Abstract
Cultural evolution is an important force in creating and maintaining behavioral variation in some species. Vocal dialects have provided a useful model for the study of cultural evolution and its interactions with genetic evolution. This study examined the acoustic and geographic changes in vocal dialects over an eleven-year span in the yellow-naped amazon, Amazona auropalliata, in Costa Rica. Contact calls were recorded at 16 communal night roosts in 1994 and 19 roosts in 2005, with 12 roosts sampled in both surveys. In both surveys three dialects were found, each characterized by a distinctive contact call type and each encompassing multiple roosts. The limits between two of these dialects, the North and South dialects, was found to be geographically stable, while at the boundary between the North and Nicaraguan dialect there was introgression of each call type into roosts in the bordering dialect. Acoustic measurements and cross-correlations of spectrograms detected no change in the acoustic structure of contact calls in the South dialect but did show significant differences in the calls of both the North and Nicaraguan dialect between 1994 and 2005. These results are consistent with the vocal convergence hypothesis that proposes that dialects are long-term features maintained through some combination of biased transmission of local call types and purifying selection against foreign call types. Migration, copying errors and cultural drift may also play a role in the more subtle changes seen in the acoustic form of dialect call types.
Keywords: Amazona auropalliata, contact calls, cultural evolution, Neotropical parrots, vocal communication, vocal dialects, yellow-naped amazon
Cultural evolution of learned traits creates and maintains behavioral variation within certain species. Learned traits may evolve in a manner analogous to genetic evolution, with population frequencies of behaviors changing due to cultural drift, selection, mutation through learning errors or innovation, biased transmission via preferential learning from certain individuals, and migration (Mundinger 1980, Boyd & Richerson 1985, Lynch 1996). Geographic variation in learned acoustic signals, commonly termed ‘vocal dialects’, represent a particularly useful setting in which to investigate the process of cultural evolution (Mundinger 1982, Lynch et al. 1989). Vocal dialects are present in a wide range of taxa, including humans (Trudgill 1983, Nettle 1999), numerous oscine and at least one suboscine songbird (Catchpole & Slater 1995, Kroodsma 2004), hummingbirds (Gaunt et al. 1994), parrots (Wright 1996, Baker 2000, Baker 2003), cetaceans (Ford 1991, Weilgart & Whitehead 1997) and bats (Davidson & Wilkinson 2002). The broad taxonomic distribution of vocal dialects offers diverse opportunities to examine the relative importance of drift, selection, mutation, migration and biased transmission in cultural evolution.
Studies of temporal stability in vocal dialects can provide particular insight into these evolutionary processes. Temporal stability in vocal dialects can be considered in two dimensions: 1) the geographic stability of dialect boundaries, and 2) the stability of acoustic properties of vocalizations. High rates of either learning errors or drift would decrease acoustic stability, as would diversifying or directional selection (Lynch 1996). Likewise, migration could reduce the geographic stability of dialect boundaries by introducing foreign call variants into dialects, or through the recolonization of regions by individuals with new dialect types after extinction of previous populations (Harbison et al. 1999). In contrast, both biased transmission, in which the most prevalent local call types are preferentially learned, and purifying selection against novel call types should promote dialect stability. Models incorporating both biased transmission and purifying selection have predicted dialects that are stable in both acoustic form and geographic pattern, even in the face of mutation and migration (Ellers & Slabbekoorn 2003, Lachlan et al. 2004).
To date, studies of dialect stability have focused primarily on songbirds. Within this group, a number of distinct patterns of dialect stability have been observed. In some cases dialects appear to be both geographically and acoustically stable (Trainer 1983, Chilton & Lein 1996, Harbison et al. 1999). In other cases acoustic form is more temporally variable, with new call forms being introduced into populations and others being lost (Ince et al. 1980, Payne 1996, Baker & Gammon 2006). In still others, some components of a vocal signal have been found to be stable while others are changing with time, suggesting that different components may be governed by different forms of selection (Audret-Hausberger 1986, Nelson et al. 2004). Relating these patterns to the function of the vocal signals in each species and to specific processes of cultural evolution remains a challenge. One approach to this challenge is to broaden the scope of dialect stability studies to other taxa such as parrots, which differ from songbirds in their social structure, sound production mechanisms, social contexts for vocal communication, and timing and neural mechanisms of vocal learning (Bradbury 2003, Jarvis 2004).
Among the parrots, vocal dialects have been most thoroughly investigated in the yellow-naped amazon, Amazona auropalliata. In 1994, a survey of contact calls recorded at sixteen communal roosting sites in Costa Rica revealed three distinct types of this call (Wright 1996). Each of these call types was shared by a number of neighboring roosts, and, in general, a single call type was recorded at a given roost, resulting in the mosaic pattern typical of vocal dialects. Notable exceptions were found at roosts on the borders of dialects, where a low proportion of birds were bilingual and used the contact calls of both neighboring dialects. Subsequent genetic studies utilizing both mitochondrial control region sequences and microsatellites detected no population genetic structure congruent with dialect boundaries, suggesting high rates of gene flow, and thus individual movements, between two dialects (Wright & Dorin 2001, Wright et al. 2005). These results suggest that dialects are maintained through time by preferential learning of local call types by immigrants, a form of biased transmission (Payne 1981). Such a process of vocal convergence is predicted to curb the pace of cultural evolution and produce stable dialects that persist over time and space.
To test this prediction, we conducted a second survey of vocal variation in the contact calls of the yellow-naped amazon in Costa Rica in 2005. Our goal was to assess the degree of change in both the geographic boundaries between vocal dialects and the acoustic structure of contact calls within dialects over the 11-year span between this resurvey and the original 1994 survey, and to utilize these patterns to infer the processes of cultural evolution that shape vocal variation in this species.
METHODS
Contact Call Recordings
In June, 2005, we sampled contact calls from yellow-naped amazons across the range of this species in Costa Rica following the methods of the original dialect survey performed from March to June, 1994 (Wright 1996). Calls were recorded at communal night roosts in the early morning or late afternoon. Communal night roosts typically contain large numbers of birds (50–300) and are located in traditional sites that are used throughout the year and over the course of decades. In 2005 we revisited 12 of the 16 roosts surveyed in 1994; four roosts from our original survey were inaccessible due to poor road conditions or lack of landowner permission. In addition, we recorded calls at seven roosts that either we had not found in 1994 or from which we had not obtained usable recordings. In total we surveyed 19 roosts in 2005, of which 12 were sampled in both the 1994 and 2005 survey (Fig. 1). In sum the two surveys sampled all the know roosts in the range of this species in Costa Rica that were accessible to us.
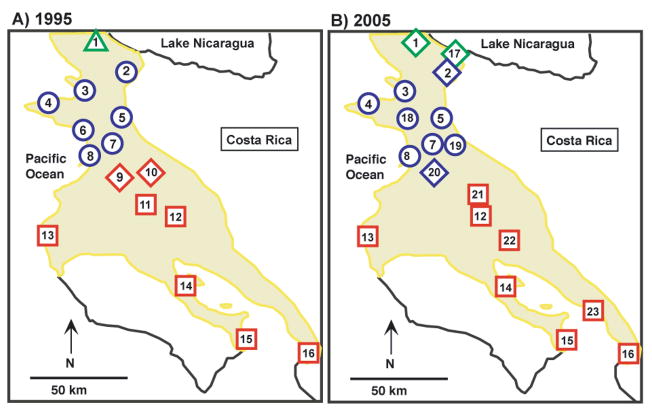
Figure 1.
Maps of northwestern Costa Rica showing the distribution of roost sites and vocal dialects surveyed in a) 1994 and b) 2005. Green triangles indicate Nicaraguan dialect roosts, blue circles indicate North dialect, red squares indicate South dialect, and the diamonds indicate bilingual roosts at which both neighboring dialects were observed, with the shading of the diamond indicating the predominant dialect recorded. The yellow shading indicates the range of the yellow-naped amazon in Costa Rica. Sixteen roosts were included in the 1994 call analysis; twelve of these roosts were also sampled in 2005: 1-Peñas Blancas, 2-Hacienda Inocentes, 3-Playa Junquillal, 4-Murcielago, 5-Pelon Altura, 6-Playa Naranjo, 7-Horizontes, 8-Playa Cabuyal, 9-Finca Gisa, 10-Hacienda San Jeronimo, 11-Finca Zapolita, 12-Pelon Bajura, 13-Playa Grande, 14-Puerto San Pablo, 15-Finca Curu, 16-Tarcoles. Seven additional roosts were sampled in 2005 only: 17-Parcelas Santa Elena, 18- Parque Santa Rosa, 19-Finca Ahogados, 20- Las Trancas, 21- Finca Palenque, 22- Taboga/Finca El Cortijo, 23-Tivives.
We recorded contact calls from unmarked birds of both sexes perched in the vicinity of roosts (generally within 500 m). In 2005, calls were recorded with an ME67 shotgun microphone (Sennheiser Electronic Corp., Old Lyme, Connecticut) onto a PMD670 solid state recorder (Marantz Corp, Mahwah, New Jersey) and saved as 16 bit wav files with a sampling rate of 22.05 kHz. In 1994 we used a Sennheiser MKH816 P48 shotgun microphone and a DAP-20 DAT recorder (TEAC Inc., Montebello, California) of comparable audio quality. Calls from 1994 were band-pass filtered from 0.25 to 8 kHz then digitized with a Macintosh Powerbook 180 internal 8 bit digitizer sampling at 22 kHz. To sample among individual variation we recorded six to eight birds per site in 2005 and two to four birds in 1994; to sample within individual variation we aimed for ten high-quality calls from each bird, although this goal was not met for all birds. At two sites where calls of more than one dialect were observed (‘bilingual sites’), we increased our sample sizes to 12 birds (Hacienda Inocentes, site 2) or 15 birds (Parcelas Santa Elena, site 17) in order to adequately sample variation within both dialect forms (see Fig. 1 for site locations). In total, in 1994 we sampled 514 calls from 54 birds at 16 sites (Table 1), with 218 calls from 23 birds using the South dialect, 256 calls from 27 birds using the North dialect, and 40 calls from 4 birds using the Nicaraguan dialect (mean ± SE of 31.2 ± 2.4 calls from a mean of 3.2 ± 0.2 birds per site). In 2005, we sampled 1173 calls from 130 birds at 19 sites (Table 1), with 424 calls from 44 birds using the South dialect, 592 calls from 69 birds using the North dialect, and 157 calls from 17 birds using the Nicaraguan dialect (mean ± SE of 61.7 ± 5.9 calls from a mean of 6.9 ± 0.7 birds per site).
Table 1.
Numbers of birds and contact calls sampled at each site in 1993 and 2005.
| 1994 | 2005 | ||||
|---|---|---|---|---|---|
| Site | Site number * | Birds | Calls | Birds | Calls |
| Peñas Blancas | 1 | 4 | 40 | 3 | 27 |
| Hacienda Inocentes | 2 | 4 | 37 | 12 | 111 |
| Playa Junquillal | 3 | 4 | 40 | 6 | 59 |
| Murcielago | 4 | 3 | 28 | 7 | 61 |
| Pelon Altura | 5 | 4 | 40 | 8 | 49 |
| Playa Narajo | 6 | 4 | 34 | ||
| Horizontes | 7 | 4 | 40 | 6 | 59 |
| Playa Cabuyal | 8 | 2 | 13 | 7 | 61 |
| Finca Gisa | 9 | 4 | 34 | ||
| Hacienda San Jeronimo | 10 | 3 | 29 | ||
| Finca Zapolita | 11 | 4 | 40 | ||
| Pelon Bajura | 12 | 4 | 40 | 6 | 60 |
| Playa Grande | 13 | 3 | 25 | 1 | 9 |
| Puerto San Pablo | 14 | 2 | 20 | 6 | 55 |
| Finca Curu | 15 | 2 | 20 | 6 | 60 |
| Tarcoles | 16 | 3 | 24 | 7 | 66 |
| Parcelas Santa Elena | 17 | 15 | 133 | ||
| Parque Santa Rosa | 18 | 7 | 63 | ||
| Finca Ahogados | 19 | 9 | 62 | ||
| Las Trancas | 20 | 7 | 64 | ||
| Finca Palenque | 21 | 6 | 57 | ||
| Taboga/Finca El Cortijo | 22 | 6 | 57 | ||
| Tivives | 23 | 6 | 60 | ||
Non-invasive techniques were used throughout this study. This project was conducted under protocol number 2005-001 of the Animal Care and Use Committee at New Mexico State University.
Acoustical Analysis
We opened sound files recorded in 2005 in Raven 1.2.1 (Cornell Lab of Ornithology, Ithaca, New York) and randomly selected up to ten contact calls for each individual from among the high-quality recordings with low levels of background noise. Similar procedures were employed with the 1994 calls (Wright 1996). We then performed two different forms of acoustic analyses of these call sets: acoustic parameter measurements and spectrogram cross correlations (SPCC).
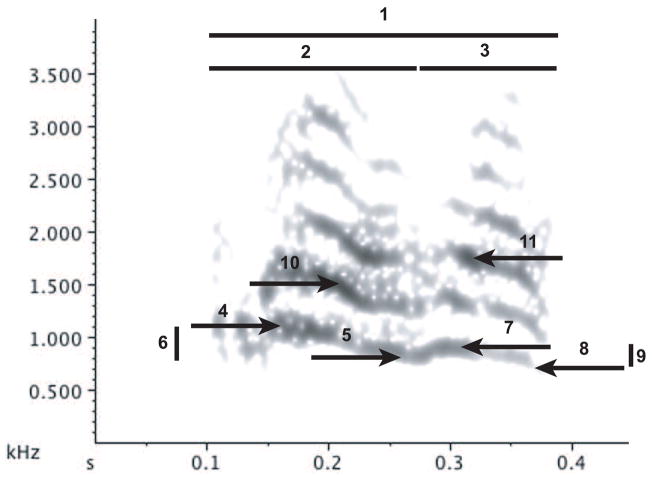
To measure acoustic parameters, we created spectrograms of 1994 and 2005 calls in Raven 1.2.1 with a Hanning window of 512 samples and 3dB filter bandwidth of 62.5 Hz, a frequency grid with DFT size of 512 samples and spacing of 43.5 Hz, a time grid with Hop size of 51 samples and 90% overlap and averaging of 12 spectra. Eleven time and frequency parameters were measured from these spectrograms for each call using onscreen cursors or automated measurements in Raven 1.2.1 (Fig. 2).
Figure 2.
Representative spectrogram of a North dialect contact call illustrating the eleven acoustic parameters measured on calls from all three dialects. Parameters are 1 = total note duration, 2 = first segment duration, 3 = second segment duration, 4 = first segment high frequency, 5 = first segment low frequency, 6 = first segment frequency range, 7 = second segment high frequency, 8 = second segment low frequency, 9 = second segment frequency range, 10 = first segment peak frequency, 11 = second segment peak frequency. Frequency parameters 4 through 9 were measured on the second harmonic.
To calculate spectrogram cross-correlations, we first equalized the sampling rates of the 1994 and 2005 calls to 22.05 kHz using the ‘resamp’ command in SIGNAL 4.03 (Engineering Design, Berkeley, California). We then performed spectrogram cross-correlations using the CORMAT routine v2.26 in SIGNAL using spectrograms with a 512 sample FFT, 100 steps, band pass filtering from 0.6 to 3.5 kHz and no frequency shifts or time normalization. We performed separate batch cross-correlations for five sets of calls: all 1994 calls, all 2005 calls, and for the calls from both years combined for each of the three dialects (North, South and Nicaraguan).
Statistical Analysis
We performed Principal Components Analysis (PCA) on the correlation matrix of the call measurements using JMP 5.1 (SAS Institute, Cary North Carolina) to reduce the number of collinear variables. We performed PCA on each of the three dialects separately and rotated the first five factors to create five orthogonal variables for each dialect. We then performed nested Analysis of Variance (ANOVA) in JMP 5.1 on each rotated factor for each dialect in which year was a fixed effect, recording site nested within year was a random effect, and individual nested within site was a random effect. We adjusted alphas within each dialect using the Bonferroni method to correct for multiple tests (Sokal & Rohlf 1995).
To visualize patterns of similarity in the matrices of peak cross-correlation values produced by each of our five batch SPCC’s we used Principal Coordinates Analysis (PCO) implemented in the R Package v 4.03 (Casgrain & Legendre 2001). To aid in the visualization of this large dataset, we averaged the eigenvector values for all calls from each individual for the first two eigenvectors and plotted these average values for each individual in bivariate space. We tested for hypothesized effects of dialect, year, and site using standard and partial Mantel tests (Mantel 1967, Smouse et al. 1986) between the matrix of similarity values for individual calls and test matrices constructed with 1’s for within classification comparisons (e.g. calls from the same year or site) and 0’s for between classification comparisons. We tested for differences among the three dialects within each year (1994 and 2005). We also combined the calls of both years and conducted separate tests within each of the three dialects for the effect of a) site, and b) year after controlling for the effects of site. All Mantel tests were implemented in the R Package v 4.03 (Casgrain & Legendre 2001) and were run for 1000 permutations.
RESULTS
Stability of Geographic Distributions
Auditory classification of sounds and visual inspection of spectrograms suggested that the three dialects observed across the range of the yellow-naped amazon in Costa Rica in 1994 persisted in 2005 (Fig. 3a–c). Provisional assignment of sites to dialects based on auditory and visual inspection revealed a pattern of geographic distribution that was similar between 1994 and 2005 (Fig. 1). The border between the South and North dialects was in the same location, with all sites that had previously been classified as North or South in 1994 retaining that classification in 2005. Furthermore, the distribution of sites at this boundary at which some birds used both neighboring dialects (were ‘bilingual’) remained similar over the eleven-year span. For example, in 2005 a few (<10) bilingual birds were observed at Las Trancas (site 20) using both North and South contact calls, but most individuals produced only North contact calls. Similar usage patterns were observed at this site in 1994, although no usable recordings were obtained at that time (T.F. Wright unpublished data). Conversely, at Finca Gisa (site 9) located 20 km to the southeast, a few bilingual birds were recorded in 1994, but most birds produced South contact calls. We were unable to obtain access to this site in 2005, but during a visit in 2000 we observed similar usage patterns with a small number of bilingual birds and majority usage of the South dialect (T.F. Wright unpublished data).
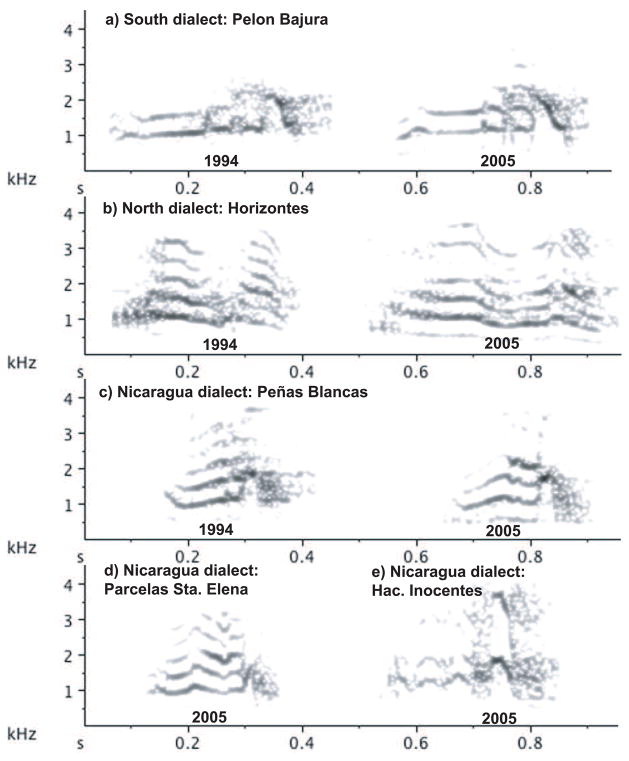
Figure 3.
Representative spectrograms of contact calls recorded in 1994 and 2005 from one site in each of the a) South, b) North, and c) Nicaraguan dialects. These calls illustrate the conservation of the basic form of the contact call and the distinctiveness of the three dialects over an eleven-year span. The bottom two spectrograms are of Nicaraguan dialect calls recorded in 2005 at two additional sites, d) Parcelas Santa Elena and e) Hacienda Inocentes, which illustrate the distinctive form of the calls from Hacienda Inocentes relative to the other two Nicaraguan dialect site.
A different pattern was apparent at the boundary between the North and Nicaraguan dialects. Here we observed sites at which all birds exclusively used one dialect in 1994 (e.g. North dialect only at Hacienda Inocentes or Nicaraguan dialect only at Peñas Blancas, site 1), while in 2005 some birds used both dialects. We also made recordings in 2005 at a new roost between these two sites at which birds were using both North and Nicaraguan dialects. Thus in 2005 there appeared to be more introgression of call types into the neighboring dialects at this dialect boundary than was observed in 1994, or was observed in either year at the boundary between North and South dialects.
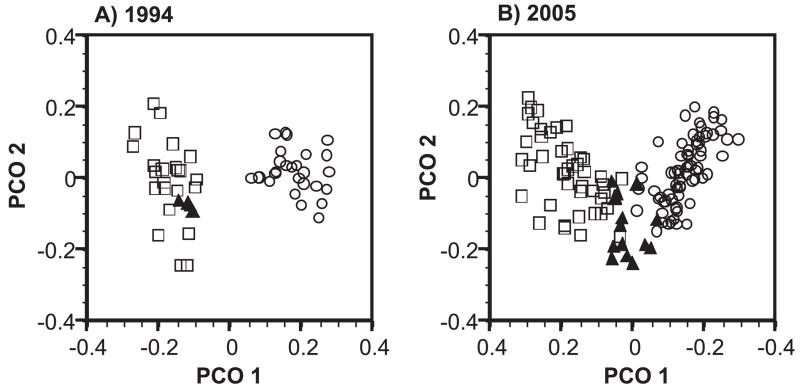
The analysis of contact call structural variation by spectrogram cross-correlation analysis confirmed the geographic stability of the three dialects. PCO plots of the matrices of peak correlation values for all calls recorded in 1994 (Fig. 4a) and 2005 (Fig. 4b) showed non-overlapping distributions of the North and South dialects in both years. The Nicaraguan dialect was nested within the South dialect in 1994 while in 2005 it appeared to more intermediate between the two dialects. Mantel tests between the spectrogram cross-correlation matrix and a 1-0 test matrix coded for provisional membership within the three dialects showed a significant and positive relationship for the 1994 calls (Mantel r = 0.51, array size = 514 calls, P < 0.001) and 2005 calls (Mantel r = 0.45, array size = 1173 calls, P < 0.001).
Figure 4.
Plots of the first and second PCO eigenvectors of the peak spectrogram cross-correlation values for calls from the a) 1994 survey and b) the 2005 survey. Points indicate the mean values for the calls of an individual (1994 = 54 individuals, 2005 = 131 individuals). Points are coded by open squares for South dialect sites, open circles for North dialect sites, and closed triangles for Nicaraguan dialect sites.
Stability of Acoustic Structure
The three dialects showed differing degrees of stability in the acoustic structure of their contact calls. In the South dialect, a PCO plot of spectrogram cross-correlations showed a high degree of overlap between calls recorded in 1994 and 2005 (Fig. 5a). Mantel tests showed an association of call variation with both site (Mantel r = 0.19, array size = 642 calls, P < 0.001) but not with year after controlling for site (partial Mantel r = 0.001, array size = 642 calls, P = 0.44). Measurements of acoustic parameters from spectrograms showed a similar pattern. The first five factors from a Principal Components Analysis explained 85 percent of the variation in the eleven measured parameters (Table 2). Nested ANOVA’s for each of these five PCA factors revealed significant effects of year for none of these factors, significant effects of site nested within year for four factors, and significant effects of individual nested within site and year for all five factors (Table 3).
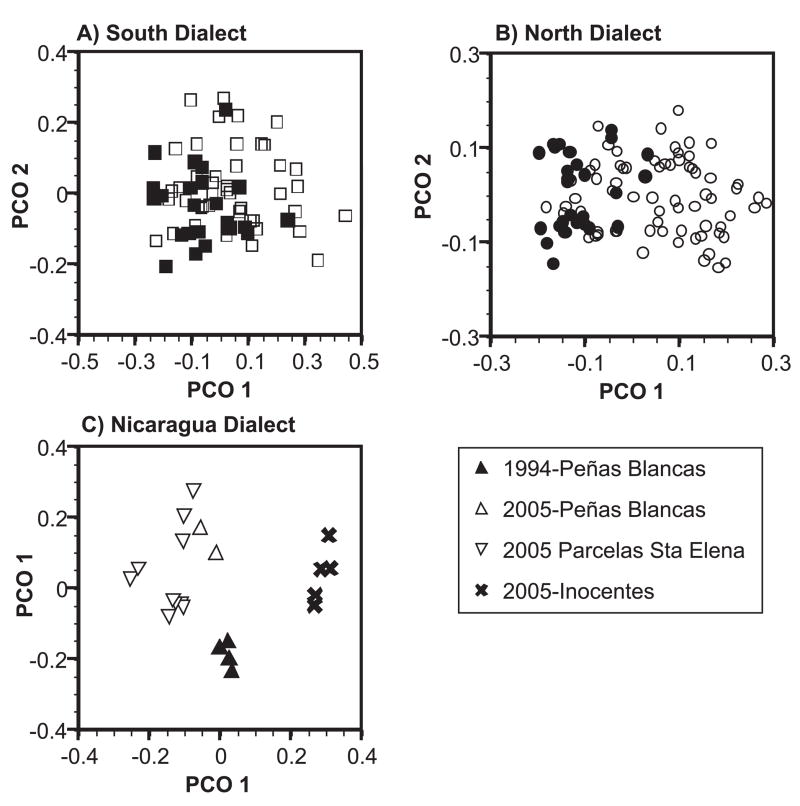
Figure 5.
Plots of the first and second PCO eigenvectors of the peak spectrogram cross-correlation values for calls from the a) South, b) North, and c) Nicaraguan dialects. Points indicate the mean values for the calls of an individual (South = 67 individuals, North = 96 individuals, Nicaraguan = 21 individuals). In a) and b), closed symbols indicate individuals from 1994 and open symbols individuals from 2005. In c) points follow the accompanying legend.
Table 2.
Factor loadings from Principal Components analysis of acoustic parameter measures from contact calls of three vocal dialects
| South Dialect | North Dialect | Nicaraguan Dialect | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measurements | PCA 1 | PCA 2 | PCA 3 | PCA 4 | PCA 5 | PCA 1 | PCA 2 | PCA 3 | PCA 4 | PCA 5 | PCA 1 | PCA 2 | PCA 3 | PCA 4 | PCA 5 |
| total note duration (ms) | 0.97 | −0.02 | −0.10 | −0.13 | −0.02 | 0.043 | −1.00 | 0.03 | 0.02 | −0.04 | 0.03 | 0.98 | −0.06 | 0.05 | −0.13 |
| first segment duration (ms) | 0.81 | 0.04 | −0.11 | −0.29 | −0.06 | −0.20 | −0.78 | 0.22 | −0.18 | 0.13 | 0.36 | 0.87 | 0.00 | 0.06 | −0.04 |
| second segment duration (ms) | 0.58 | −0.13 | 0.45 | 0.32 | 0.07 | 0.28 | −0.68 | −0.20 | 0.22 | −0.21 | −0.43 | 0.77 | −0.11 | 0.01 | −0.21 |
| first segment high freq. (Hz) | 0.08 | −0.05 | 0.07 | −0.78 | 0.59 | 0.21 | −0.06 | 0.89 | 0.26 | −0.10 | 0.87 | 0.13 | 0.02 | −0.09 | −0.43 |
| first segment low freq. (Hz) | −0.10 | −0.05 | −0.01 | 0.03 | 0.96 | 0.28 | −0.03 | −0.11 | 0.82 | −0.08 | 0.95 | 0.01 | 0.02 | −0.09 | 0.04 |
| first segment freq. range (Hz) | 0.19 | −0.01 | 0.09 | −0.90 | −0.24 | −0.03 | −0.03 | 0.88 | −0.41 | −0.02 | 0.34 | 0.24 | 0.01 | −0.04 | −0.90 |
| second segment high freq. (Hz) | −0.08 | −0.11 | 0.91 | −0.03 | 0.07 | 0.87 | −0.02 | 0.10 | 0.37 | 0.03 | 0.92 | 0.16 | 0.00 | −0.16 | −0.23 |
| second segment low freq. (Hz) | −0.01 | 0.85 | 0.48 | 0.03 | −0.01 | −0.11 | 0.02 | 0.03 | 0.87 | 0.08 | 0.85 | −0.26 | 0.10 | −0.10 | −0.12 |
| second segment freq. range (Hz) | −0.04 | −0.96 | 0.13 | −0.06 | 0.06 | 0.97 | −0.03 | 0.08 | −0.15 | −0.02 | 0.68 | 0.47 | −0.08 | −0.15 | −0.25 |
| first segment peak freq. (Hz) | 0.14 | −0.50 | 0.07 | 0.12 | 0.47 | −0.05 | 0.02 | −0.11 | 0.11 | 0.73 | 0.03 | −0.09 | 0.98 | −0.13 | 0.00 |
| second segment peak freq. (Hz) | −0.16 | 0.25 | 0.81 | −0.12 | −0.05 | 0.054 | 0.02 | 0.02 | −0.09 | 0.80 | 0.23 | −0.08 | 0.14 | −0.95 | −0.04 |
| Eigenvalue | 2.48 | 2.16 | 1.82 | 1.25 | 1.21 | 2.56 | 2.16 | 1.98 | 1.44 | 1.20 | 4.86 | 2.80 | 1.07 | 0.70 | 0.68 |
| Variation explained (%) | 23.3 | 19.6 | 18.0 | 13.1 | 10.9 | 22.5 | 19.6 | 16.5 | 11.4 | 11.0 | 44.2 | 25.5 | 9.8 | 6.4 | 6.2 |
| Total variation explained (%) | 85 | 81 | 92 | ||||||||||||
Table 3.
Results of nested Analyses of Variance testing the effects of year, site and individuals on acoustic parameters for the South, North and Nicaraguan dialects
| F- value* |
||||
|---|---|---|---|---|
| Dialect | PCA Factor | Year† | Site nested within Year** | Individual nested within Site and Year†† |
| South | PCA 1 | 0.2 | 9.8 * | 6.3 * |
| PCA 2 | 2.3 | 6.1 * | 4.5 * | |
| PCA 3 | 3.2 | 2.5 * | 8.9 * | |
| PCA 4 | 1.8 | 7.2 * | 3.3 * | |
| PCA 5 | 4.8 | 0.7 | 7.4 * | |
| North | PCA 1 | 4.5 | 5.3 * | 4.4 * |
| PCA 2 | 9.0 * | 2.4 * | 6.2 * | |
| PCA 3 | 6.2 | 7.8 * | 3.2 * | |
| PCA 4 | 0.6 | 3.4 * | 5.0 * | |
| PCA 5 | 18.1 * | 1.2 | 2.0 * | |
| Nicaraguan | PCA 1 | 0.1 | 93.6 * | 9.9 * |
| PCA 2 | 1.8 | 0.9 | 69.9 * | |
| PCA 3 | 26.7 * | 0.1 | 2.5 * | |
| PCA 4 | 3.2 | 1.4 | 1.8 | |
| PCA 5 | 0.04 | 6.0 | 2.9 * | |
F-values with P < 0.05 after Bonferroni correction for multiple tests within each dialect
df = 1 for all three dialects
df = 14 for South, 18 for North, and 2 for Nicaraguan dialect
df = 51 for South, 81 for North, and 17 for Nicaraguan dialect
In the North dialect, a PCO plot of spectrogram cross-correlations showed a lower degree of overlap between 1994 and 2005 calls than was found in the South dialect (Fig. 5b). Here Mantel tests showed an association of call variation with both site (Mantel r = 0.13, array size = 838 calls, P < 0.001) and with year after controlling for site (partial Mantel r = 0.16, array size = 838 calls, P < 0.001). Results from acoustic parameter measurements were consistent with those from the cross-correlations. The first five PCA factors explained 81 percent of the variation in the eleven parameters (Table 2), and nested ANOVA’s on these PCA factors revealed significant effects of year for two factors, significant effects of site nested within year for four factors, and significant effects of individual nested within site and year for all five factors (Table 3).
To examine the patterns of acoustic change between 1994 and 2005 in the North dialect in more detail, we averaged the PCA factor values across calls for each individual and performed unpaired t-tests comparing mean values in the two years at each of the six sites that were surveyed in both years. Results from these post-hoc comparisons indicated that all sites but one had significant differences between the two years for at least one PCA factor (Table 4). Notably, the one site that showed no differences between 1994 and 2005 was Hacienda Inocentes (see below).
Table 4.
Results of post-hoc t-tests examining variation in acoustic parameters between calls recorded in 1994 and 2005 at six sites in the North dialect
| d.f. for all tests | PCA 1 | PCA 2 | PCA 3 | PCA 4 | PCA 5 | |
|---|---|---|---|---|---|---|
| Hacienda Inocentes | 9 | 1.5 | 0.2 | −0.1 | −0.6 | 0.4 |
| Playa Junquillal | 8 | −2.9 * | 1.2 | 0.1 | 2.2 | 1.5 |
| Murcielago | 8 | −3.3 * | 3.7 * | −3.0 * | 2.0 | 2.0 |
| Pelon Altura | 11 | 1.0 | −1.6 * | −4.0 * | −0.9 | 0.7 |
| Horizontes | 8 | −1.4 | 5.4 * | −6.3 * | −1.0 | 2.5 * |
| Playa Cabuyal | 7 | 0.8 | 1.3 | −2.2 | 1.9 | 2.8 * |
| All six sites combined | 61 | −2.0 * | 3.1 * | −4.8 * | 0.8 | 3.2 * |
t-values with P < 0.05
Although the Nicaraguan dialect was represented by fewer sites in the 1994 and 2005 surveys (one and three sites, respectively), it did show an intriguing pattern of geographic differentiation among these sites. A Mantel test for the effect of site on spectrogram cross-correlation values was significant with a Mantel r value comparable in magnitude to those from the comparisons among all three dialects (Mantel r = 0.48, array size = 197 calls, P < 0.001). The PCO plot of the cross-correlation values (Fig. 5c) revealed that this result was driven largely by calls from Hacienda Inocentes, a site at which we recorded exclusively North dialect calls in 1994. Closer inspection of the Nicaraguan type calls recorded from this site in 2005 suggested that they were qualitatively different from those recorded at the other two sites. Calls from Hacienda Inocentes did share some characteristics of the Nicaraguan calls recorded at the other two sites, particularly in the pattern of frequency modulation in the second segment, but they also showed a pattern of modulation in the first segment that is unique to this site (Fig. 3c–e). Results from acoustic parameter measurements were more difficult to interpret given that only one site that we sampled in both 1994 and 2005 (Peñas Blancas) exhibited the Nicaraguan dialect in both years. The first five PCA factors explained 92 percent of the variation in the eleven parameters (Table 2), and nested ANOVA’s on these PCA factors revealed significant effects of year for one factor, significant effects of site nested within year for one factor, and significant effects of individual nested within site and year for four of the five factors (Table 3).
DISCUSSION
This study of vocal dialects in the yellow-naped amazon revealed a general picture of both acoustic and geographic stability over the eleven-year span between surveys conducted in 1994 and 2005. The three different forms of contact calls (South, North and Nicaraguan) were clearly recognizable in both years and were distributed in the same general spatial pattern in both surveys. Closer examination of call structure, however, revealed subtle changes in call structure between the two surveys in two of the three dialects, and geographic introgression of call types at the boundary between the same two dialects. Below we summarize these changes and discuss their implications for the maintenance and origin of vocal dialects and the process of cultural evolution.
Acoustic and Geographic Change
Acoustic structure of the South dialect did not differ between the two surveys after controlling for variation among different sites, and there were no observed changes in the boundary between this dialect and its neighboring North dialect, although it should be noted that some sites along this boundary were not sampled in both surveys. In contrast, there was bidirectional introgression of the North and Nicaraguan call types along their shared border, such that two sites on either side of the boundary between the two dialects that had exhibited only a single call type in 1994 exhibited both dialects in 2005. Calls of both the North and Nicaraguan dialects recorded in 2005 differed in many measures of acoustic variation from those recorded in 1994, but the call types of the two dialects remained different from each other, with little evidence of coalescence of the two types. In the North dialect changes in acoustic form were not concentrated at any particular site but occurred throughout the dialect, while in the Nicaraguan dialects these changes were most evident at a single site, Hacienda Inocentes. Patterns of temporal change at this site are particularly notable. In 1994 only North calls were recorded at this site, while in 2005 both North and Nicaraguan calls were recorded there. This result is unlikely to be an artifact of increased sampling effort in 2005 as we only increased our sample sizes there in 2005 after observing bilingual birds among the first birds recorded. The acoustic structure of the North calls recorded at this site did not differ between 1994 and 2005. In contrast, the Nicaraguan calls at Hacienda Inocentes were strikingly different from those recorded at the other two Nicaraguan sites in 2005 and may represent an incipient dialect (see below).
Temporal Persistence and Dialect Maintenance
Our data confirm that dialects in the yellow-naped amazon are maintained through time as distinctive geographic features. Such temporal stability in geographic and acoustic patterns has been found in dialects in a range of songbird species, including white crowned sparrows, Zonotrichia leucophrys (Trainer 1983, Chilton & Lein 1996, Harbison et al. 1999, Nelson et al. 2004), brown-headed cowbirds, Molothrus ater (Anderson et al. 2005), redwings, Turdus iliacus (Bjerke 1980), and black-capped chickadees, Poecile atricapillus (Ficken & Popp 1995, Baker & Gammon 2006), and in some cetaceans including killer whales, Orcinus orca (Deecke 2000, Riesch et al. 2006), and sperm whales, Physeter macrocephalus (Rendell & Whitehead 2005). The temporal stability of dialects in this range of species suggests that they are long-term, population-level phenomena that are maintained by one or more evolutionary processes that promote stability. Yet the high estimates of gene flow in many (Fleischer & Rothstein 1988, Lynch 1996, MacDougall-Shackleton & MacDougall-Shackleton 2001, Wright & Wilkinson 2001, Soha et al. 2004, Wright et al. 2005), but not all (Yurk et al. 2002), of these taxa imply high rates of migration of individuals from one dialect to another (assuming dialects have not arisen very recently). High rates of migration would be expected to homogenize vocal patterns if vocalizations were purely genetic phenomena. The fact that homogenization is not observed in most systems suggest that processes of cultural evolution, specifically patterns of vocal learning, are responsible for the maintenance of vocal dialects.
One caveat that should be noted is that the eleven-year time span of our study is relatively short compared to the expected lifespan of the yellow-naped amazon, which has a maximum recorded lifespan in captivity of 49 years (Brouwer et al. 2000). Although we do not have lifespan data for our population, genetic evidence from one site indicates that one mated pair successfully renested at the same site for a minimum of seven years (T.F. Wright, A.M. Rodriguez, R.C. Fleischer, unpublished data). This indirect evidence of long lifespans suggests that we may have sampled the same individuals in both surveys. On the other hand, the number of birds recorded was often less than 10% of the birds observed at a given site, suggesting that resampling of individuals was relatively rare.
In the yellow-naped amazon system, biased transmission of local call types to immigrant birds could account for the observed pattern of temporal stability (Boyd & Richerson 1985, Lachlan et al. 2004), provided that learning is still possible post-dispersal. Although formal studies of the developmental time course of vocal learning have not been conducted in this species, it is well known for its lifelong vocal mimicry abilities in captivity (Forshaw 1989). Other species of parrot have been shown to rapidly learn new vocalizations when placed in novel social situations (Farabaugh et al. 1994, Vehrencamp et al. 2003, Wanker et al. 2005, Pepperberg 2006), suggesting that open-ended learning is common in parrots. Stabilizing selection against foreign call types could act in concert with biased transmission. Such selection could arise from negative social interactions with local birds, and could take the form of either reduced mating success or even reduced survival if this depends on access to social groups. We are currently examining these possibilities through experimental translocations of birds within and across dialects.
Acoustic Change and Cultural Evolution
Although dialects appear generally stable, we did find evidence of some change in the acoustic structure of the North dialect between 1994 and 2005. In contrast, the South dialect showed no measurable change in acoustic structure over the same time interval. Although there are several possible explanations for these differences, at present we don’t have strong evidence in support of any particular one. Cultural drift might be greater in the North dialect; such drift is enhanced by smaller population sizes, increased population isolation or reduced opportunity to learn from models (Mundinger 1980, Lynch et al. 1989, Harbison et al. 1999, Gammon et al. 2005). Higher levels of migration into the North dialect could also contribute to more rapid change in call types in this dialect. Both microsatellites and mtDNA sequences, however, show similar levels of genetic diversity in the South and North dialects, and rates of gene flow between the two dialects are estimated to be equivalent (Wright & Wilkinson 2001, Wright et al. 2005). The North dialect does differ from the South dialect in that it shares borders with two other dialects and thus may receive more migrants in total than the South dialect. If these immigrants are not capable of complete convergence to their new dialect, or have some reciprocal influence on the vocalizations of their new neighbors, then they may contribute to the greater change observed in the North dialect.
One force that may have acted to change calls to a greater degree in the North than South dialects is selection arising from the acoustic transmission properties of different habitats (Morton 1975, Wiley & Richards 1982, Bradbury et al. 2001, Seddon 2005). Both dialects are a mosaic of primary and regenerating secondary tropical dry forests and agricultural land under a variety of regimes from intensive sugar cane and rice farms to extensive cattle ranching (Edelman 1992, van Laake & Sanchez-Azofeifa 2004, Kleinn et al. 2005). Furthermore, the yellow-naped amazon is found in most of these habitats within each dialect, and telemetry data indicate that a given individual will range widely through, and call in, a variety of habitats in any given day (A. Salinas-Melgoza, unpublished data.) suggesting that directional selection for specific acoustic characteristics is not strong.
Finally, the possibility that the observed differences simply represent a sampling artifact should be acknowledged. If individuals tend to remain static in their vocalizations over time, and if by chance we rerecorded more of the same birds in the second survey in the South dialect than in the North, then it may give the appearance of greater stasis in the South dialect. While this scenario is possible, we do not have any reason to think that it is true; our sampling procedures and numbers of birds sampled were equivalent in the two dialects. Playback experiments comparing the responses of birds to calls recorded in 1994 versus 2005 would be useful in determining whether the degree of change observed in either dialect is salient to the birds themselves (Derryberry 2007).
Dialect Origins
The question of how dialects originate is more difficult to address than is the question of dialect maintenance because it is essentially a historical question (Baker & Cunningham 1985). In a direct analogy to speciation models (Coyne & Orr 2004), most hypotheses for the formation of dialects focus on the importance of allopatry (Payne 1981, Catchpole & Slater 1995). These models for dialect formation invoke a scenario of divergence among geographically separated populations (Payne 1981, Baker & Cunningham 1985), perhaps accelerated by colonization of new peripheral habitat islands by juveniles with incompletely formed repertoires (Thielcke 1973). These differences accrue in isolation and then are maintained when dialects come into secondary contact. Processes analogous to sympatric speciation (Coyne & Orr 2004) are less commonly posited, perhaps because the origin of new dialects within existing dialects would be inconsistent with the process of dialect maintenance through vocal convergence described above. Theoretical models have provided some support for these general processes but have not suggested any one form as being more likely than others (Ellers & Slabbekoorn 2003, Lachlan et al. 2004). Distinguishing between these hypotheses empirically would be aided by the identification of dialects that are newly arisen or in the process of formation.
The presence of a distinctly different version of the Nicaraguan contact call at the Hacienda Inocentes site may represent a rare opportunity to observe directly the process of dialect formation. The new Nicaraguan call form observed only at Hacienda Inocentes is qualitatively different in both spectrograms (Fig. 3) and to the human ear. As noted above, it is uncertain whether the newly documented call variant represents a truly novel call type or is present in unsurveyed portions of this species’ range, which extends into southern Mexico. Spectrograms of calls from sound libraries reveal considerable variation in calls of the yellow-naped amazon consistent with the presence of dialects throughout its range (Wright & Wilkinson 2001), but none of these variants match this new Nicaraguan call form.
Likewise, it is presently unclear whether the birds recorded using this call variant at Hacienda Inocentes represent only newly arrived immigrants, perhaps in the process of convergence to the Northern dialect, or also include residents of this site who have converted from the North dialect call to the novel Nicaraguan call variant. Genetic data obtained from nests reused over several years, and telemetry data from the non-nesting season indicate a high degree of site fidelity by adults, but more widely-ranging movements by juvenile birds (A. Salinas Melgoza, unpublished data). These data would suggest that the new Nicaraguan call form was brought to Hacienda Inocentes by an influx of birds, likely juveniles, from other parts of the range of the yellow-naped amazon. Further observation of call use and ranging patterns at this site will address these questions and may provide insight into the process by which vocal dialects originate.
Acknowledgments
We gratefully acknowledge the assistance of Sarah Garcia and Hugo Guadamuz with the collection of vocal recordings and June Coughlin, Sarah Garcia, Afiwa Midambegbe and Daniel Acosta with spectrogram measurements. We thank the Area de Conservacíon Guanacaste (in particular Roger Blanco), the Area de Conservacíon Tempisque, and numerous landowners in Costa Rica for permitting and facilitating fieldwork. Jaime Guerra, Kathryn Hanley and two anonymous reviewers provided valuable comments on the manuscript. Funding for the 1994 fieldwork was provided by the National Geographic Society, the Animal Behavior Society and Sigma Xi. Funding for the 2005 fieldwork and data analyses was provided by an NMSU College of Arts and Sciences Research Center mini grant (TFW), an EPA GRO Fellowship (CRD), a CONACyT Graduate Fellowship (ASM), NIH grant S06 GM008136 (TFW) and NSF grant IOS-0725032 (TFW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson KE, Rothstein SI, Fleischer RC, O’Loghlen AL. Large-scale movement patterns between song dialects in brown-headed cowbirds (Molothrus ater) Auk. 2005;122:803–818. [Google Scholar]
- Audret-Hausberger M. Temporal dynamics of dialects in the whistled songs of starlings. Ethology. 1986;71:140–152. [Google Scholar]
- Baker MC. Cultural diversification in the flight call of the ringneck parrot in Western Australia. Condor. 2000;102:905–910. [Google Scholar]
- Baker MC. Local similarity and geographic differences in a contact call of the galah (Cacatua roseicapilla assimilis) in Western Australia. Emu. 2003;103:233–237. [Google Scholar]
- Baker MC, Cunningham MA. The biology of bird-song dialects. Behavioral and Brain Science. 1985;8:85–100. [Google Scholar]
- Baker MC, Gammon DE. Persistence and change of vocal signals in natural populations of chickadees: annual sampling of the gargle calls over eight seasons. Behaviour. 2006;143:1473–1509. [Google Scholar]
- Bjerke TK. Continuity and change in dialect structure of redwing (Turdus iliacus) Fauna Norvegica Series C Cinclus. 1980;3:73–79. [Google Scholar]
- Boyd R, Richerson PJ. Culture and the Evolutionary Process. Chicago: University of Chicago Press; 1985. [Google Scholar]
- Bradbury JW. Vocal communication in wild parrots. In: DeWaal FBM, Tyack PL, editors. Animal Social Complexity: Intelligence, Culture and Individualized Societies. Cambridge, MA: Harvard University Press; 2003. pp. 293–316. [Google Scholar]
- Bradbury JW, Cortopassi KA, Clemmons JR. Geographical variation in the contact calls of orange-fronted parakeets. Auk. 2001;118:958–972. [Google Scholar]
- Brouwer K, Jones ML, King CE, Schifter H. Longevity records for Psittaciformes in captivity. International Zoo Yearbook. 2000;37:299–316. [Google Scholar]
- Casgrain P, Legendre P. Montreal: Département de sciences biologiques; Université de Montréal: 2001. The R package for multivariate and spatial analysis, version 4.0 d3 – user’s manual. Available on the WWWeb site< http://www.fas.umontreal.ca/BIOL/legendre/>. [Google Scholar]
- Catchpole CK, Slater PJB. Bird Song: Biological Themes and Variations. Cambridge: Cambridge University Press; 1995. [Google Scholar]
- Chilton G, Lein MR. Long-term changes in songs and song dialect boundaries of Puget Sound white-crowned sparrows. Condor. 1996;98:567–580. [Google Scholar]
- Coyne JA, Orr HA. Speciation. Sunderland, Mass: Sinauer Assoc; 2004. [Google Scholar]
- Davidson SM, Wilkinson GS. Geographic and individual variation in vocalizations by male Saccopteryx bilineata (Chiroptera: Emballonuridae) Journal of Mammalogy. 2002;83:526–535. [Google Scholar]
- Deecke VBFJKB, Spong P. Dialect change in resident killer whales: implications for vocal learning and cultural transmission. Animal Behaviour. 2000;60:629–638. doi: 10.1006/anbe.2000.1454. [DOI] [PubMed] [Google Scholar]
- Derryberry E. Evolution of bird song affects signal efficacy: an experimental test using historical and current signals. Evolution. 2007;61:1938–1945. doi: 10.1111/j.1558-5646.2007.00154.x. [DOI] [PubMed] [Google Scholar]
- Edelman M. The Logic of Latifundio: the Large Estates of Northwestern Costa Rica Since the Late Nineteenth Century. Palo Alto, CA: Stanford University Press; 1992. [Google Scholar]
- Ellers J, Slabbekoorn H. Song divergence and male dispersal among bird populations: a spatially explicit model testing the role of vocal learning. Animal Behaviour. 2003;65:671–681. [Google Scholar]
- Farabaugh SM, Linzenbold A, Dooling RJ. Vocal plasticity in budgerigars (Melopsittacus undulatus): evidence for social factors in the learning of contact calls. Journal of Comparative Psychology. 1994;108:81–92. doi: 10.1037/0735-7036.108.1.81. [DOI] [PubMed] [Google Scholar]
- Ficken MS, Popp JW. Long-term persistence of a culturally transmitted vocalization of the black-capped chickadee. Animal Behaviour. 1995;50:683–693. [Google Scholar]
- Fleischer RC, Rothstein SI. Known secondary contact and rapid gene flow among subspecies and dialects in the brown-headed cowbird. Evolution. 1988;42:1146–1158. doi: 10.1111/j.1558-5646.1988.tb04175.x. [DOI] [PubMed] [Google Scholar]
- Ford JKB. Vocal traditions among resident killer whales (Orcinus orca) in coastal waters of British Columbia. Canadian Journal of Zoology. 1991;69:1454–1483. [Google Scholar]
- Forshaw JM. Parrots of the World. Willoughby, NSW: Landsdowne Editions; 1989. [Google Scholar]
- Gammon DE, Baker MC, Tipton JR. Cultural divergence within novel song in the black-capped chickadee (Poecile atricapillus) Auk. 2005;122:853–871. [Google Scholar]
- Gaunt SLL, Baptista LF, Sánchez JE, Hernandez D. Song learning as evidenced from song sharing in two hummingbird species (Colibri coruscans and C. thalassinus) Auk. 1994;111:87–103. [Google Scholar]
- Harbison H, Nelson DA, Hahn TP. Long-term persistence of song dialects in the mountain white-crowned sparrow. Condor. 1999;101:133–148. [Google Scholar]
- Ince SA, Slater PJB, Weisnmann C. Changes with time in the songs of a population of chaffinches. Condor. 1980;82:285–290. [Google Scholar]
- Jarvis ED. Brains and birdsong. In: Marler P, Slabbekoorn H, editors. Nature’s Music: the Science of Birdsong. San Diego: Elsevier; 2004. pp. 226–271. [Google Scholar]
- Kleinn C, Ramirez C, Holmgren P, Valverde SL, Chavez G. A national forest resources assessment for Costa Rica based on low intensity sampling. Forest Ecology and Management. 2005;210:9–23. [Google Scholar]
- Kroodsma DE. The diversity and plasticity of birdsong. In: Marler P, Slabbekoorn H, editors. Nature’s Music: the Science of Birdsong. San Diego: Elsevier; 2004. pp. 108–131. [Google Scholar]
- Lachlan RF, Janik VM, Slater PJB. The evolution of conformity-enforcing behaviour in cultural communication systems. Animal Behaviour. 2004;68:561–570. [Google Scholar]
- Lynch A. The population memetics of birdsong. In: Kroodsma DE, Miller EH, editors. Ecology and Evolution of Acoustic Communication in Birds. Ithaca: Cornell University Press; 1996. pp. 181–197. [Google Scholar]
- Lynch A, Plunkett GM, Baker AJ, Jenkins PF. A model of cultural evolution of chaffinch song derived with the meme concept. American Naturalist. 1989;133:634–653. [Google Scholar]
- MacDougall-Shackleton EA, MacDougall-Shackleton SA. Cultural and genetic evolution in mountain white-crowned sparrows: song dialects are associated with population structure. Evolution. 2001;55:2568–2575. doi: 10.1111/j.0014-3820.2001.tb00769.x. [DOI] [PubMed] [Google Scholar]
- Mantel N. The detection of disease clustering and a generalized regression approach. Cancer Research. 1967;27:209–220. [PubMed] [Google Scholar]
- Morton ES. Ecological sources of selection on avian sounds. American Naturalist. 1975;109:17–34. [Google Scholar]
- Mundinger PC. Animal cultures and a general theory of cultural evolution. Ethology and Sociobiology. 1980;1:183–223. [Google Scholar]
- Mundinger PC. Microgeographic and macrogeographic variation in the acquired vocalizations of birds. In: Kroodsma DE, Miller EH, editors. Acoustic Communication in Birds, Volume 2: Song learning and its consequences. San Diego: Academic Press; 1982. pp. 147–208. [Google Scholar]
- Nelson DA, Hallberg KI, Soha JA. Cultural evolution of Puget Sound white-crowned sparrow song dialects. Ethology. 2004;110:879–908. [Google Scholar]
- Nettle D. Linguistic Diversity. New York: Oxford University Press; 1999. [Google Scholar]
- Payne RB. Population structure and social behavior: Models for testing the ecological significance of song dialects in birds. In: Alexander RD, Tinkle DW, editors. Natural Selection and Social Behavior: Recent research and new theory. New York: Chiron Press; 1981. pp. 108–120. [Google Scholar]
- Payne RB. Song traditions in indigo buntings: origin, improvisation, dispersal, and extinction in cultural evolution. In: Kroodsma DE, Miller EH, editors. Ecology and Evolution of Acoustic Communication in Birds. Ithaca: Cornell University Press; 1996. pp. 198–220. [Google Scholar]
- Pepperberg IM. Cognitive and communicative abilities of grey parrots. Applied Animal Behaviour Science. 2006;100:77–86. [Google Scholar]
- Rendell L, Whitehead H. Spatial and temporal variation in sperm whale coda vocalizations: stable usage and local dialects. Animal Behaviour. 2005;70:191–198. [Google Scholar]
- Riesch R, Ford JBK, Thomsen F. Stability and group specificity of stereotyped whistles in resident killer whales, Orcinus orca, off British Columbia. Animal Behaviour. 2006;71:79–91. [Google Scholar]
- Seddon N. Ecological adaptation and species recognition drives vocal evolution in Neotropical suboscine birds. Evolution. 2005;59:200–215. [PubMed] [Google Scholar]
- Smouse PE, Long JC, Sokal RR. Multiple regression and correlation extensions of the Mantel test of matrix correspondence. Systematic Zoology. 1986;35:627–632. [Google Scholar]
- Soha JA, Nelson DA, Parker PG. Genetic analysis of song dialect populations in Puget Sound white-crowned sparrows. Behavioral Ecology. 2004;15:636–646. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. New York: W. H. Freeman and Company; 1995. [Google Scholar]
- Thielcke G. On the origin of divergence in learned signals (songs) in isolated populations. Ibis. 1973;115:511–516. [Google Scholar]
- Trainer JM. Changes in song dialect distributions and microgeographic variation in song of white-crowned sparrows (Zonotrichia leucophrys nuttalli) Auk. 1983;100:568–582. [Google Scholar]
- Trudgill P. On Dialect. New York: New York University Press; 1983. [Google Scholar]
- van Laake PE, Sanchez-Azofeifa GA. Focus on deforestation: zooming in on hot spots in highly fragmented ecosystems in Costa Rica. Agriculture Ecosystyems & Environment. 2004;102:3–15. [Google Scholar]
- Vehrencamp SL, Ritter AF, Keever M, Bradbury JW. Responses to playback of local vs. distant contact calls in the orange-fronted conure, Aratinga canicularis. Ethology. 2003;109:37–54. [Google Scholar]
- Wanker R, Sugama Y, Prinage S. Vocal labelling of family members in spectacled parrotlets, Forpus conspicillatus. Animal Behaviour. 2005;70:111–118. [Google Scholar]
- Weilgart L, Whitehead H. Group-specific dialects and geographical variation in coda repertoire in South Pacific sperm whales. Behavioral Ecology and Sociobiology. 1997;40:277–285. [Google Scholar]
- Wiley RH, Richards DG. Adaptations for acoustic communication in birds: sound transmission and signal detection. In: Kroodsma DE, Miller EH, editors. Acoustic Communication in Birds. New York: Academic Press; 1982. pp. 131–181. [Google Scholar]
- Wright TF. Regional dialects in the contact call of a parrot. Proceedings of the Royal Society of London, series B. 1996;263:867–872. [Google Scholar]
- Wright TF, Dorin M. Pair duets in the yellow-naped amazon (Psittaciformes: Amazona auropalliata): responses to playbacks of different dialects. Ethology. 2001;107:111–124. [Google Scholar]
- Wright TF, Rodriguez AM, Fleischer RC. Vocal dialect, sex-biased dispersal and microsatellite population structure in the parrot Amazona auropalliata. Molecular Ecology. 2005;14:1197–1205. doi: 10.1111/j.1365-294X.2005.02466.x. [DOI] [PubMed] [Google Scholar]
- Wright TF, Wilkinson GS. Population genetic structure and vocal dialects in an amazon parrot. Proceedings of the Royal Society of London, series B. 2001;268:609–616. doi: 10.1098/rspb.2000.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurk H, Barrett-Lennard L, Ford JBK, Matkin CO. Cultural transmission within maternal lineages: vocal clans in resident killer whales in southern Alaska. Animal Behaviour. 2002;63:1103–1119. [Google Scholar]