Abstract
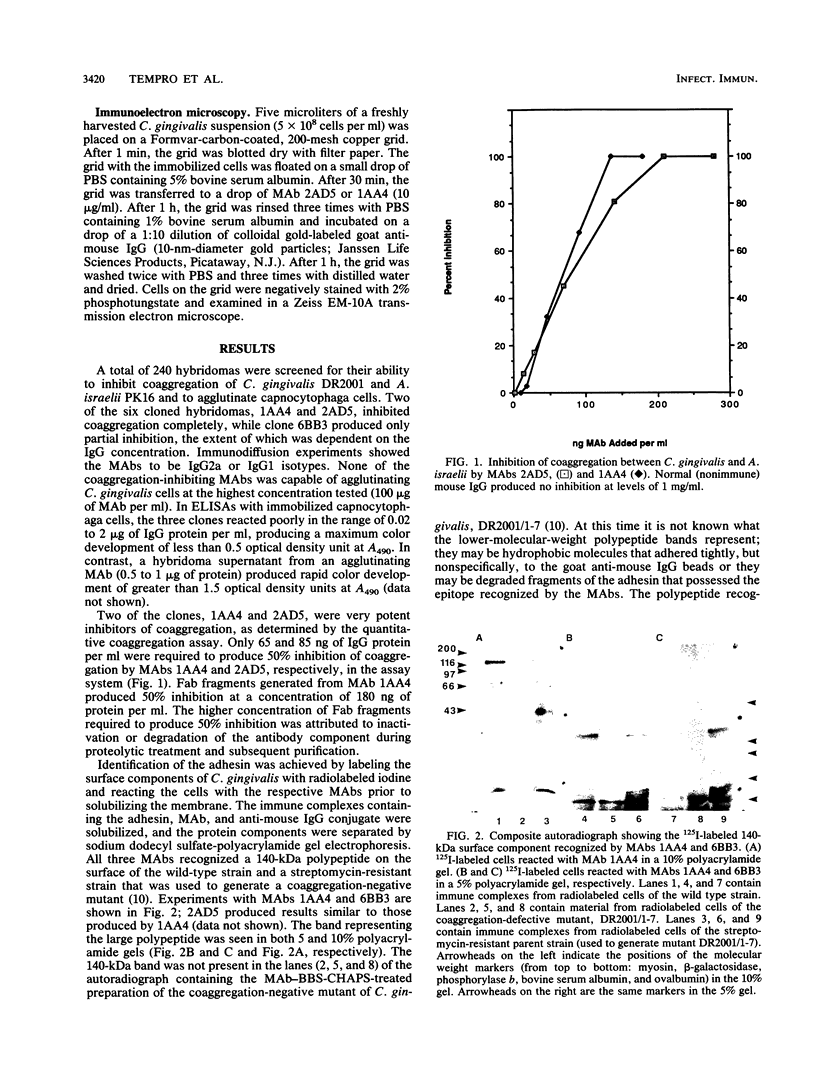
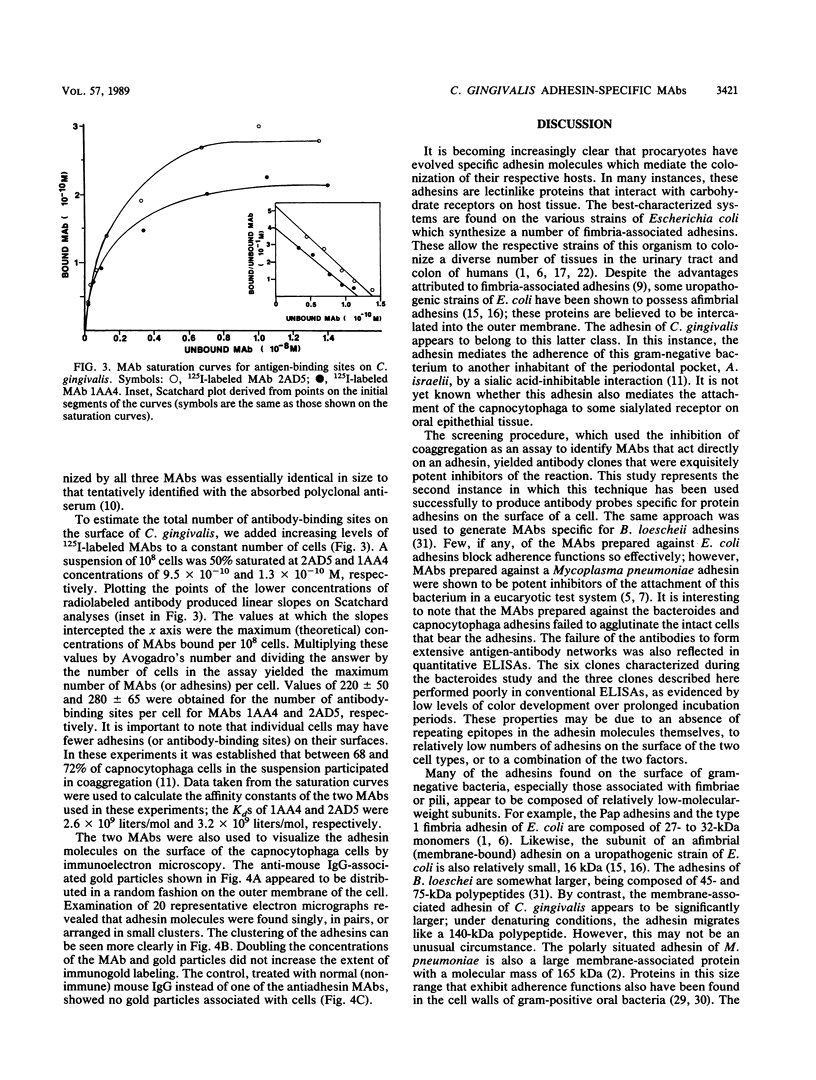
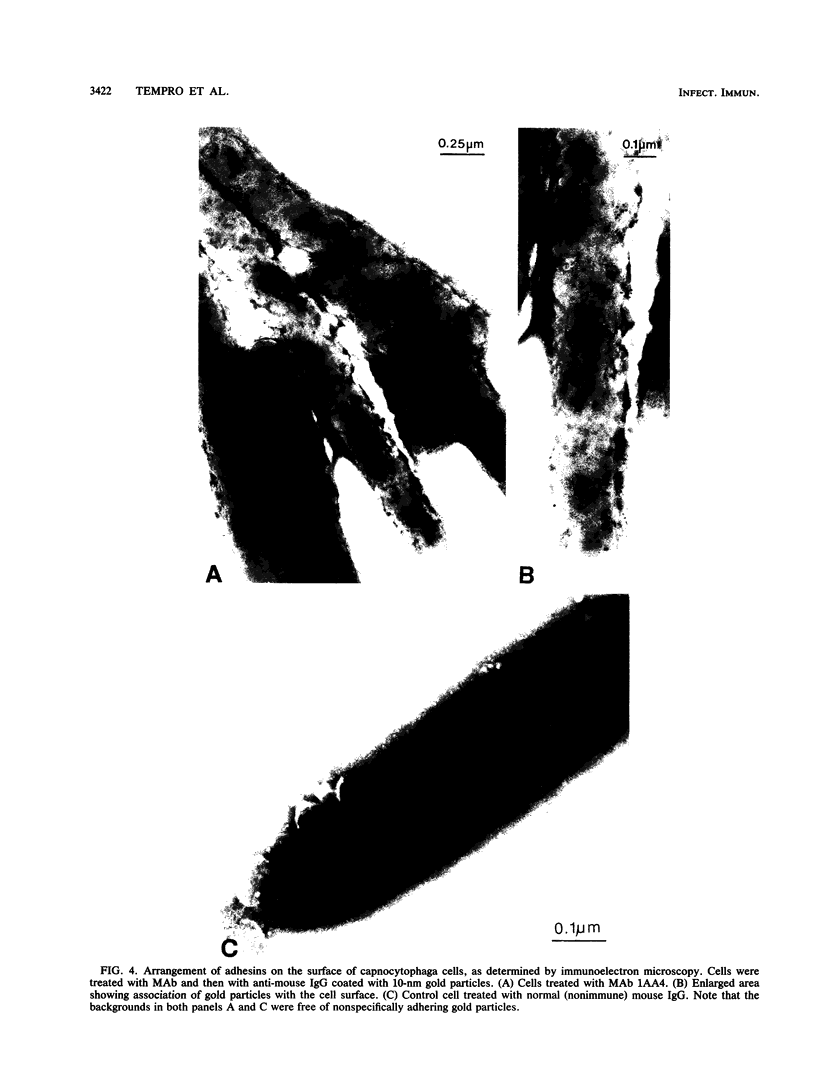
Monoclonal antibodies capable of inhibiting coaggregation between Capnocytophaga gingivalis DR2001 and Actinomyces israelii PK16 were used to identify the adhesin on C. gingivalis that mediates the interaction. The monoclonal antibodies were used to demonstrate that a 140-kilodalton polypeptide found in the outer membrane of C. gingivalis was the adhesin responsible for coaggregation. A coaggregation-defective mutant that was unable to coaggregate with A. israelii lacked this large polypeptide. The monoclonal antibodies were also used to estimate the number of binding sites on the surfaces of individual cells and show how the adhesin molecules were arranged on the outer membrane. Values of between 220 and 280 were obtained for the number of adhesin molecules per cell. Immunoelectron microscopy performed with the monoclonal antibodies revealed that the adhesin molecules were arranged nonuniformly on the bacterial surface and occurred singly, in pairs, and in small clusters.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham S. N., Goguen J. D., Sun D., Klemm P., Beachey E. H. Identification of two ancillary subunits of Escherichia coli type 1 fimbriae by using antibodies against synthetic oligopeptides of fim gene products. J Bacteriol. 1987 Dec;169(12):5530–5536. doi: 10.1128/jb.169.12.5530-5536.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celesk R. A., London J. Attachment of oral Cytophaga species to hydroxyapatite-containing surfaces. Infect Immun. 1980 Aug;29(2):768–777. doi: 10.1128/iai.29.2.768-777.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldner J., Göbel U., Bredt W. Mycoplasma pneumoniae adhesin localized to tip structure by monoclonal antibody. Nature. 1982 Aug 19;298(5876):765–767. doi: 10.1038/298765a0. [DOI] [PubMed] [Google Scholar]
- Hanson M. S., Brinton C. C., Jr Identification and characterization of E. coli type-1 pilus tip adhesion protein. Nature. 1988 Mar 17;332(6161):265–268. doi: 10.1038/332265a0. [DOI] [PubMed] [Google Scholar]
- Hu P. C., Cole R. M., Huang Y. S., Graham J. A., Gardner D. E., Collier A. M., Clyde W. A., Jr Mycoplasma pneumoniae infection: role of a surface protein in the attachment organelle. Science. 1982 Apr 16;216(4543):313–315. doi: 10.1126/science.6801766. [DOI] [PubMed] [Google Scholar]
- Hughes C. V., Kolenbrander P. E., Andersen R. N., Moore L. V. Coaggregation properties of human oral Veillonella spp.: relationship to colonization site and oral ecology. Appl Environ Microbiol. 1988 Aug;54(8):1957–1963. doi: 10.1128/aem.54.8.1957-1963.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Isaacson R. E. Proteinaceous bacterial adhesins and their receptors. Crit Rev Microbiol. 1983;10(3):229–260. doi: 10.3109/10408418209113564. [DOI] [PubMed] [Google Scholar]
- Kagermeier A. S., London J., Kolenbrander P. E. Evidence for the participation of N-acetylated amino sugars in the coaggregation between Cytophaga species strain DR2001 and Actinomyces israelii PK16. Infect Immun. 1984 May;44(2):299–305. doi: 10.1128/iai.44.2.299-305.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagermeier A., London J. Identification and preliminary characterization of a lectinlike protein from Capnocytophaga gingivalis (emended). Infect Immun. 1986 Feb;51(2):490–494. doi: 10.1128/iai.51.2.490-494.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- Kolenbrander P. E., Andersen R. N. Multigeneric aggregations among oral bacteria: a network of independent cell-to-cell interactions. J Bacteriol. 1986 Nov;168(2):851–859. doi: 10.1128/jb.168.2.851-859.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Celesk R. A. Coaggregation of human oral Cytophaga species and Actinomyces israelii. Infect Immun. 1983 Jun;40(3):1178–1185. doi: 10.1128/iai.40.3.1178-1185.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labigne-Roussel A. F., Lark D., Schoolnik G., Falkow S. Cloning and expression of an afimbrial adhesin (AFA-I) responsible for P blood group-independent, mannose-resistant hemagglutination from a pyelonephritic Escherichia coli strain. Infect Immun. 1984 Oct;46(1):251–259. doi: 10.1128/iai.46.1.251-259.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labigne-Roussel A., Schmidt M. A., Walz W., Falkow S. Genetic organization of the afimbrial adhesin operon and nucleotide sequence from a uropathogenic Escherichia coli gene encoding an afimbrial adhesin. J Bacteriol. 1985 Jun;162(3):1285–1292. doi: 10.1128/jb.162.3.1285-1292.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F., Lund B., Johansson L., Normark S. Localization of the receptor-binding protein adhesin at the tip of the bacterial pilus. Nature. 1987 Jul 2;328(6125):84–87. doi: 10.1038/328084a0. [DOI] [PubMed] [Google Scholar]
- Mashimo P. A., Yamamoto Y., Slots J., Park B. H., Genco R. J. The periodontal microflora of juvenile diabetics. Culture, immunofluorescence, and serum antibody studies. J Periodontol. 1983 Jul;54(7):420–430. doi: 10.1902/jop.1983.54.7.420. [DOI] [PubMed] [Google Scholar]
- McIntire F. C., Vatter A. E., Baros J., Arnold J. Mechanism of coaggregation between Actinomyces viscosus T14V and Streptococcus sanguis 34. Infect Immun. 1978 Sep;21(3):978–988. doi: 10.1128/iai.21.3.978-988.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moch T., Hoschützky H., Hacker J., Kröncke K. D., Jann K. Isolation and characterization of the alpha-sialyl-beta-2,3-galactosyl-specific adhesin from fimbriated Escherichia coli. Proc Natl Acad Sci U S A. 1987 May;84(10):3462–3466. doi: 10.1073/pnas.84.10.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Smibert R. M., Cato E. P., Burmeister J. A., Palcanis K. G., Ranney R. R. Bacteriology of experimental gingivitis in children. Infect Immun. 1984 Oct;46(1):1–6. doi: 10.1128/iai.46.1.1-6.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parenti D. M., Snydman D. R. Capnocytophaga species: infections in nonimmunocompromised and immunocompromised hosts. J Infect Dis. 1985 Jan;151(1):140–147. doi: 10.1093/infdis/151.1.140. [DOI] [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Shurin S. B., Socransky S. S., Sweeney E., Stossel T. P. A neutrophil disorder induced by capnocytophaga, a dental micro-organism. N Engl J Med. 1979 Oct 18;301(16):849–854. doi: 10.1056/NEJM197910183011601. [DOI] [PubMed] [Google Scholar]
- Siraganian R. P., Fox P. C., Berenstein E. H. Methods of enhancing the frequency of antigen-specific hybridomas. Methods Enzymol. 1983;92:17–26. doi: 10.1016/0076-6879(83)92005-0. [DOI] [PubMed] [Google Scholar]
- Slots J., Genco R. J. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival, and tissue destruction. J Dent Res. 1984 Mar;63(3):412–421. doi: 10.1177/00220345840630031101. [DOI] [PubMed] [Google Scholar]
- Weerkamp A. H., van der Mei H. C., Liem R. S. Structural properties of fibrillar proteins isolated from the cell surface and cytoplasm of Streptococcus salivarius (K+) cells and nonadhesive mutants. J Bacteriol. 1986 Mar;165(3):756–762. doi: 10.1128/jb.165.3.756-762.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. I., London J., Kolenbrander P. E., Andersen R. N., Fischler C., Siraganian R. P. Characterization of monoclonal antibodies to fimbria-associated adhesins of Bacteroides loescheii PK1295. Infect Immun. 1988 Jan;56(1):219–224. doi: 10.1128/iai.56.1.219-224.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. I., London J., Kolenbrander P. E., Hand A. R., Siraganian R. Localization and enumeration of fimbria-associated adhesins of Bacteroides loescheii. J Bacteriol. 1988 Mar;170(3):1123–1128. doi: 10.1128/jb.170.3.1123-1128.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]