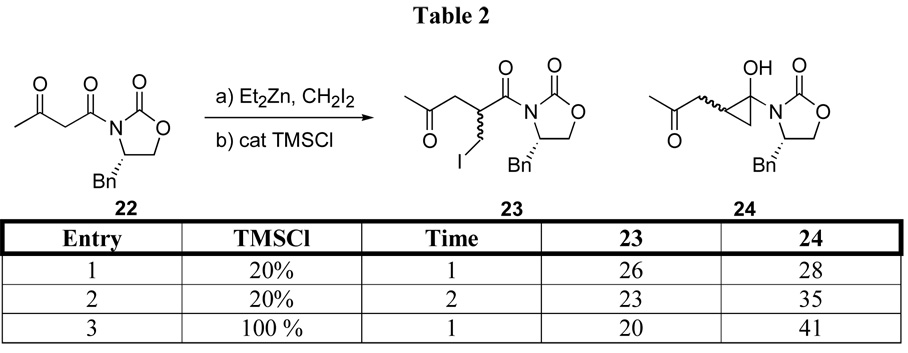
We also explored the chain extension-iodomethylation of chiral oxazolidinone 22 (Scheme 5). A mixture of diastereomeric iodomethylated products 23 and cyclopropanols 24 was generated. Consistent with the reactivity of compound 16, amounts of cyclopropane and iodomethylated products were influenced by reaction time and the amount of TMSCl. Independent of the reaction time and TMSCl equivalencies, the crude reaction mixture revealed that the iodomethylated compounds 23 were formed in an approximate 5:1 diastereomeric ratio, although it was not possible to identify the stereochemistry. Since the facial selectivity in tandem-chain extension aldol reaction of β-keto imides are known3a and the preferred facial selectivities in tandem-chain extension-aldol and the tandem chain extension-homoenolate generation reactions on amino acid-derived β-keto amides are identical,3b the stereochemistry of 23 is tentatively assigned as S,S. Interestingly, the diastereoselectivity in the formation of cyclopropane 24 was much higher, on the order of 19:1, although stereochemical assignment has not been made. If cyclopropane formation takes place by intramolecular cyclization of the homoenolate, one of the homoenolates appears to cyclize to the cyclopropane more quickly. Isolated yields for the iodomethylated material and the cyclopropanes are listed in Table 2.
Scheme 5.
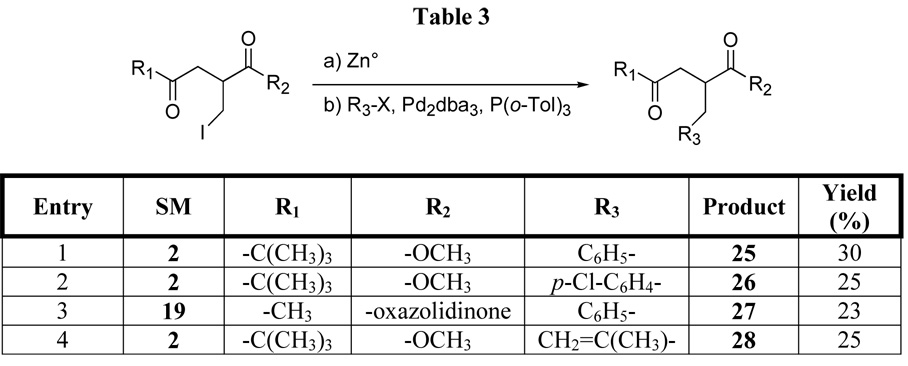
The attractiveness of an iodomethylated product is that a number of functionalized products are available from the primary iodide. One method involves palladium-catalyzed Negishi cross-coupling between an organozinc intermediate available from the primary iodide and an aryl iodide. The cross coupling reaction was successfully and reproducibly performed with different aryl iodides and alkenyl bromides and starting γ-dicarbonyl compounds, albeit in rather disappointing yields (Scheme 6, Table 3). Nevertheless, the 30 % yield achieved in the reaction between 2 and iodobenzene is comparable to the 35–39% yield reported14 on a similar system. A major by-product in this reaction is the α-methylated material 4, which indicates that the homoenolate was formed, but that cross-coupling was not efficient. The proposed intermediacy of a zinc homoenolate (ie 18) in the iodomethylation reaction suggested that the palladium-catalyzed cross-coupling might be able to be performed on the zinc homoenolate generated in the chain extension reaction. Unfortunately, all efforts, which included variations in concentrations, temperatures and solvents, to effect the one-pot chain extension-homoenolate generation-cross coupling reaction were unsuccessful. The major by-product isolated under these conditions was the α-methylated material, indicating that the cross-coupling step does not take place under these reaction conditions.
Scheme 6.
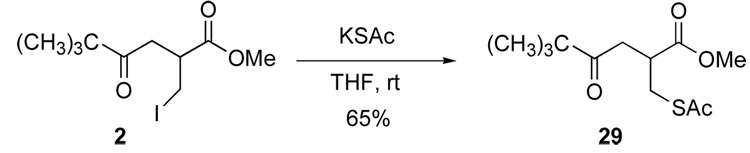
One of the motivations for this study was the desire to expand the library of α-substituents that would mimic α-amino acid side chains in a ketomethylene isostere. For example, the Negishi cross coupling chemistry provided access to α-substituents that could serve as phenylalanine side-chain mimics and, upon hydrogenation of 28, isoleucine mimics. We also felt that the iodomethyl substituents provided a resource for addressing other side chains. For example, cysteine mimics could be accessed through sulfur displacement of the iodide. A number of nucleophilic sulfur reagents have been used for this displacement,15 although we found that use of potassium thioacetate provide the most efficient formation of a protected cysteine mimic 29. Deprotection of the thioacetate functionality is well-precedented.16
3. Conclusion
In summary, a tandem chain extension-iodomethylation reaction has been developed for β-keto esters and β-keto imides. The yields for this reaction are modest, in large part, due to the performance of three sequential transformations in one reaction vessel. This variation on the zinc carbenoid-mediated chain extension reaction provides the ability to convert readily available β-keto ester starting materials into products that are nicely functionalized at the ester’s α-carbon, which is the least thermodynamically-acidic site of the γ-keto ester. The long-term utility of the iodomethylated materials available from this reaction remains to be seen, although palladium-mediated coupling and nucleophilic displacement reactions are possible. A by-product observed in this reaction when performed on β-keto imides is a substituted-cyclopropyl alcohol, most likely resulting from cyclization of homoenolate. The uses of this cyclopropyl alcohol as a chiral homoenolate-equivalent and a chiral cyclopropanone equivalent are under investigation.
4. Experimental
4.1 General Information
All reactions were run in oven-dried glassware and stirred with teflon-coated magnetic stir-bars. The term concentrated under reduced pressure refers to the use of a rotary-evaporator or vacuum pump. Melting points are uncorrected. Optical rotations were conducted in the specified solution and concentrations are given in g/mL. Methylene chloride was dried by passing the solvent through a column of alumina or molecular sieves using an Innovative Technology Inc. solvent delivery system. Ethyl acetate (EtOAc) and hexanes used for chromatography were distilled prior to use. Iodine was sublimed prior to use. Column chromatography was performed with flash silica gel (32–63µm). The mobile phase was prepared as noted in the individual experimental section. Thin Layer Chromatography (TLC) was carried out on glass-backed silica plates with the indicated mobile phase and visualized by UV and anisaldehyde or KMnO4 stains. Nuclear Magnetic Resonance (NMR) spectroscopy was performed on a Varian Mercury operating at 399.768 MHz for 1H nuclei and 100.522 MHz for 13C nuclei, or a Varian Inova operating at 499.766 MHz for 1H nuclei and 125.679 MHz for 13C nuclei. All 13C are 1H-decoupled. All chemical shifts are reported in parts per million (ppm) downfield of tetramethylsilane (TMS) internal standard.
4.2 Experimental procedures
General Procedure for the Preparation of α-Iodomethyl γ-Keto Esters
An oven-dried, one-necked, 50-mL round-bottomed flask equipped with a rubber septum and magnetic stir-bar was cooled to room temperature under a nitrogen atmosphere, and charged with 15 mL of methylene chloride. The solvent was cooled to 0 °C in an ice bath and methylene iodide (0.32 mL, 4.0 mmol) was added dropwise into the flask. Diethyl zinc (4.0 mL, 1.0 M in hexane, 4.0 mmol) was added slowly to the reaction mixture at 0 °C over two min. The resulting white suspension was stirred for 10 min, and then the starting β-keto ester (1.0 mmol) was added rapidly. After the solution was stirred for 30 min, trimethylsilylchloride (25 µL, 0.2 mmol) was added in one portion by micro-syringe. The mixture was allowed to stir for 45 min at room temperature followed by the addition of iodine (1.269 g, 5.0 mmol) to the reaction mixture. The reaction mixture quickly became a pink suspension and was stirred for 10 min, quenched with 10 mL of saturated aqueous ammonium chloride, and extracted with diethyl ether (3×10 mL). The combined organic layers were washed with saturated sodium thiosulfate solution (2×15 mL), and brine (3×20 mL), dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure (25 °C, 30 torr). The residue was purified by column chromatography on silica to provide the α-iodomethyl-γ-keto ester.
2-Iodomethyl-5, 5-dimethyl-4-oxo-hexanoic acid methyl ester 2
Column chromatography on silica (30:1, hexane: ethyl acetate, Rf = 0.16) provided 0.181 g (58%) of 2-iodomethyl-5,5-dimethyl-4-oxo-hexanoic acid methyl ester (2) as a clear yellow liquid. 1H NMR (400 MHz, CDCl3) δ 3.73 (s, 3 H), 3.45 (dd, J = 6.0, 10.0 Hz, 1 H), 3.38 (dd, J = 5.4, 10.0 Hz, 1 H), 3.20 (m, 1 H), 3.11 (dd, J = 7.0, 17.9 Hz, 1 H), 2.80 (dd, J = 5.4, 18.0 Hz, 1 H), 1.18 (s, 9 H); 13C NMR (100 MHz, CDCl3) δ 213.3, 172.5, 52.6, 44.3, 42.0, 39.2, 26.8, 6.3; IR (neat, cm−1): 2967, 1738, 1705, 1477, 1366, 1232. HRMS (EI) m/z calcd 312.0222, found 312.0225.
2-Iodomethyl-4-oxo-pentanoic acid tert-butyl ester 9
Column chromatography on silica (15:1, hexane: ethyl acetate, Rf = 0.15) provided 0.201 g (63%) of 2-iodomethyl-4-oxo-pentanoic acid tert-butyl ester (9) as a clear yellow liquid. 1H NMR (400 MHz, CDCl3) δ 3.46 (dd, J = 5.6, 9.9 Hz, 1 H), 3.35 (dd, J = 4.4, 9.9 Hz, 1 H), 3.07 (m, 1 H), 3.00 (dd, J = 5.5, 17.6 Hz, 1 H), 2.62 (dd, J = 5.0, 17.6 Hz, 1 H), 2.20 (s, 3H), 1.46 (s, 9 H); 13C NMR (100 MHz, CDCl3) δ 206.0, 170.8, 82.0, 45.3, 42.5, 30.4, 28.1, 7.3. HRMS (EI) m/z calcd for C6H8IO3 (M-tBu) 254.9518, found 254.9507.
2-Iodomethyl-4-oxo-pentanoic acid benzyl ester 11
Column chromatography on silica (25:1, hexane: ethyl acetate, Rf = 0.21) provided 0.208 g (58%) of 2-iodomethyl-4-oxo-pentanoic acid benzyl ester (11) as a clear yellow liquid. 1H NMR (400 MHz, CDCl3) δ 7.36 (s, 5 H), 5.15-5.14 (m, 2 H), 3.48 (dd, J = 7.5, 11.4 Hz, 1 H), 3.39 (dd, J = 4.6, 10.1 Hz, 1 H), 3.23 (m, 1 H), 3.06 (dd, J = 7.4, 18.1 Hz, 1 H), 2.71 (dd, J = 5.4, 18.2 Hz, 1 H), 2.18 (s, 3 H); 13C NMR (100 MHz, CDCl3) δ 205.8, 171.7, 135.6, 128.8, 128.7, 67.5, 45.3, 41.9, 30.4, 6.1. HRMS (EI) m/z calcd 346.0066, found 346.0070.
2-Iodomethyl-1-(2-oxo-oxazolidin-3-yl)-pentane-1, 4-dione 19
An oven-dried, one-necked, 100-mL round-bottomed flask equipped with a rubber septum and magnetic stir-bar was cooled to room temperature under a nitrogen atmosphere, and charged with 1-(2-oxo-oxazolidin-3-yl)-butane-1,3-dione 16 (0.346 g, 2.0 mmol) dissolved in 30 mL of methylene chloride. The solvent was cooled to 0 °C in an ice bath and diethyl zinc (10.0 mL, 1.0 M in hexane, 10.0 mmol) was added slowly to the flask at 0 °C over two min to form an enolate. The reaction mixture was stirred for 15 min, and then methylene iodide (0.48 mL, 6.0 mmol) was added into the flask. An additional portion of methylene iodide (0.32 mL, 4.0 mmol) was added to the reaction mixture at 0 °C after 15 to 20 min. The resulting solution was stirred for 30 min, and trimethylsilylchloride (50 µL, 0.4 mmol) was added by micro-syringe in one portion. The mixture was allowed to stir for 30 min at room temperature followed by the addition of iodine (2.58 g, 10.0 mmol) to the reaction mixture. The reaction mixture quickly became a pink suspension and was stirred for 10 min, quenched with 10 mL of saturated aqueous ammonium chloride, and extracted with ethyl acetate (5×20 mL). The combined organic layers were washed with saturated sodium thiosulfate solution (2×25 mL), and brine (3×25 mL), dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure (25 °C, 20 torr). The residue was purified by column chromatography on silica (2:1, hexane: ethyl acetate, Rf = 0.16) to yield 0.101 g (31%) of 2-iodomethyl-1-(2-oxo-oxazolidin-3-yl)-pentane-1,4-dione (19) as a yellow solid. mp 71–75 °C; 1H NMR (400 MHz, CDCl3) δ 4.50-4.42 (m, 2 H), 4.31 (m, 1 H), 4.12-3.97 (m, 2H), 3.39 (dd, J = 5.4, 10.2 Hz, 1H), 3.32 (dd, J = 6.5, 10.1 Hz, 1H), 3.16 (dd, J = 9.9, 18.1 Hz, 1H), 2.75 (dd, J = 4.1, 18.0 Hz, 1H), 2.18 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 206.1, 172.3, 153.4, 62.4, 46.6, 42.9, 39.0, 29.9, 4.4. HRMS (EI) m/z calcd 324.9811, found 324.9813.
3-[1-Hydroxy-2-(2-oxo-propyl)-cyclopropyl]-oxazolidin-2-one 20 and 2-Methyl-1- (2-oxo-oxazolidin-3-yl)-pentane-1, 4-dione 21
An oven-dried, one-necked, 100-mL round-bottomed flask equipped with a rubber septum and magnetic stir-bar was cooled to room temperature under a nitrogen atmosphere, and charged with 1-(2-oxo-oxazolidin-3-yl)-butane-1,3-dione 16 (0.171 g, 1 mmol) dissolved in 15 mL of methylene chloride. The solvent was cooled to 0 °C in an ice bath and diethyl zinc (5.0 mL, 1.0 M in hexane, 5.0 mmol) was added slowly to the flask at 0 °C over two min to form an enolate. The reaction mixture was stirred for 15 min, and then methylene iodide (0.24 mL, 3.0 mmol) was added into the flask. An additional portion of methylene iodide (0.16 mL, 2.0 mmol) was added to the reaction mixture at 0 °C after 15 to 20 min. The resulting solution was stirred for 30 min, and trimethylsilylchloride (50 µL, 0.4 mmol) was added by micro-syringe in one portion. The mixture was allowed to stir for 45 min, quenched with 10 mL of saturated aqueous ammonium chloride, and extracted with ethyl acetate (3×20 mL). The combined organic layers were washed with brine (3×15 mL), dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure (25 °C, 20 torr). The residue was purified by column chromatography on silica (2:1, hexane: ethyl acetate, Rf = 0.08) to yield 0.06 g (30%) of 20 as a colorless liquid. 1H NMR (400 MHz, CDCl3) δ 4.28-4.16 (m, 2 H), 3.76-3.66 (m, 2 H), 2.56 (dd, J = 4.1, 17.4 Hz, 1 H ), 2.35 (dd, J = 8.8, 18.2 Hz, 1 H), 2.09 (s, 3 H), 1.64-1.59 (m, 2 H), 1.18 (m, 1 H), 0.78 (t, J = 6.9 Hz, 1 H); 13C NMR (100 MHz, CDCl3) δ 209.1, 158.2, 65.2, 62.8, 43.4, 42.3, 30.3, 20.9, 20.5; IR (neat, cm−1): 3442(b), 2927, 1775, 1699, 1390, 1227, 1039. HRMS (CI, NH3) [M+H]+ m/z calcd 200.0923, found 200.0917.
Compound 21 was also isolated from the crude reaction mixture by column chromatography on silica (Rf = 0.16) in a 22% yield (0.043 g) as a colorless liquid. 1H NMR (400 MHz, CDCl3) δ 4.43-4.30 (m, 2 H), 4.41-3.95 (m, 3 H), 3.10 (dd, J = 10.3, 18.1 Hz, 1 H), 2.53 (dd, J = 4.1, 18.2, 1 H), 2.14 (s, 3 H), 1.18 (d, J = 7.0 Hz, 3 H); 13C NMR (100 MHz, CDCl3) δ 207.1, 176.8, 153.4, 62.2, 47.4, 42.9, 33.3, 29.8, 17.3. IR (neat, cm−1): 1775, 1698, 1392, 1264,1202. HRMS (EI) m/z calcd 199.0845, found 199.0841.
1-((S) 4-Benzyl-2-oxo-oxazolidin-3-yl)-butane-1, 3-dione 22
An oven-dried, one-necked, 100-mL round-bottomed flask equipped with a rubber septum and magnetic stir-bar was cooled to room temperature under a nitrogen atmosphere, and charged with a solution of (S)-4-benzyl-oxazolidin-2-one (1.4 g, 8.0 mmol) in 50 mL of anhydrous tetrahydrofuran. The THF solution was cooled to −78 °C and n-butyl lithium (4.0 mL, 10.0 mmol of a 2.5 M solution in hexane) was added dropwise to the flask. After the completion of the addition, the reaction mixture was warmed to 0 °C for 10 min. The mixture was cooled to −78 °C again, and diketene (1.23 mL, 16.0 mmol) was added slowly to the mixture. The resultant orange solution was maintained at −78 °C for an additional 30 min after the addition was complete, and then allowed to stir at 0 °C for 2 h. The mixture was quenched with 15 mL of saturated NH4Cl solution and concentrated under reduced pressure (25 °C, 18 torr) to remove the solvent THF. The residue was extracted by methylene chloride (3×20 mL), and the combined organic layers were washed with sat. NaHCO3 solution (2×20 mL) and brine (3×20 mL), dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The residue was purified by column chromatography on silica (4:1, hexane: ethyl acetate, Rf = 0.14) to yield 0.80 g (38%) of 22 as a light yellow solid. mp 97–98.5 °C; lit mp 97–98 °C;17 1H NMR (400 MHz, CDCl3) δ 7.36-7.22 (m, 5 H), 4.72 (m, 1 H), 4.25-4.16 (m, 2 H ), 4.11-4.06 (m, 2 H), 3.37 (dd, J = 3.4, 13.5 Hz, 1 H), 2.80 (dd, J = 9.6, 13.5 Hz, 1 H), 2.29 (s, 3 H); 13C NMR (100 MHz, CDCl3) δ 201.1, 166.6, 153.9, 135.3, 129.7, 129.2, 127.6, 66.6, 55.2, 51.6, 38.4, 30.4; enol form: δ 180.5, 153.9, 135.3, 129.7, 129.2, 127.6, 90.1, 66.3, 54.9, 38.4, 22.4.
1-((S)-4-Benzyl-2-oxo-oxazolidin-3-yl)-2-iodomethyl-pentane-1, 4-dione 23 and (S)-4-Benzyl-3-[1-hydroxy-2-(2-oxo-propyl)-cyclopropyl]-oxazolidin-2-one 24
An oven-dried, one-necked, 50-mL round-bottomed flask equipped with a rubber septum and magnetic stir-bar was cooled to room temperature under a nitrogen atmosphere, and charged with a solution of 1-((S) 4-benzyl-2-oxo-oxazolidin-3-yl) -butane-1,3-dione 112 ( 0.261 g, 1.0 mmol) in 15 mL of methylene chloride. The solvent is cooled to 0 °C in an ice bath and diethyl zinc (5.0 mL, 1.0 M in hexane, 5.0 mmol) was added slowly to the flask at 0 °C over two min to form an enolate. The reaction mixture was stirred for 15 min, and then methylene iodide (0.24 mL, 3.0 mmol) was added into the flask. An additional portion of methylene iodide (0.16 mL, 2.0 mmol) was added to the mixture at 0 °C after 15 to 20 min. The resultant solution was stirred for 30 min, and trimethylsilylchloride (25 µL, 0.2 mmol) was added by micro-syringe in one portion. The mixture was allowed to stir for 30 min at room temperature followed by adding iodine (1.265 g, 5.0 mmol) to the reaction. The reaction mixture quickly became a pink suspension and was stirred for 10 min, quenched with 10 mL of saturated aqueous ammonium chloride, and extracted with ethyl acetate (3×20 mL). The combined organic layers were washed with saturated sodium thiosulfate solution (2×15 mL) and brine (3×15 mL), dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The residue was purified by column chromatography on silica (3:1, hexane: ethyl acetate, Rf = 0.28) to yield 0.11 g (26%) of 23 as a light yellow liquid of two diastereoisomers with a ratio of 2.5:1, as determined by integration of the reaction material’s 1H NMR spectrum.. 1H NMR (400 MHz, CDCl3) δ 7.33-7.20 (m, 5 H), 4.80-4.67 (m, 1 H), 4.31-4.16 (m, 3 H), 3.42 (d, J = 5.5 Hz, 2 H), 3.36-3.33 (m, 1 H), 3.20 (dd, J = 10.1, 18.1 Hz, 1 H), 2.87-2.69 (m, 2 H), 2.19 (s, 3 H); 13C NMR (100 MHz, CDCl3) δ 206.0, 172.0, 153.3, 140.9, 129.6, 129.2, 127.7, 66.8, 55.5, 46.4, 40.0, 38.5, 37.8, 29.9, 4.9; LRMS (MALDI): calcd for C16H18O4N I (M+-I) 288.2: found, 287.7. HRMS (EI) m/z calcd 415.0281, found 415.0285. Small quantities of the α,β-unsaturated compound 1-((S)-4-Benzyl-2-oxo-oxazolidin-3-yl)-pent-2-ene-1,4-dione was also present in the reaction mixture. Resonances observed for this compound: 1H NMR (400 MHz, CDCl3) δ 8.0 (d, J = 15.9 Hz, 1 H), 7.1 (d, J = 15.9 Hz, 1 H); 13C NMR (100 MHz, CDCl3) δ 198.1, 164.4, 135.1, 27.8.
One predominant stereoisomer of 24 was also isolated in the amount of 0.08 g (28%) as a colorless crystal during column chromatography on silica (TLC data: 1:1, hexane: ethyl acetate, Rf = 0.12). mp 102–103 °C; [α]25 D = +4.8 (c = 0.0053 g/mL, CHCl3). 1H NMR (400 MHz, CDCl3) δ 7.39-7.25 (m, 5 H), 4.75 (s, 1 H), 4.22 (m, 1 H), 4.07-3.98 (m, 2 H), 3.54 (dd, J = 3.8, 13.4 Hz, 1H), 2.83 (dd, J = 4.5, 18.4 Hz, 1 H), 2.71-2.62 (m, 2 H), 2.22 (s, 3 H), 1.54 (m, 1 H), 1.38 (dd, J = 5.9, 10.1 Hz, 1 H), 0.96 (t, J = 6.2 Hz, 1 H); 13C NMR (100 MHz, CDCl3) δ 208.5, 157.8, 136.7, 129.7, 129.2, 127.1, 66.9, 64.8, 57.6, 42.1, 38.4, 30.2, 21.8, 19.6. IR (KBr, cm−1): 3317 (b), 3029, 2921, 1745, 1603, 1413, 1246. LRMS (MALDI): calcd for C16H19O4N (M+ +Na+) 312.2: found, 312.1; calcd for C16H19O4N (M+ +K+) 328.3: found, 328.1. HRMS (CI, NH3) [M+H]+ m/z calcd 290.1392, found 290.1391.
General procedure for the palladium-catalyzed Negishi coupling
A mixture of zinc dust (0.09 g, 1.4 mmol) and iodine (2.0 mg, 0.005 mmol) was placed into an oven-dried, 25-mL round-bottomed flask equipped with a rubber septum and magnetic stir bar. The flask was allowed to cool to room temperature under nitrogen gas. Dimethylformamide (1 mL) was added via syringe into the flask, followed by the addition of a solution of the α-iodomethyl-γ-keto carbonyl starting material (2 or 19) (1.0 mmol) in 4 mL of DMF dropwise by syringe. The reaction mixture was stirred at 0 °C (ice bath) for 30 min. The ice bath was removed and the septum was replaced with a reflux condenser after the sp2-hybridized halide (1.1 – 1.3 mmol), tris(dibenzylideneacetone)dipalladium(0) (45 mg, 0.05 mmol) and tri-o-tolylphosphine (60 mg, 0.2 mmol) were added. The reaction mixture was heated to 60 °C and stirred for 5 h. The resulting black mixture was decanted to an Erlenmeyer flask containing 10 mL of deionized water. An additional 5 mL of saturated citric acid solution was added in order to break the emulsion. The mixture was extracted with diethyl ether (3×10 mL), and the combined organic layers were washed with deionized water (2×10 mL) and brine (2×10 mL), dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The residue was purified by column chromatography.
2-Benzyl-5, 5-dimethyl-4-oxo-hexanoic acid methyl ester 25
The residue was purified by column chromatography (30:1, hexane: ethyl acetate, Rf = 0.14) to yield 0.79 g (30%) of 2-benzyl-5,5 -dimethyl-4-oxo-hexanoic acid methyl ester 25 as a clear orange liquid. 1H NMR (400 MHz, CDCl3) δ 7.26 (t, J = 7.4 Hz, 2 H), 7.19 (t, J = 7.3 Hz, 1 H), 7.13 (d, J = 6.8 Hz, 2H), 3.61 (s, 3 H), 3.14 (m, 1H), 2.99 (dd, J = 6.6, 13.5 Hz, 1 H), 2.92 (dd, J = 9.0, 18.2 Hz, 1 H), 2.72 (dd, J = 8.3, 13.6 Hz, 1 H), 2.56 (dd, J = 4.6, 18.1 Hz, 1 H), 1.08 (s, 9 H); 13C NMR (100 MHz, CDCl3) δ 214.3, 175.6, 138.8, 129.4, 129.1, 126.8, 51.9, 44.1, 42.2, 37.9, 26.6; IR (neat, cm−1): 2966, 1736, 1704, 1476, 1366, 1230; MS (ESI): calcd for C16H22O3 (M+ +Na+) 285.1919: found, 285.1. HRMS (EI) m/z calcd 262.1569, found 262.1568.
2-(4-Chloro-benzyl)-5, 5-dimethyl-4-oxo-hexanoic acid methyl ester 26
The residue was purified by column chromatography (20:1, hexane: ethyl acetate, Rf = 0.18) to yield 0.075 g (25%) of 2-(4-chloro-benzyl)-5,5 -dimethyl- 4-oxo-hexanoic acid methyl ester 26 as a clear orange liquid. 1H NMR (400 MHz, CDCl3□ δ 7.25 (d, J = 8.6 Hz, 2 H), 7.09 (d, J = 8.3 Hz, 2 H), 3.62 (s, 3 H), 3.15 (m, 1 H), 2.96 (dd, J = 7.0, 11.9 Hz, 1 H), 2.92 (dd, J = 8.5, 18.0 Hz, 1 H), 2.71 (dd, J = 7.8, 13.8 Hz, 1 H), 2.54 (dd, J = 4.9, 18.1 Hz, 1 H), 1.09 (s, 9 H); 13C NMR (100 MHz, CDCl3) δ 214.0, 175.6, 137.3, 132.6, 130.5, 128.8, 52.1, 44.3, 42.2, 38.5, 36.6, 26.6; IR (neat, cm−1): 2967, 1736, 1704, 1492, 1169. HRMS (EI) m/z calcd 296.1179, found 296.1173.
2-Benzyl-1-(2-oxo-oxazolidin-3-yl)-pentane-1,4-dione 27
The residue was purified by column chromatography (1:1, hexane: ethyl acetate, Rf = 0.47) to yield 0.066 g (24%) of 2-benzyl-1-(2-oxo-oxazolidin -3-yl)-pentane- 1,4-dione 27 as a white solid. mp 108–111 °C; 1H NMR (400 MHz, CDCl3) δ 7.30-7.27 (m, 5 H), 4.45-4.30 (m, 3 H); 4.05 (dd, J = 6.7, 9.4 Hz, 1 H), 3.93 (dd, J = 6.9, 9.3 Hz, 1 H), 3.09 (dd, J = 5.1, 13.1 Hz, 1 H), 3.03 (dd, J = 10.7, 18.0 Hz, 1 H), 2.51 (dd, J = 10.0, 13.1 Hz, 1 H), 2.45 (dd, J = 3.5, 18.3 Hz, 1 H), 2.07 (s, 3 H); 13C NMR (100 MHz, CDCl3) δ 207.2, 175.7, 153.4, 138.2, 129.4, 128.7, 126.9, 62.2, 44.4, 42.9, 40.2, 37.9, 29.9. IR (KBr, cm−1): 2921, 1773, 1715, 1480, 1395, 1263. HRMS (EI) m/z calcd 275.1158, found 275.1152.
5, 5-Dimethyl-2-(2-methyl-allyl)-4-oxo-hexanoic acid methyl ester 28
The residue was purified by column chromatography (20:1, hexane: ethyl acetate, Rf = 0.15) to yield 0.075 g (12%) of 5,5-dimethyl-2-(2-methyl-allyl)-4-oxo-hexanoic acid methyl ester 28 as a clear orange liquid. 1H NMR (400 MHz, CDCl3) δ 4.79 (s, 1 H), 4.69 (s, 1 H), 3.66 (s, 3 H), 3.07 (m, 1 H), 2.92 (dd, J = 9.3, 18.1 Hz, 1 H), 2.58 (dd, J = 4.2, 18.1 Hz, 1 H), 2.38 (dd, J = 6.8, 14.2 Hz, 1 H), 2.14 (dd, J = 8.5, 14.6 Hz, 1 H), 1.79 (s, 3 H), 1.14 (s, 9 H); 13C NMR (100 MHz, CDCl3) δ 214.4, 175.9, 142.8, 113.2, 51.9, 44.2, 40.5, 38.4, 38.1, 26.5, 21.9. HRMS (EI) m/z calcd 226.1569, found 226.1575.
2-Acetylsulfanylmethyl-5, 5-dimethyl-4-oxo-hexanoid acid methyl ester 29
Into an oven-dried, one-necked, 10-mL round-bottomed flask equipped with a rubber septum and magnetic stir-bar with a flow of nitrogen were placed potassium thioacetate (0.23 g, 2.0 mmol), compound 2 (0.312 g, in 1 mL of tetrahydrofuran, 1.0 mmol) and 4.0 mL of tetrahydrofuran. The light yellow suspension was allowed to stir overnight at room temperature. The resultant brown suspension was extracted with diethyl ether (3×15 mL) and the organic layer was removed using a pipette. The combined ether extracts were washed with brine (3×15 mL), dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The residue was purified by column chromatography on silica (15:1, hexane: ethyl acetate, Rf = 0.10) to yield 0.17 g (65%) of 2-acetylsulfanylmethyl-5,5-dimethyl-4-oxo-hexanoid acid methyl ester (29) as a orange liquid. 1H NMR (400 MHz, CDCl3) δ 3.63 (s, 3 H), 3.16 (dd, J = 8.0, 15.6 Hz, 1 H), 3.08-3.02 (m, 2 H), 2.96 (dd, J = 7.2, 18.0 Hz, 1 H), 2.64 (dd, J = 4.2, 17.8 Hz, 1 H), 2.27 (s, 3 H), 1.08 (s, 9 H); 13C NMR (100 MHz, CDCl3) δ 213.8, 194.9, 173.8, 52.3, 44.1, 40.3, 37.5, 30.5, 30.2, 26.6. HRMS (CI, NH3) [M+H]+ m/z calcd 261.1161, found 261.1164.
Supplementary Material
Scheme 7.
Acknowledgements
We are grateful to the National Institutes of Health (R15 GM060967-02) for their support of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Brogan JB, Zercher CK. J. Org. Chem. 1997;62:6444–6446. [Google Scholar]
- 2.Furstner A. In: Organozinc Reagents: A Practical Approach. Knochel P, Jones P, editors. Oxford University Press; 1999. pp. 287–305. [Google Scholar]
- 3.a) Lai S-J, Zercher CK, Jasinski JP, Reid SN, Staples RJ. Org. Lett. 2001;3:4169–4171. doi: 10.1021/ol016788n. [DOI] [PubMed] [Google Scholar]; b) Lin W, Tryder N, Su F, Zercher CK, Jasinski JP, Butcher RJ. J. Org. Chem. 2006;71:8140–8145. doi: 10.1021/jo061184o. [DOI] [PubMed] [Google Scholar]
- 4.a) Ronsheim MD, Zercher CK. J. Org. Chem. 2003;68:1878–1885. doi: 10.1021/jo0264776. [DOI] [PubMed] [Google Scholar]; b) Lin W, Zercher CK. J. Org. Chem. 2007;72:4390–4395. doi: 10.1021/jo0701379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hilgenkamp R, Zercher CK. Org. Lett. 2001;3:3037–3040. doi: 10.1021/ol016485t. [DOI] [PubMed] [Google Scholar]
- 6.Lin W, McGinness RJ, Wilson E, Zercher CK. Synthesis. 2007:2404–2408. [Google Scholar]
- 7.Furukawa J, Kawabata N, Nishimura J. Tetrahedron. 1968;24:53–58. [Google Scholar]
- 8.a) Orsini F, Pelizzoni F, Ricca G. Tetrahedron. 1984;40:2781–2787. [Google Scholar]; b) Orsini F, Pelizzoni F. Synth. Commun. 1984;14:805–816. [Google Scholar]
- 9.Ronsheim MD, Zercher CK. J. Org. Chem. 2003;68:4535–4538. doi: 10.1021/jo026299g. [DOI] [PubMed] [Google Scholar]
- 10.Ronsheim MD, Hilgenkamp R, Zercher CK. Org. Synth. 2002;79:146–153. [Google Scholar]
- 11.Aiken KA. Ph.D Thesis. University of New Hampshire; 2006. [Google Scholar]
- 12.Saigo K, Yamashita T, Hongu A, Hasegawa M. Synth. Commun. 1985:715–721. [Google Scholar]
- 13.Palombi L, Feroci M, Orsini M, Rossi L, Inesi A. Tetrahedron Lett. 2002;43:2881–2884. [Google Scholar]
- 14.Jackson RFW, Perez-Gonzalez M. Org. Synth. 2005;81:77–88. [Google Scholar]
- 15.a) Schmittberger T, Uguen D. Tetrahedron Lett. 1996;37:29–32. [Google Scholar]; b) Kaloustian MK, Khouri FF. J. Am. Chem. Soc. 1986;108:6683–6695. [Google Scholar]; c) Ashby EC, Park WS, Goel AB, Su W-Y. J. Org. Chem. 1985;50:5184–5193. [Google Scholar]
- 16.Zervasa L, Ghelis N. J. Am. Chem. Soc. 1963;85:1337–1341. [Google Scholar]
- 17.Galvez N, Moreno-Manas M, Padros I, Sebastian RM, Serra N, Vallribera A. Polyhedron. 1995;14:1397–1399. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.