Abstract
The successful function of cochlear prostheses depends on activation of auditory nerve. The survival of auditory nerve neurons, however, can vary widely in candidates for cochlear implants and influence implant efficacy. Stem cells offer the potential for improving the function of cochlear prostheses and increasing the candidate pool by replacing lost auditory nerve. The first phase of studies for stem cell replacement of auditory nerve has examined the in vitro survival and differentiation as well as in vivo differentiation and survival of exogenous embryonic and tissue stem cells placed into scala tympani and/or modiolus. These studies are reviewed and new results on in vivo placement of B-5 mouse embryonic stem cells into scala tympani of the guinea pig cochleae with differentiation into a glutamatergic neuronal phenotype are presented. Research on the integration and connections of stem cell derived neurons in the cochlea is described. Finally, an alternative approach is considered, based on the use of endogenous progenitors rather than exogenous stem cells, with a review of promising findings that have identified stem cell-like progenitors in cochlear and vestibular tissues to provide the potential for auditory nerve replacement.
Keywords: spiral ganglion neurons, cochlea, auditory, deafness, embryonic stem cells
1. Introduction
1.1 – Rationale and Challenges
Cochlear implants now provide an increasingly successful therapy to restore hearing to a large number of the severely deaf. Cochlear prostheses function by directly activating the auditory nerve in the cochlea. When there is loss of auditory nerve, as is often associated with deafness, this can influence the efficacy of cochlear prostheses (e.g. Blamey et al., 1996; Kileny et al., 1991; Incesulu and Nadol, 1998; Rubinstein et al., 1999). The minimal amount of remaining auditory nerve necessary for effective use of cochlear prostheses has not yet been established and the correlation between total amount of auditory nerve survival and performance remains in contention. Even a low number of surviving auditory neurons may still allow good speech perception (Blamey, 1997). Psychophysical measures in monkeys demonstrated increased thresholds and smaller dynamic range from cochlear electrical stimulation, associated with increased sensorineural damage (Pfingst and Sutton, 1983; Pfingst et al, 1981). Shepherd and Javel (1997) compared physiological responses to cochlear electrical stimulation with the status of the auditory nerve in the cat and reported elevation of thresholds in animals with extensive auditory nerve pathology but only moderate reductions in response amplitudes. They also found decreases in entrainment at higher stimulation rates and increases in bursting. Sly et al (2007) recently reported a decrease in spike latency and a reduction in threshold as auditory nerve survival decreased following ototoxic deafening in guinea pig. Certainly in cases of auditory neuropathology, where there is very large loss of auditory nerve, cochlear prostheses could be expected to be less effective. Replacement of lost auditory nerve could therefore improve the function of cochlear prostheses as well as increase the candidate pool. Stem cell technology provides a potential mechanism to accomplish replacement of lost auditory nerve neurons. There are three basic challenges which must be met for successful use of stem cells to replace auditory nerve for use with cochlear prostheses: 1) Differentiation into an appropriate phenotype, 2) long-term survival after reaching appropriate phenotype, and 3) functional central connection.
1.2. Differentiation into an appropriate phenotype
In order to accomplish auditory nerve replacement, the stem cells must be induced to differentiate into a neuronal phenotype. However, even if stem cells successfully reach a neuronal phenotype, if they are inhibitory, they would not be useful for activating the central auditory pathways. Therefore, it is important that an auditory nerve-like excitatory glutamatergic phenotype be obtained. If differentiated stem cells can also exhibit a molecular signature reflecting appropriate ion channels and other characteristics of auditory nerve, likely they could be even more capable of substituting for its function.
Several studies have examined the survival and differentiation of embryonic and tissue stem cells following their placement into the scala tympani and/or modiolus of the cochlea. Iguchi et al. (2003) placed neural stem cells from fetal mouse neuroepithelium into the mouse cochlea and found 10% survival at 25 days following placement, with the majority showing a glial phenotype based on glial fibrillary acidic protein (GFAP) immunostaining and a few showing a pre-neural phenotype based on nestin immunostaining. Tamura et al. (2004) placed mouse neural stem cells into the modiolus of mouse cochleae following deafening by treatment with cisplatin. They also found the majority had differentiation into a glial phenotype, based on GFAP immunostaining and only a small percentage reached a neuronal type phenotype. Coleman et al. (2006) placed mouse embryonic stem cells (mESCs) into guinea pig cochlea and found poor survival, with 4–6 mESCs per profile through scala tympani and 1 per profile through Rosenthal’s canal, with a significant decline in the number in scala tympani between 2 and 4 weeks following placement. A portion of the surviving mESCs (not specified in the text) showed a neuronal phenotype based on neurofilament immunolabeling. Hu et al. (2005) placed neural stem cells from the lateral wall of mouse lateral ventricle into normal or deafened guinea pig cochlea and found only a few reached a neuronal phenotype, based on TUJ1 (neuronal class III β-tubulin) immunostaining, with many showing a glial fated based on GFAP immunostaining. More recently, Parker et al. (2007) used neural stem cells derived from mouse fetal cerebellum, placed into noise exposed mouse or guinea pig cochleae. They found moderate levels of survival with differentiation into satellite cell-, Spiral Ganglion Neurons (SGN)-, Schwann cell-, hair cell- and supporting cell-like phenotypes. Interestingly, they found that this differentiation was influenced by the microenvironment, with cerebellum derived cells often expressing genes characteristics of local endogenous cells. Matsuoka et al. (2006) tested the placement of mouse bone marrow derived stem cells into the gerbil cochlea and found these stem cells would survive 7 days of placement into scala tympani or modiolus. None of these studies examined the transmitter phenotype of the cells reaching a neuronal phenotype.
Two approaches have been applied to increase the number of neuronal stem cells following transplantation into the cochlea. One approach is to apply cell signaling factors in vitro to differentiate the stem cells toward the desired neural phenotype prior to their placement in the cochlea. The second approach is to place undifferentiated stem cells in the cochlea and then apply growth factors in vivo, using the cochlear fluids for delivery. Advantages of the first approach include that differentiation can be monitored and that cell sorting can be applied to further refine the population before placement. Advantages of the second approach include that undifferentiated stem cells can induce less of an immune response to their placement and that their differentiation can be influenced and modified by “natural” endogenous factors, as suggested by Parker et al. (2007), to form the desired lineage.
Rivolta et al. (2006) used the “in vitro” approach and were successful in inducing mouse ESCs to form a variety of inner ear-like phenotypes, with a focus on generating a hair cell-like phenotype. Coleman et al. (2007) found that the in vitro differentiation of stem cells into neuron-like cells was facilitated by co-culture with auditory neurons or hair cell explants, isolated from post-natal day five rats. This in vitro approach has also been applied to bone marrow derived stem cells. Kondo et al. (2005) demonstrated sonic hedgehog and retinoic acid when in combination induced differentiation of marrow stromal cells to a neuronal phenotype with a greater than 200-fold increase in GATA3, Irx2, Sox10, calretinin and GluR4 mRNAs (all expressed during normal development in auditory nerve neurons) and Wnt 1 induced a 400-fold increase in the expression level of Brn3a, Ngn2 (neuroD) and Ngn1, which play a role in the differentiation of auditory neurons during normal development. Falk et al. (2002) found that transduction of stem cells with Neurogenin 2 (Ngn2) induced over 90% towards a neuronal phenotype. Hu et al. (2005) tested this approach in the cochlea, transfecting Ngn2 into neural stem cells prior to placement and found the number reaching a neuronal phenotype (based on immunostaining for TUJ1) increased from 15% to over 50%.
Hu et al. (2004) co-transplantated mouse ESCs and a neuronal co-graft of dorsal root ganglion tissue into guinea pig cochlea, to influence stem cell differentiation in vivo. They found improved survival of TUJ1 immunolabeled stem cells at 4 weeks following placement with the co-graft. The in vivo approach can also take advantage of the opportunity that the cochlear fluids offer for intrascalar delivery. Undifferentiated or partially differentiated stem cells can be placed into the cochlea and then exogenous neurotrophic factors or induction of genetic signaling applied in vivo. Our studies, described in a later section, showed intrascalar delivery of the neurotrophic factor (NTF) glial cell line derived neurotrophic factor (GDNF) increased the percentage of undifferentiated mouse embryonic stem cells reaching a neuronal phenotype in vivo, based on TUJ1 immunolabeling.
1.3. Challenge Two - Survival
In addition to generating the appropriate phenotype, a second challenge is survival after reaching this phenotype. Long-term survival is necessary, of course, for auditory nerve replacement to have therapeutic value. Hu et al. (2004) found moderate survival of mouse stem cell derived neurons several weeks following placement into guinea pig modiolus, but also found that the survival time of stem cells transduced into neural phenotype by expression of Ngn2 was decreased compared to the non-transduced stem cells (Hu et al.., 2005). These neural stem cells demonstrated good survival at 2 weeks; but this was greatly diminished at 4 weeks post-placement (Hu et al., 2005). This diminished survival could have been due to the neurophysiologically “unnatural” sustained rather than transient expression of Ngn2 and/or that the site of integration of the transgene interfered with differentiation. These studies also did not provide intracochlear infusion of NTF(s) or other survival factors that Ulfendahl et al. (2007) suggested could be needed for continued survival. Just as SGN require trophic support for survival and begin to degenerate within weeks following their deafferentation from inner hair cell loss (see Shepherd article this volume, which addresses this in detail), when stem cells assume a neuronal phenotype they may also require appropriate trophic support. This is supported by results from our lab, which show that chronic intrascalar infusion of glial cell line derived neurotrophic factor (GNDF) (see later section) and/or brain derived neurotrophic factor (BDNF) (Hernandez et al., 2007) provided for excellent survival of neuronal stem cells in the cochlea at five to six weeks following their placement. Clearly longer survival times and the efficacy of various NTFs and electrical stimulation alone and combined should be assessed in future studies.
1.4. Challenge Three – Integration and Functional Central Connection
The stem cells that differentiate into a neuronal phenotype in cochlear compartments are usually seen to extend processes (e.g. Figure 1c,d). We find the processes from ESC derived neurons in scala tympani are most often directed towards the organ of Corti (or the scar replacing it) (Figure 1b) or towards remaining SGN neurons in the modiolus. Matsumoto et al.., (2005) found that mouse embryonic stem cells co-cultured (in vitro) with mouse auditory epithelium send processes towards hair cells. Corrales et al., (2005) placed mouse neural stem cells into the cochlear nerve trunk of the gerbil 1 week after oubain injection which eliminated all SGN without loss of hair cells. Twelve days following placement these stem cells expressed neuronal phenotype markers and sent processes into the space formerly occupied by the auditory nerve. Neurites projected peripherally towards the organ of Corti and contacted hair cells.
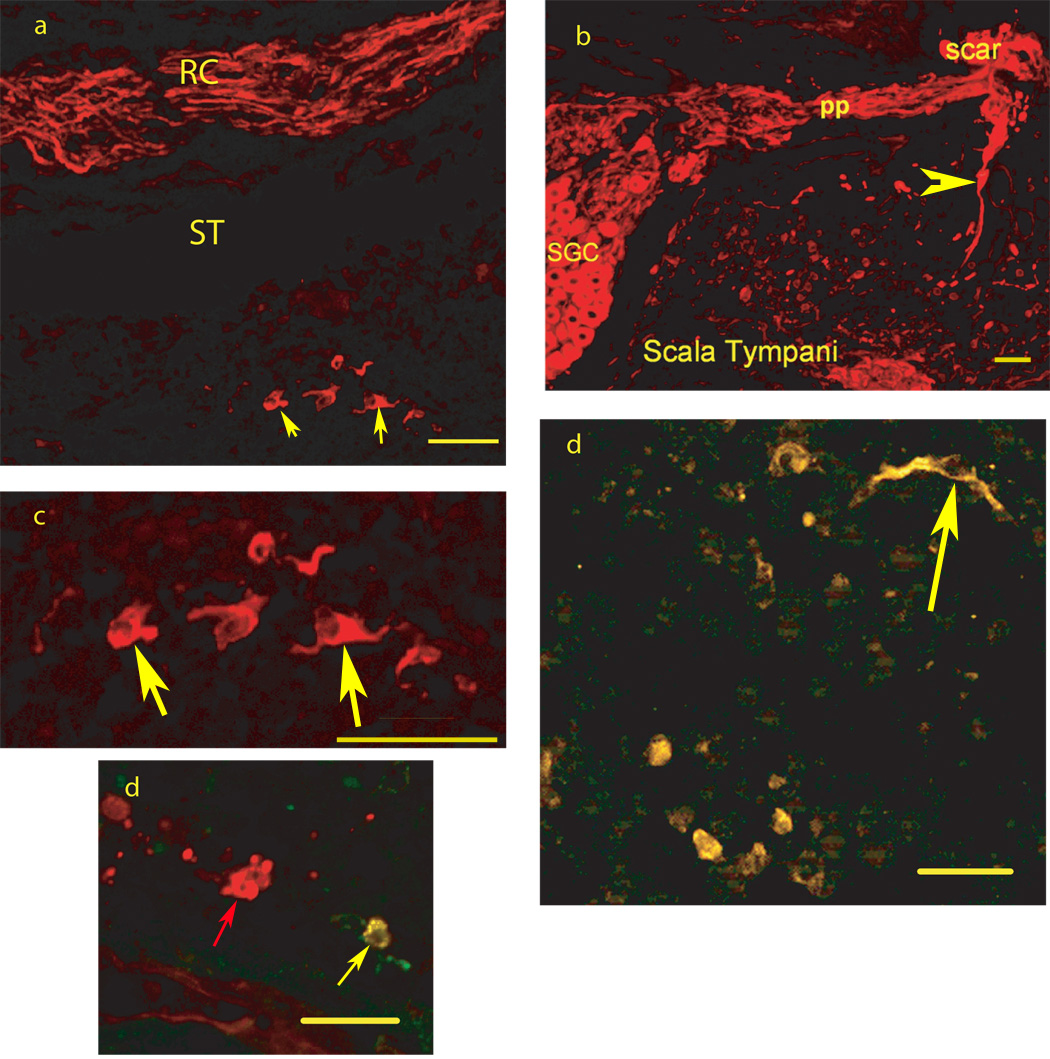
FIGURE 1.
Photomicrographs from mid-modiolar cryostat sections through the guinea pig cochlea two weeks following placement of mouse embryonic stem cells in scala tympani/modiolus immunostained with antibody to the early neuronal phenotype marker TUJ1. 1a: TUJ1 immunolabels cells (red) (see arrows for examples) with a neuronal appearance in scala tympani (ST) from the basal turn of the cochlear spiral, seen below TUJ1 immunolabeled (red) peripheral processes of endogenous SGN in Rosenthals canal (RC), 1b: TUJ1 immunolabels endogenous spiral ganglion neuron cells (SGC) and their peripheral processes (pp) in Rosenthals canal running towards the scar that replaced the organ of Corti following deafening. There is also TUJ1 immunolabeling of stem cells in Scala Tympani as well fibers between scala tympani and the scar region (arrowhead); 1c: Higher magnification view of the TUJ1 immunolabeled cells in 1a (see arrows for examples).. 2d: Co-immunostaining for TUJ1 and VGLUT2, co-localization is yellow (see arrow for examples) and found in all labeled cells in this field, 3d: Co-immunostaining for TUJ1 (Red) and GAT (green), co-localization is yellow and found in one cell (yellow arrow) in this field, while another cell in the center of the field (red arrow) is TUJ1 but not GAT immunolabeled. Bars = 50 microns.
To achieve functional replacement of lost auditory nerve, the stem cell derived neurons must also send a central axonal projection that crosses the peripheral nervous system – central nervous system barrier and connects with central auditory neurons in the Cochlear Nucleus (CN). Hu et al. (2004) have demonstrated migration of mouse embryonic dorsal root ganglion cells from the site of placement in the rat scala tympani into the modiolus with projections both peripherally toward the hair cells, as well as centrally, and formation of axonal connections between the projections of the implants and SGN central projections. Hu et al. (2005) also observed mouse embryonic stem cells placed into rat scala tympani migrating into the auditory nerve and entering into the CN. While this suggests there might not be a PNS/CNS barrier constraint, the less differentiated embryonic stem cells may be less inhibited by this barrier than processes with a more mature neuronal phenotype. Recent studies suggest that central connections can be enhanced by blocking the natural inhibition at the PNS/CNS barrier from proteins such as Nogo and myelin inhibitors (He and Koprivica, 2004 for review). Koprivica et al. (2005) have developed small molecules to block the downstream pathway in this inhibition, for example by blocking EGFR kinase. This resulted in greatly improved regeneration of optic nerve fibers. Gharabaghi and Tatagiba (2005) applied IN-1, a Nogo-A inhibitor, and growth factors to the auditory nerve through a cannula at the cerebellopontine angle and found improved functional regeneration of the auditory nerve after axotomy. It may be necessary to apply such factors to enhance central connections to the cochlear nucleus from new stem cell derived neurons in the cochlea.
We have done studies addressing the critical first challenge of increasing the number of stem cells reaching an appropriate phenotype for auditory nerve replacement. Coleman et al.., (2006) implanted B-5 mESCs into guinea pig cochlea and found moderate survival with most differentiated into a glial phenotype. We used the same B-5 cell line of undifferentiated mESCs and added intrascalar application of the neurotrophic factor (NTF) glial cell line derived neurotrophic factor (GDNF) following the mESC placement into guinea pig cochlea. Rask-Anderson et al.., (2005) had used GDNF to successfully induce neurospheres gathered from human or guinea pig spiral ganglion into a neuronal phenotype. We hypothesized that GDNF could also induce mESCs into a SGN-like phenotype. GDNF could also increase the number of mESCs with a neuronal phenotype found at 4 weeks following placement by acting as a survival factor, as it does for de-afferented spiral ganglion neurons (Altschuler et al., 1999; Kanzaki et al., 2002; Miller et al., 1997; Miller et al., 1998; Shepherd et al., this issue).
2. Methods
All experimental procedures were approved by the University of Michigan Committee on the Use and Care of Animals. Eight normal hearing adult NIH strain guinea pigs (Elm Hill) weighing 300 to 400 grams, were systemically deafened by administration of 450-mg/kg kanamycin s.c., followed by anesthesia with ketamine/xylazine and 60 mg/kg ethacrynic acid, administered i.v. (jugular vein) 2 hours later (West et al., 1973). Three days following deafening, mouse ESCs from the B-5 line (Hadjantonakis et al., 1998) that express enhanced green fluorescent protein (eGFP) were were aspirated into a 1cc syringe and carefully transferred to a glass microliter syringe. A 30g needle was attached to the syringe and inserted into a micro-cannula (Prieskorn & Miller, 2000). The fine cannula tip was then inserted through a cochleostomy in the basal turn of the cochlear spiral with a silastic ball serving as a stop to plug the hole during the injection to prevent leakage. A 5µl bolus containing approximately 500,000 B-5 mouse ESCs was slowly injected into the modiolus and scala tympani and the cannula kept in place for an additional 10 minutes. The cannula was then removed and replaced by a second cannula into scala tympani primed with GDNF (10 µg/ml) attached to a miniosmotic pump (Alza Corporation, model 2002, 14 day reservoir, flow rate 0.5 µl/hr). The GDNF was chronically infused over two weeks. At 14 days following placement the guinea pigs were heavily anesthetized with 0.5 ml i.p. sodium pentobarbital (Fatal Plus, Vortech, Dearborn, MI) and terminated with a vascular fixation of phosphate buffer followed by 4% paraformaldehyde fixative in phosphate buffer. Immunocytochemistry was carried out on 6 µm paramodiolar cryostat sections through the cochleae. Mouse ESCs in different compartments of the guinea pig cochlear were identified by their eGFP expression and also by in situ hybridization for genomic mouse DNA. Differentiation of the mouse ESCs into a neuronal or glial phenotype was assessed using TUJ1 as a marker for neuronal phenotype and GFAP as a marker for an astrocyte phentotype. Mouse monoclonal antibody to TUJ1 (Covance, Berkeley, CA) was applied at a 1:300 dilution in phosphate buffered saline (PBS) with 0.2% triton X-100 and polyclonal rabbit antibody to GFAP (MP Biomedicals, Aurora, OH) was used at a 1:500 dilution) in PBS with triton X100. The TUJ1 immunolabeled stem cells were further assessed for neurotransmitter phenotype with co-immunolabeling for vesicular glutamate transporters 1 or 2 (VGLUT1,2) or the GABA transporter (VGAT) using rabbit polyclonal antibodies (Synaptic Systems, SYSY, Germany) all used at a 1:500 dilution in PBS with 0.2% triton X-100. Secondary anti-mouse or anti-rabbit antibodies (Molecular Probes, Carlsbad, CA) were linked to Alexa 350 (TUJ1) or Alexa 594 (anti-rabbit) for co-localization of TUJ1, VGLUTs and VGAT. Other sections were co-labeled with in fluorescent in situ hybridization (FISH) for genomic mouse DNA and immunostaining for TUJ1 using mouse COT-1 DNA (Invitogen Corporation, Carlsbad, CA) as the DNA probe for mouse genomic DNA. The probe was labeled with digoxigenin-16-dUTP using a nick translation kit (both from Roche Applied Science, Indianapolis, IN). Sections were incubated with the COT-1 DNA probe, diluted 1:20 in hybridization buffer (Enzo Life Sciences, Farmingdale, NY) for 5 minutes at 92°C. Sections were then rinsed in PBS, followed by in situ hybridization wash reagent (Enzo Life Sciences) for 15 minutes at 37°C and then additional rinses in PBS at room temperature. Slides were incubated with rhodamine conjugated antibody to digoxigenin (1:200, Roche Applied Science, Indianapolis, IN) for 1 hour at room temperature in the dark. The sections were co-immunolabeled with antibody to TUJ1 (as above) and the TUJ1 antibody visualized with an anti-mouse antibody conjugated to Alexa 594. Confocal fluorescent images were acquired on Olympus FV-500 or Zeiss LSM510 confocal microscope.
3. Results
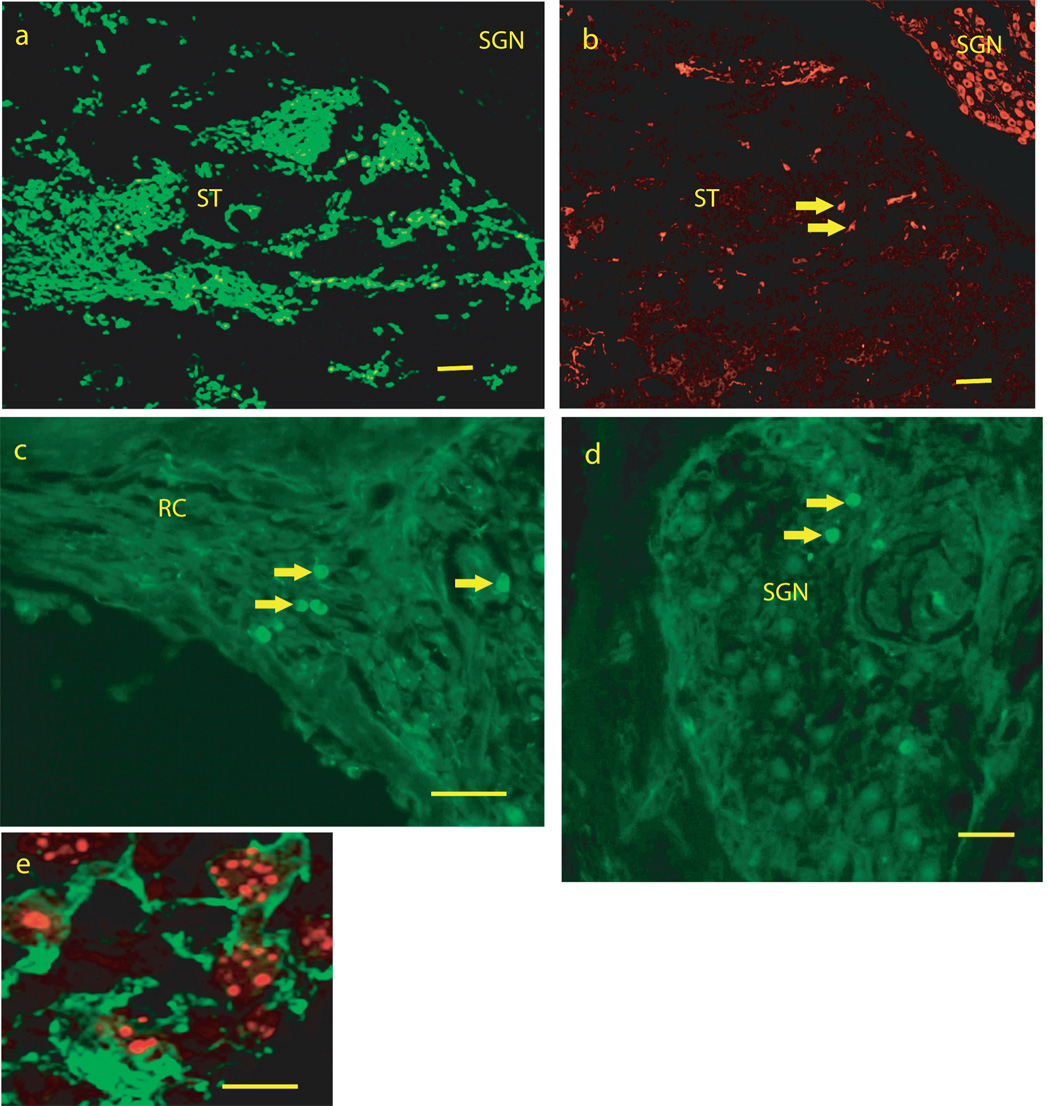
Many cells in sections could be identified as mouse ESCs, based on eGFP labeling (Figure 2a,c,d) or expression of mouse genomic DNA (Figure 2e). A typical profile through scala tympani of the basal turn of the cochlear spiral, contained 100–200 eGFP labeled cells, with fewer in more apical turns and scala vestibuli. Smaller numbers of eGFP labeled cells (5–12 per section) were seen in the modiolus, most often among SGN (Figure 2d) or their peripheral processes (Figure 2c) in Rosenthal’s canal. Most of the eGFP labeled cells were small (10 microns) and round and without processes; they did not have a neuronal-like appearance.
FIGURE 2.
Photomicrographs from mid-modiolar cryostat sections through the guinea pig cochlea at two weeks following placement of mouse embryonic stem cells into the scala tympani/modiolus. 2a: low magnification image from scala tympani in the basal turn of the cochlea spiral showing enhanced green fluorescent protein (eGFP) fluorescence. 2B: An adjacent section to the section in 2a immunostained for TUJ1, showing TUJ1 immunostaining of spiral ganglion neurons (SGN) in Rosenthals canal and of cells (see arrows for examples) in scala tympani (ST). Note that there is little or no overlap of eGFP fluorescence in 1a and TUJ1 immunofluoresence in 1b. Figures 2c and d show eGFP fluorescent cells (arrows for examples) among SGN (2c) and SGN peripheral processes (2d) in Rosenthals canal. 1e shows co-labeling for TUJ1 immunostaining (green and cytoplasmic) and in situ hybridization labeling of mouse genomic DNA (red, punctuate and nuclear) in cells in scala tympani of the basal turn of the cochlear spiral. SGN = region of Spiral Ganglion Neurons, RC = region of SGN peripheral processes in Rosenthals Canal, ST = Scala Tympani. Bars for 1 a–c = 100 microns, 1d = 50 microns, 1 e = 10 microns.
There were many cells in scala tympani that were immunolabeled for TUJ1 (30–40 in a typical section through basal scala tympani (Figure 1). These had a more neuronal-like appearance, often with a fusiform shape and processes. The majority of the TUJ1 immunolabeled cells in scala tympani did not co-label with eGFP (Figure 1a–c) and it required co-labeling with FISH for mouse genomic DNA (Figure 2e) to show they had differentiated from the mouse ESCS, although any other origin would have been unlikely. Fewer cells were identified with an astrocyte phenotype based on GFAP immunolabeling, with 8 – 20 per profile through basal scala tympani (Figure 2b). These also did not co-label for eGFP. This suggests that the B-5 mouse embryonic stem cells also down regulate eGFP following in vivo differentiation.
Co-labeling of TUJ1 with glutamate or GABA vesicular transporters was used to assess the neurotransmitter phenotype of the embryonic stem cell derived neurons. The majority of TUJ1 cells in scala tympani (50–75%) were co-labeled for VGLUT1 or VGLUT2, with VGLUT2 co-immunolabeling most common (Figure 1d). The endogenous SGN were brightly immunolabeled with VGLUT1 and had only light VGLUT2 immunolabeling. Fewer TUJ1 cells (7–12%) in basal scala tympani were found co-immunolabeled for GAT (Figure 1e), a marker for GABA. GABA is inhibitory and would not be a suitable transmitter for providing replacement of auditory nerve.
4. Discussion
Our results show that chronic intrascalar infusion of GDNF following mESC placement into scala tympani/modiolus results in a much higher percentage of mESCs reaching a neuronal rather than a glial phenotype and increased survival at 4 weeks compared to an earlier study (Coleman et al., 2006) which did not supply NTFs. It also found that most of the mESCs differentiating into a neuronal phenotype were glutamatergic and therefore suitable for auditory nerve replacement. The question still remains as to how such an in vivo approach compares in efficacy to the vitro approach of inducing stem cell differentiation prior to cochlear placement and this awaits future study. It will also be important to determine which approach provides best long-term survival as well as integration and central connections, the other two challenges.
An alternative approach to implanting differentiated or undifferentiated exogenous stem cells into the cochlea is to mobilize and activate endogenous stem cells already present in the cochlea to differentiate into a neuronal phenotype and form a central connection. In the last few years a number of observations support the potential of this approach. Li et al. (2003) found spheres of inner ear stem cells could be isolated and generated from adult utricular epilethelium in vitro. Rask-Anderson et al. (2005) identified progenitor stem cells in cultures from human and guinea pig spiral ganglion. These neurospheres could be induced to differentiate into a neuronal or glial phenotype with application of neurotrophic factors such as GDNF. Yerukhimovich et al. (2007) found spheres isolated from mouse neonate cochleae that could differentiate into astrocytes and oligodendrocytes in vitro. Oshima et al. (2007) were able to generate sphere-forming stem cells from early postnatal organ of Corti, vestibular sensory epithelium, SGN region, and stria vascularis of the mouse cochlea. Those from the SGN region displayed features of neural stem cells or glial cells. This ability to form spheres decreased with postnatal age and was greatly reduced by the third postnatal week. Lou et al. (2007) found that otospheres could be derived from the cochleae of young rats when cultured in vitro with EGF and FGF2 and that these could differentiate into neuron, glial, hair cell and supporting cell phenotypes. These studies show that endogenous stem cells are indeed present in the cochlea, although the numbers decrease with age. The challenge remains to prospectively identify these cells so that their location, identity and growth factor responsiveness can be determined. With those data it should be possible to identify mechanisms to induce and control their differentiation into an auditory nerve-like phenotype and to form a central auditory connection.
While the potential for stem cell replacement of auditory nerve is therefore receiving increasing support, critical questions remain regarding the challenges of inducing appropriate phenotype, long term survival, functional central connections and ability to provide improvement of the function of cochlear prostheses.
The auditory nerve phenotype is well suited for its normal function of providing an excitatory connection between inner hair cells and the cochlear nucleus and this phenotype should be the target for stem cell differentiation for connection to regenerated hair cells. While an excitatory phenotype is still necessary for cochlear electrical stimulation, however, the auditory nerve properties may not be optimal. Perhaps stem cells can be differentiated into a phenotype with different ion channels and different firing properties that would be even better than the auditory nerve for being excited and coded by electrical stimulation.
Challenges also remain for long term survival. Loss of auditory nerve following inner hair cell loss is most likely due to loss of survival factors such as neurotrophic factors (see Shepherd article, this issue). Stem cell derived neurons in the cochlea may be subject to the same dependence on survival factors once they reach the mature auditory nerve-like phenotype. In that case it would be likely that chronic cochlear electrical stimulation would enhance their survival as has been shown for auditory nerve following deafness (Hartshorn et al., 1991; Leake et al., 1991, 1992, 1995, this issue; Lousteau, 1987; Mitchell et al., 1997; Miller et al., 2003; Miller & Altschuler, 1995). It may also be the case that neurotrophic factors would also enhance survival, as for auditory nerve following deafness (Altschuler et al., 1999; Ernfors et al., 1996; Malgrange et al., 1999; Marzella and Clark, 1999; McGuinness and Shepherd, 2005; Miller et al., 1997; Miller et al., 1998; Schindler et al., 1995; Shepherd et al., this issue; Staecker et al., 1996; Van de Water et al., 1996) with the combination of neurotrophic factors and chronic electrical stimulation potentially the most effective (Kanzaki et al., 2002; Shepherd et al., 2005, this issue).
There are also challenges for establishing functional central connections. Remaining auditory nerve may provide a substrate and cues for guiding new central processes, but the PNS-CNS barrier may play a critical role limiting the extent and functional integrity of the integration of stem cells with CNS. With partial survival of the auditory nerve, an infrastructure may exist to help overcome this barrier; however in the absence of most or all of the auditory neurons, this PNS-CNS interface may be a key limiting barrier. Studies with NoGo suggest there may be molecules that will allow us to overcome this barrier. Clearly, additional studies will be necessary to investigate this and if necessary develop clinically applicable interventions to encourage central projections.
The presence of precursor cells in the cochlea that have many characteristics of stem cells also offers promise. These could be used to develop new human cell lines for implantation in human subjects. The endogenous precursors might also be induced to differentiate into an auditory neuron phenotype in vivo and form central connections to replace lost auditory nerve allowing the damaged cochlea to help “repair itself”.
While challenges remain, these studies to date show the great potential for using both exogenous as well as endogenous stem cells to replace lost auditory nerve. There is an obvious clinical application to improve the efficacy of cochlear prostheses which are dependent on remaining auditory nerve for their function. Still another approach for clinical application would be to place stem cells reaching a neuronal glutamatergic phenotype directly onto sites on the cochlear prostheses and direct their processes towards new synapses with remaining SGN along the cochlear spiral. This addition of this “extra” neuron could lower thresholds by bringing the neuronal target for excitation closer to the stimulation site. Further, placing these stem cells close to different stimulation sites could also increase channel separation with different groups of stem cells preferentially excited by different stimulation sites. Lower threshold and increased channel separation could be expected to improve cochlear implant function. The remaining challenges and the multiple approaches will be the focus of many interesting future studies in our and other laboratories of the field.
Acknowledgements
We would like to thank Mat Velkey, Diane Prieskorn and Noel Wys for their contributions to generating the new data described in this manuscript. These studies were supported by NIH grants DC03820 (RAA, JMM), GM069985 (KSO), NS04187 (KSO), P30 DC05188 and by GM/UAW funds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Altschuler RA, Cho Y, Ylikoski J, Pirvola U, Magal E, Miller JM. Rescue and regrowth of sensory nerves following deafferentation by neurotrophic factors. Ann. N. Y. Acad. Sci. 1999;884:305–311. doi: 10.1111/j.1749-6632.1999.tb08650.x. [DOI] [PubMed] [Google Scholar]
- Blamey P, Arndt P, Bergeron F, Bredberg G, Brimacombe J, Facer G. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants. Audiol. Neurootol. 1996;1:293–306. doi: 10.1159/000259212. [DOI] [PubMed] [Google Scholar]
- Blamey P. Are spiral ganglion cell numbers important for speech perception with a cochlear implant? Am. J. Otol. 1997;18(6 Suppl):S11–S12. [PubMed] [Google Scholar]
- Coleman B, Hardman J, Coco A, Epp S, de Silva M, Crook J, Shepherd R. Fate of embryonic stem cells transplanted into the deafened mammalian cochlea. Cell Transplant. 2006;15:369–380. doi: 10.3727/000000006783981819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman B, Fallon JB, Pettingill LN, de Silva MG, Shepherd RK. Auditory hair cell explant co-cultures promote the differentiation of stem cells into bipolar neurons. Exp. Cell Res. 2007;313:232–243. doi: 10.1016/j.yexcr.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales CE, Pan L, Li H, Liberman MC, Heller S, Edge AS. Engraftment and differentiation of embryonic stem cell-derived neural progenitor cells in the cochlear nerve trunk: growth of processes into the organ of Corti. J. Neurobiol. 2006;66:1489–1500. doi: 10.1002/neu.20310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P, Duan ML, El Shamy WM, Canlon B. Protection of auditory neurons from aminoglycoside toxicity by NT3. Nat. Med. 1996;2:463–467. doi: 10.1038/nm0496-463. [DOI] [PubMed] [Google Scholar]
- Falk A, Holmstrom N, Carlen M, Cassidy R, Lundberg C, Frisen J. Gene delivery to adult neural stem cells. Exper. Cell Res. 2002;279:34–39. doi: 10.1006/excr.2002.5569. [DOI] [PubMed] [Google Scholar]
- Gharabaghi A, Tatagiba M. Functional regeneration of the axotomized auditory nerve with combined neurotrophic and anti-inhibitory strategies. Acta Neurochir. 2005;93:89–91. doi: 10.1007/3-211-27577-0_14. [DOI] [PubMed] [Google Scholar]
- Hadjantonakis A-K, Gertsenstein M, Ikawa M, Okabe M, Nagy A. Generating green fluorescent mice by germline transmission of green fluorescent ES cells. Mech. Dev. 1998;76:79–90. doi: 10.1016/s0925-4773(98)00093-8. [DOI] [PubMed] [Google Scholar]
- Hartshorn DO, Miller JM, Altschuler RA. Protective effect of electrical stimulation in the deafened guinea pig cochlea. Otolaryngol. Head Neck Surg. 1991;04(3):311–319. doi: 10.1177/019459989110400305. [DOI] [PubMed] [Google Scholar]
- He Z, Koprivica V. The NOGO signaling pathway for regeneration block. Ann. Rev. Neurosci. 2004;27:341–368. doi: 10.1146/annurev.neuro.27.070203.144340. [DOI] [PubMed] [Google Scholar]
- Hernandez Reyes J, Wys NL, Velkey M, Prieskorn DM, Wesolowski K, O’Shea KS, Miller JM, Altschuler RA. Neuronal differentiation of mouse embryonic stem cells following transient neurogenin 1 expression and treatment with BDNF and GDNF: in vitro and in vivo with placement into guinea pig cochlea. Soc. Neurosci. Abst. 2007 doi: 10.1523/JNEUROSCI.0563-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Ulfendahl M, Olivius NP. Central migration of neuronal tissue and embryonic stem cells following transplantation along the auditory nerve root. Brain Res. 2004;1026:68–73. doi: 10.1016/j.brainres.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Hu Z, Wei D, Johansson CB, Holmstrom N, Duan M, Frisen J, Ulfendahl M. Survival and neural differentiation of adult neural stem cells transplanted into the mature inner ear. Exp. Cell Res. 2005;302:40–47. doi: 10.1016/j.yexcr.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Iguchi F, Nakagawa T, Tateya I, Kim TS, Endo T, Taniguchi Z, Naito Y, Ito J. Trophic support of mouse inner ear by neural stem cell transplantation. Neuroreport. 2003;14(1):77–80. doi: 10.1097/00001756-200301200-00015. [DOI] [PubMed] [Google Scholar]
- Incesulu A, Nadol JB., Jr Correlation of acoustic threshold measures and spiral ganglion cell survival in severe to profound sensorineural hearing loss: implications for cochlear implantation. Ann. Otol. Rhinol. Laryngol. 1998;107:906–913. doi: 10.1177/000348949810701102. [DOI] [PubMed] [Google Scholar]
- Kanzaki S, Stover T, Kawamoto K, Prieskorn DM, Altschuler RA, Miller JM, Raphael Y. Glial cell line-derived neurotrophic factor and chronic electrical stimulation prevent VIII cranial nerve degeneration following denervation. J. Comp. Neurol. 2002;454:350–360. doi: 10.1002/cne.10480. [DOI] [PubMed] [Google Scholar]
- Kileny PR, Zimmerman-Phillips S, Kemink JL, Schmaltz SP. Effects of preoperative electrical stimulability and historical factors on performance with multichannel cochlear implant. Ann. Otol. Rhinol. Laryngol. 1991;100:563–568. doi: 10.1177/000348949110000708. [DOI] [PubMed] [Google Scholar]
- Kondo T, Johnson SA, Yoder MC, Romand R, Hashino E. Sonic hedgehog and retinoic acid synergistically promote sensory fate specification from bone marrow-derived pluripotent stem cells. Proc. Natl. Acad. Sci. U. S. A. 2005;102:4789–4799. doi: 10.1073/pnas.0408239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprivica V, Cho KS, Park JB, Yiu G, Atwal J, Gore B, Kim JA, Lin E, Tessier-Lavigne M, Chen DF, He Z. EGFR activation mediates inhibition of axon regeneration by myelin and chondroitin sulfate proteoglycans. Science. 2005;310:106–110. doi: 10.1126/science.1115462. [DOI] [PubMed] [Google Scholar]
- Li H, Liu H, Heller S. Pluripotent stem cells from the adult mouse inner ear. Nat. Med. 2003;9(10):1293–1299. doi: 10.1038/nm925. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Rebscher SJ, Snyder RL. Chronic intracochlear electrical stimulation induces selective survival of spiral ganglion neurons in neonatally deafened cats. Hear. Res. 1991;54(2):251–257. doi: 10.1016/0378-5955(91)90120-x. [DOI] [PubMed] [Google Scholar]
- Leake PA, Snyder RL, Hradek GT, Rebscher SJ. Chronic intracochlear stimulation in neonatally deafened cats: Effects of intensity and stimulating electrode position. Hear. Res. 1992;64:99–117. doi: 10.1016/0378-5955(92)90172-j. [DOI] [PubMed] [Google Scholar]
- Leake PA, Snyder RL, Hradek GT, Rebscher SJ. Consequences of chronic extracochlear electrical stimulation in neonatally deafened cats. Hear. Res. 1995;82(1):65–80. doi: 10.1016/0378-5955(94)00167-o. [DOI] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Sttakhovskaya O. Factors influencing neurotrophic effects of electrical stimulation in the deafened, developing auditory system. Hear. Res. doi: 10.1016/j.heares.2008.06.002. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou X, Zhang Y, Yuan C. Multipotent stem cells from the young rat inner ear. Neurosci. Lett. 2007;416:28–33. doi: 10.1016/j.neulet.2006.12.061. [DOI] [PubMed] [Google Scholar]
- Lousteau RJ. Increased spiral ganglion cell survival in electrically stimulated, deafened guinea pig cochleae. Laryngoscope. 1987;97:836–842. [PubMed] [Google Scholar]
- Malgrange B, Rigo JM, Van de Water TR, Staecker H, Moonen G, Lefebvre PP. Growth factor therapy to the damaged inner ear: clinical prospects. Inter. J. Ped. Otorhinolaryngol. 1999;49 Suppl 1:S19–S25. doi: 10.1016/s0165-5876(99)00126-3. [DOI] [PubMed] [Google Scholar]
- Marzella PL, Clark GM. Growth factors, auditory neurones and cochlear implants: a review. Acta Otolaryngol. 1999;119:407–412. doi: 10.1080/00016489950180919. [DOI] [PubMed] [Google Scholar]
- Matsuoka AJ, Kondo T, Miyamoto RT, Hashino E. In vivo and in vitro characterization of bone marrow-derived stem cells in the cochlea. Laryngoscope. 2006;116:1363–1367. doi: 10.1097/01.mlg.0000225986.18790.75. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Nakagawa T, Higashi T, Kim TS, Kojima K, Kita T, Sakamoto T, Ito J. Innervation of stem cell-derived neurons into auditory epithelia of mice. Neuroreport. 2005;16(8):787–790. doi: 10.1097/00001756-200505310-00001. [DOI] [PubMed] [Google Scholar]
- McGuinness SL, Shepherd RK. Exogenous BDNF rescues rat spiral ganglion neurons in vivo. Otol. Neurotol. 2005;26:1064–1072. doi: 10.1097/01.mao.0000185063.20081.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AL, Yamasoba T, Altschuler RA. Hair cell and spiral ganglion neuron preservation and regeneration - influence of growth factors. Curr. Opin. Otolaryngol. Head Neck Surg. 1998;6:301–307. [Google Scholar]
- Miller JM, Altschuler RA. Effectiveness of different electrical stimulation conditions in preservation of spiral ganglion cells following deafness. Ann. Otol. Rhinol. Laryngol. 1995;166:57–60. [PubMed] [Google Scholar]
- Miller JM, Chi DH, O'Keeffe LJ, Kruszka P, Raphael Y, Altschuler RA. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Inter. J. Develop. Neurosci. 1997;15:631–643. doi: 10.1016/s0736-5748(96)00117-7. [DOI] [PubMed] [Google Scholar]
- Miller AL, Prieskorn DM, Altschuler RA, Miller JM. Mechanism of electrical stimulation-induced neuroprotection: effects of verapamil on protection of primary auditory afferents. Brain Res. 2003;966(2):218–230. doi: 10.1016/s0006-8993(02)04170-7. [DOI] [PubMed] [Google Scholar]
- Mitchell A, Miller JM, Finger PA, Heller JW, Raphael Y, Altschuler RA. Effects of chronic high-rate electrical stimulation on the cochlea and eighth nerve in the deafened guinea pig. Hear. Res. 1997;105(1–2):30–43. doi: 10.1016/s0378-5955(96)00202-x. [DOI] [PubMed] [Google Scholar]
- Oshima K, Grimm CM, Corrales CE, Senn P, Martinez Monedero R, Geleoc GS, Edge A, Holt JR, Heller S. Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J. Assoc. Res. Otolaryngol. 2007;8:18–31. doi: 10.1007/s10162-006-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MA, Corliss DA, Gray B, Anderson JK, Bobbin RP, Snyder EY, Cotanche DA. Neural stem cells injected into the sound-damaged cochlea migrate throughout the cochlea and express markers of hair cells, supporting cells, and spiral ganglion cells. Hear. Res. 2007;232:29–43. doi: 10.1016/j.heares.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Sutton D, Miller JM, Bohne BA. Relation of psychophysical data to histopathology in monkeys with cochlear implants. Acta Otolaryngol. 1981;92:1–13. doi: 10.3109/00016488109133232. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Sutton D. Relation of cochlear implant function to histopathology in monkeys. Ann. N. Y. Acad. Sci. 1983;405:224–239. doi: 10.1111/j.1749-6632.1983.tb31635.x. [DOI] [PubMed] [Google Scholar]
- Prieskorn DM, Miller JM. Technical report: chronic and acute intracochlear infusion in rodents. Hear. Res. 2000;140:212–215. doi: 10.1016/s0378-5955(99)00193-8. [DOI] [PubMed] [Google Scholar]
- Rask-Andersen H, Bostrom M, Gerdin B, Kinnefors A, Nyberg G, Engstrand T, Miller JM, Lindholm D. Regeneration of human auditory nerve. In vitro/in video demonstration of neural progenitor cells in adult human and guinea pig spiral ganglion. Hear. Res. 2005;203:180–191. doi: 10.1016/j.heares.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Rivolta MN, Li H, Heller S. Generation of inner ear cell types from embryonic stem cells. Methods Mol. Biol. 2006;330:71–79. doi: 10.1385/1-59745-036-7:71. [DOI] [PubMed] [Google Scholar]
- Rubinstein JT, Parkinson WS, Tyler RS, Gantz BJ. Residual speech recognition and cochlear implant performance: effects of implantation criteria. American J. Otol. 1999;20(4):445–452. [PubMed] [Google Scholar]
- Schindler RA, Gladstone HB, Scott N, Hradek GT, Williams H, Shah SB. Enhanced preservation of the auditory nerve following cochlear perfusion with nerve growth factors. Amer. J. Otol. 1995;16:304–309. [PubMed] [Google Scholar]
- Shepherd RK, Coco A, Epp SB, Crook JM. Chronic depolarizes enhances the trophic effects of BDNF in rescuing auditory neurons following a sensorineural hearing loss. J. Comp. Neurol. 2005;486:145–158. doi: 10.1002/cne.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK, Javel E. Electrical stimulation of the auditory nerve. I. Correlation of physiological responses with cochlear status. Hear. Res. 1997;108:112–144. doi: 10.1016/s0378-5955(97)00046-4. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Coco A, Epp SN. Neurotrophins and electrical stimulation for protection and repair of spiral ganglion neurons following sensorineural hearing loss. Hear. Res. doi: 10.1016/j.heares.2007.12.005. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly DJ, Heffer LF, White MW, Shepherd RK, Birch MG, Minter RL, Nelson NE, Wise AK, O'Leary SJ. Deafness alters auditory nerve fibre responses to cochlear implant stimulation. Eur J Neurosci. 2007;26(2):510–522. doi: 10.1111/j.1460-9568.2007.05678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staecker H, Kopke R, Malgrange B, Lefebvre P, Van de Water TR. NT-3 and/or BDNF therapy prevents loss of auditory neurons following loss of hair cells. Neuroreport. 1996;7(4):889–894. doi: 10.1097/00001756-199603220-00011. [DOI] [PubMed] [Google Scholar]
- Tamura T, Nakagawa T, Iguchi F, Tateya I, Endo T, Kim TS, Dong Y, Kita T, Kojima K, Naito Y, Omori K, Ito J. Transplantation of neural stem cells into the modiolus of mouse cochleae injured by cisplatin. Acta Otolaryngol. Suppl. 2004;551:65–68. doi: 10.1080/03655230310016780. [DOI] [PubMed] [Google Scholar]
- Ulfendahl M, Hu Z, Olivius P, Duan M, Wei D. A cell therapy approach to substitute neural elements in the inner ear. Physiol. Behav. 2007;92:75–79. doi: 10.1016/j.physbeh.2007.05.054. [DOI] [PubMed] [Google Scholar]
- Van de Water TR, Staecker H, Ernfors P, Moonen G, Lefebvre PP. Neurotrophic factors as pharmacological agents for the treatment of injured auditory neurons; Ciba Foundation Symposium; 1996. pp. 149–162. [DOI] [PubMed] [Google Scholar]
- Yerukhimovich MV, Bai L, Chen DH, Miller RH, Alagramam KN. Identification and characterization of mouse cochlear stem cells. Dev. Neurosci. 2007;29:251–260. doi: 10.1159/000096415. [DOI] [PubMed] [Google Scholar]
- West BA, Brummett RE, Himes DL. Interaction of kanamycin and ethacrynic acid. Arch. Otolaryngol. 1973;98:32–37. doi: 10.1001/archotol.1973.00780020036009. [DOI] [PubMed] [Google Scholar]