Abstract
We describe a concise and convergent route to the core matrix of the cortistatin steroidal alkaloids. The salient features of the synthesis are the Snieckus cascade methodology and the Masamune alkylative dearomatization. This chemistry lends itself to a total synthesis of the cortistatins and to the development of a SAR program based on diverted total synthesis.
In 2006, Kobayashi and coworkers reported the isolation of a novel class of steroidal alkaloids, the cortistatins, from the marine sponge, Corticium simplex.1 Among them, cortistatin A, in particular, exhibited cytostatic antiproliferative activity against human umbilical vein endothelial cells (HUVECs) at concentrations as low as 100 pM. The high selectivity observed for the endothelial (HUVEC) cell line, in comparison with other normal and cancerous cell lines, suggests that cortistatin A (1) could, in principle, be a selective angiogenesis inhibitor.2 Inspired by the potent biological activity and unusual structural elements of the cortistatins, a number of groups have been engaged in total synthetic efforts directed at this challenging family of natural products.3 The recent success of Baran and coworkers in converting prednisone to cortistatin A (1) is a milestone in this field.4 Even more recently, a total synthesis of cortistatin A (1) was accomplished by Nicolaou and coworkers.5
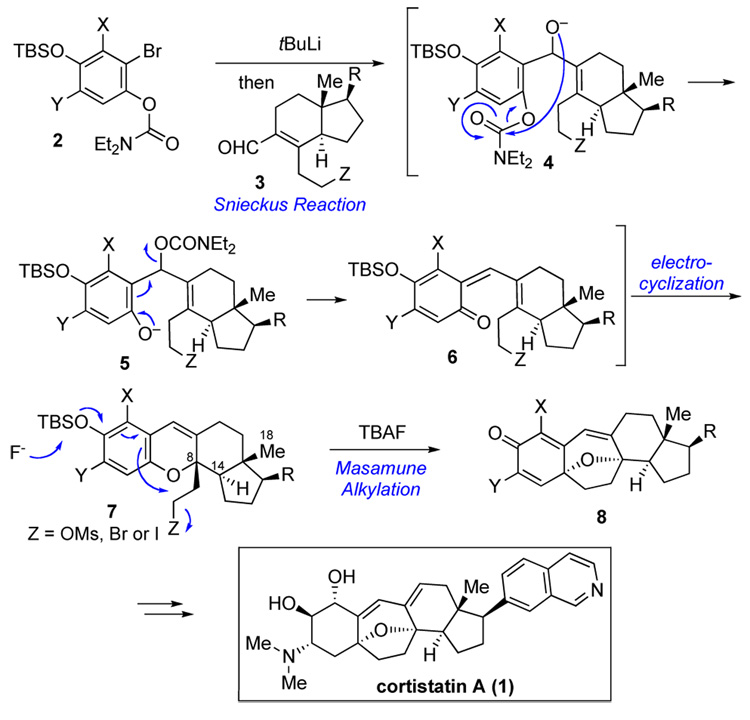
Our own initial approach to the synthesis of cortistatin A6 focused on the preparation of a key pentacyclic intermediate, cf. 8. This advanced compound would hopefully serve as a precursor to the natural product itself. Even more importantly, it would provide a useful synthetic platform from which to gain entry to a range of cortistatin analogs through diverted total synthesis.7 Unexpected problems encountered in our initial synthetic route6 led us to consider an alternative and perhaps more interesting approach to the synthesis of intermediate 8. This modified route would culminate in a Masamune-inspired alkylative dearomatization8 of compound 7. As outlined in Scheme 1, we envisioned taking advantage of the elegant Snieckus cascade methodology9 for construction of tetracyclic compound 7 from the relatively simple precursors, 2 and 3. The progression would commence with 1,2-addition of the aryllithium derived from 2 to α,β-unsaturated aldehyde 3, thereby generating alkoxide 4. Subsequent intramolecular carbamate migration, followed by 1,4-elimination would give rise to quinomethide 6. We anticipated that the latter would undergo 6π-electrocyclization to produce an intermediate of the type 7.
Scheme 1.
Synthetic strategy toward cortistatin A (1).
At the planning level, we could not be certain of the stereochemical outcome of the 6π-electrocyclization (at C8). We postulated that intermediate 7 would likely be the thermodynamically favored epimer, due to the trans/anti stereoconnectivity of the angular methyl group (C18), the hydrogen on C14 and the angular 2-carbon chain. Finally, intramolecular alkylation of 7 should provide access to the key pentacyclic core system (8) of the cortistatins.
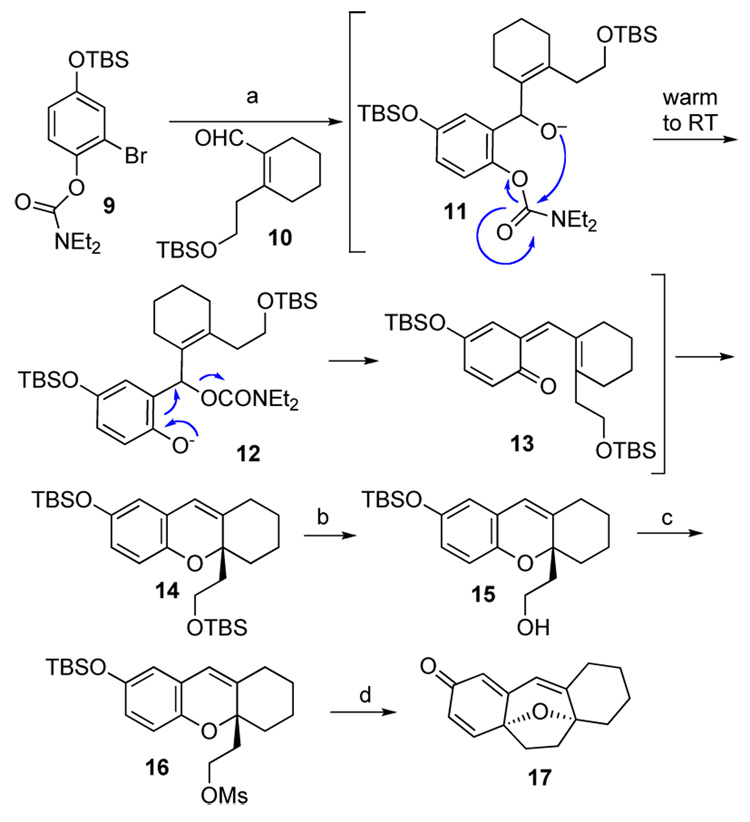
A model study was conducted to evaluate the likelihood of the applicability of the Snieckus paradigm to our system. As shown in Scheme 2, aryl bromide 910 was exposed to the action of tBuLi in ether for 30 min at −78 °C. Following addition of compound 10,11 the reaction mixture was warmed to room temperature and stirred overnight. We were pleased to find that the desired product, 14, could be isolated, albeit at the time, in only 33% yield. Selective deprotection of the primary TBS-ether afforded compound 15,12 which was transformed to mesylate 16 in excellent yield. Finally, the phenoxide, presumably generated by treatment of compound 16 with anhydrous TBAF in THF at room temperature, was further heated to 130 °C to give the desired product 17 in 85% yield.
Scheme 2.
Model Study. Reagents and conditions: (a) tBuLi, Et2O, −78 °C, 30 min, then 10, then warm to RT, overnight, 33%; (b) I2, MeOH / THF (1 / 1), RT, 3 h, 80%; (c) MsCl, pyridine, CH2Cl2, 0 °C → RT, 6 h, 98%; (d) TBAF, THF, RT, 5 min, then 130 °C, 20 min, 85%.
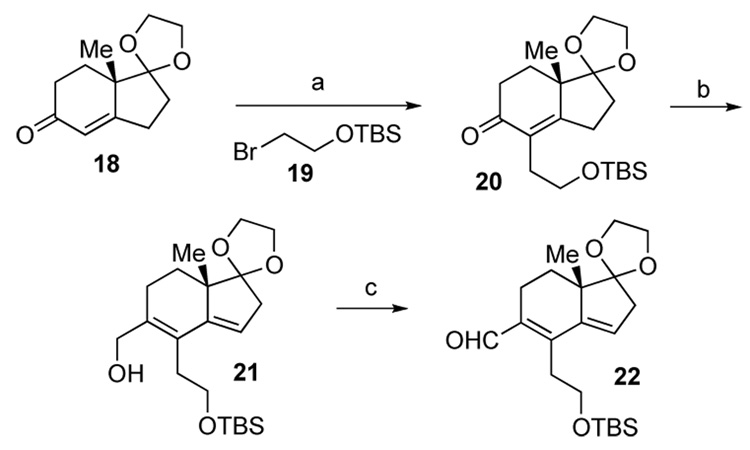
Having established the viability, at least in principle, of our general vision of the problem it was now appropriate to turn to the synthesis of aldehyde 22. As outlined in Scheme 3, alkylation of the Hajos-Parrish mono-ketal 18 with bromide 19 afforded 20 in 58% yield.13 Extended triflate formation,14 followed by Pd-catalyzed carboxylation15 of the crude triflate gave the methyl carboxylate, which was further reduced by DIBAL-H to furnish compound 21 (50% yield over three steps starting from 20). The resultant allylic alcohol was oxidized to the desired aldehyde 22 through treatment with IBX.16
Scheme 3.
Synthesis of aldehyde 22. Reagents and conditions. (a) NaH, DMSO; then 19, RT, 4 h, 58%; (b) (i) Tf2O, 2,6-di-tert-butyl-4-methylpyridine, CH2Cl2, 0 °C, 30 min; (ii) Pd(OAc)2, PPh3, iPr2NEt, CO, MeOH, 50 °C, 48 h; (iii) DIBAL-H, CH2Cl2, 0 °C, 30 min, 50% from 20; (c) IBX, ethyl acetate, reflux, 7 h, 90%.
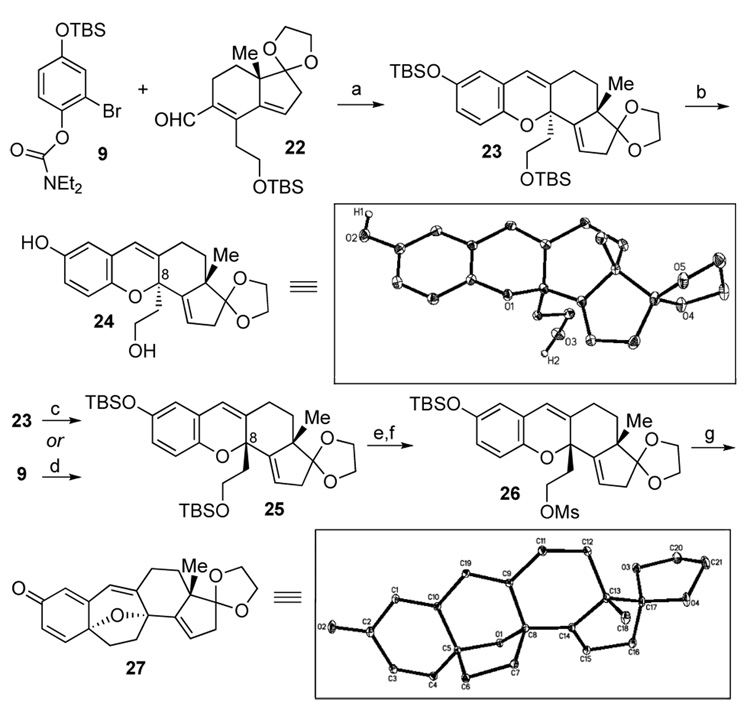
When aldehyde 22 was exposed to the kind of reaction conditions described above (see Scheme 2), only the 1,2-addition product was isolated, as a 1:1 mixture of diastereomers. However, when the reaction mixture was further heated overnight at 80 °C, the tetracyclic product 23 was obtained in 44% yield, though with the undesired stereochemistry at C8, as evidenced by X-ray crystallography of the deprotected product 24 (Scheme 4).17 However, when compound 23 was heated at 130 °C in THF, it epimerized to the desired compound 25 in quantitative yield, presumably through a sequence comprised of retro-6π-electrocyclization and 6π-electrocyclization. This transformation is consistent with our expectation that compound 25, possessing a 1,3-cis diaxial relation between the angular methyl and the angular 2-carbon chain (−CH2CH2OTBS), is the thermodynamically favored epimer. We further envisioned that, if the reaction mixture arising from treatment of aldehyde 22 with aryllithium derived from 9 were heated at 130 °C, the desired product, 25 could be obtained. Indeed, upon heating the reaction mixture at 130 °C overnight, the hoped for product 25 was obtained in high yield (71%). Following selective deprotection and subsequent mesylation of the primary alcohol, intermediate 26 was in hand. The latter smoothly underwent the desired alkylative dearomatization to produce the pentacyclic core of the cortistatins in excellent (ca. 88%) yield. The structure of compound 27 was unambiguously confirmed by X-ray crystallography.18
Scheme 4.
Synthesis of the pentacyclic core of cortistatin A. Reagents and conditions. (a) tBuLi, Et2O, −78 °C, 30 min, then 22, then heated to 80 °C, overnight, 44%; (b) TBAF, THF, 0 °C, 2 h, 96%; (c) THF, 130 °C, overnight, 100%; (d) tBuLi, Et2O, −78 °C, 30 min, then 22, then heated to 130 °C, overnight, 71%; (e) I2, MeOH / THF (1 / 1), RT, 2 h, 83%; (f) MsCl, Pyridine, CH2Cl2, 0 °C → RT, 94%; (g) TBAF, THF, RT, 5 min, then 130 °C, 20 min, 88%.
In summary, we have devised and reduced to practice a concise and efficient route to the core matrix of the cortistatins. Critical to its success was an orchestration of carbanion chemistry, an O→O acyl transfer driven rearrangement, quinonemethide formation, and electrocyclic re-aromatization setting the stage for alklyative dearomatization (see 9→27). This chemistry could well be extended to a total synthesis of the cortistatin family of steroids. Equally important, it sets the stage for realistic SAR work based on diverted total synthesis.7
Supplementary Material
Acknowledgments
Financial support for this research was provided by the National Institutes of Health (HL25848 and CA103823). M.D. thanks the generous support of the Guthikonda Fellowship in Organic Chemistry, the Bristol-Myers Squibb Graduate Fellowship in Synthetic Organic Chemistry, and the Sylvia & Victor Fourman Fellowship. We thank Daniela Buccella and Aaron Sattler (Parkin Group) for the crystal structure analysis and the National Science Foundation (CHE-0619638) for acquisition of an X-ray diffractometer. Ms. Rebecca Wilson is thanked for valuable help in editing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.(a) Aoki S, Watanabe Y, Sanagawa M, Setiawan A, Kotoku N, Kobayashi M. J. Am. Chem. Soc. 2006;128:3148. doi: 10.1021/ja057404h. [DOI] [PubMed] [Google Scholar]; (b) Watanabe Y, Aoki S, Tanabe D, Setiawan A, Kobayashi M. Tetrahedron. 2007;63:4074. [Google Scholar]; (c) Aoki S, Watanabe Y, Tanabe D, Setiawan A, Arai M, Kobayashi M. Tetrahedron Lett. 2007;48:4485. [Google Scholar]
- 2.(a) Folkman J. New Engl. J. Med. 1995;333:1757. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]; (b) Folkman J, Shing Y. J. Biol. Chem. 1992;267:10931. [PubMed] [Google Scholar]
- 3.When we were preparing this manuscript, three elegant syntheses of the cortistatin core were reported, see: Yamashita S, Iso K, Hirama M. Org. Lett. 2008;10:3413. doi: 10.1021/ol8012099. Simmons EM, Hardin AR, Guo X, Sarpong R. Angew. Chem. Int. Ed. 2008;47:6650. doi: 10.1002/anie.200802203. Craft DT, Gung BW. Tetrahedron Lett. 2008 doi: 10.1016/j.tetlet.2008.07.155.
- 4.Shenvi RA, Guerrero CA, Shi J, Li C-C, Baran PS. J. Am. Chem. Soc. 2008;130:7241. doi: 10.1021/ja8023466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicolaou KC, Sun Y-P, Peng X-S, Polet D, Chen DY-K. Angew. Chem. Int. Ed. 2008 doi: 10.1002/anie.200803550. [DOI] [PubMed] [Google Scholar]
- 6.Dai M, Danishefsky SJ. Heterocycles. published online, April 17th, 2008. COM-08-S(F)6. [Google Scholar]
- 7.(a) Njardarson JT, Gaul C, Shan D, Huang X-Y, Danishefsky SJ. J. Am. Chem. Soc. 2004;126:1038. doi: 10.1021/ja039714a. [DOI] [PubMed] [Google Scholar]; (b) Wilson RM, Danishefsky SJ. J. Org. Chem. 2006;71:8329. doi: 10.1021/jo0610053. [DOI] [PubMed] [Google Scholar]
- 8.(a) Masamune S. J. Am. Chem. Soc. 1961;83:1009. [Google Scholar]; (b) Lalic G, Corey E. J. Org. Lett. 2007;9:4921. doi: 10.1021/ol702323s. [DOI] [PubMed] [Google Scholar]
- 9.(a) Chauder BA, Kalinin AV, Taylor NJ, Snieckus V. Angew. Chem. Int. Ed. 1999;38:1435. doi: 10.1002/(SICI)1521-3773(19990517)38:10<1435::AID-ANIE1435>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]; (b) Chauder BA, Kalinin AV, Snieckus V. Synthesis. 2001:140. [Google Scholar]
- 10.(a) Kranich R, Eis K, Geis O, Mühle S, Bats JW, Schmalz H-G. Chem. Eur. J. 2000;6:2874. doi: 10.1002/1521-3765(20000804)6:15<2874::aid-chem2874>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]; (b) Alvarez-Manzaneda EJ, Chahboun R, Pérez IB, Cabrera E, Alvarez E, Alvarez-Manzaneda R. Org. Lett. 2005;7:1477. doi: 10.1021/ol047332j. [DOI] [PubMed] [Google Scholar]
- 11.(a) Gagnier SV, Larock RC. J. Am. Chem. Soc. 2003;125:4804. doi: 10.1021/ja0212009. [DOI] [PubMed] [Google Scholar]; (b) Chandraratna RAS, Okamura WH. J. Am. Chem. Soc. 1982;104:6114. [Google Scholar]; (c) Yadav VK, Senthil G, Babu KG, Parvez M, Reid JL. J. Org. Chem. 2002;67:1109. doi: 10.1021/jo0106400. [DOI] [PubMed] [Google Scholar]
- 12.Smith AB, III, Sperry JB, Han Q. J. Org. Chem. 2007;72:6891. doi: 10.1021/jo071146k. [DOI] [PubMed] [Google Scholar]
- 13.Hajos ZG, Micheli RA, Parrish DR, Oliveto EP. J. Org. Chem. 1967;32:3008. [Google Scholar]
- 14.Senanayake CH, Bill TJ, DiMichele LM, Chen CY, Larsen RD, Verhoeven TR, Reider PJ. Tetrahedron Lett. 1993;34:6021. [Google Scholar]
- 15.Snider BB, Vo NH, O’Neil SV, Foxman BM. J. Am. Chem. Soc. 1996;118:7644. [Google Scholar]
- 16.More JD, Finney NS. Org. Lett. 2002;4:3001. doi: 10.1021/ol026427n. [DOI] [PubMed] [Google Scholar]
- 17.CCDC 696105 contains the supplementary crystallographic data for compound 24.
- 18.CCDC 696762 contains the supplementary crystallographic data for compound 27.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.