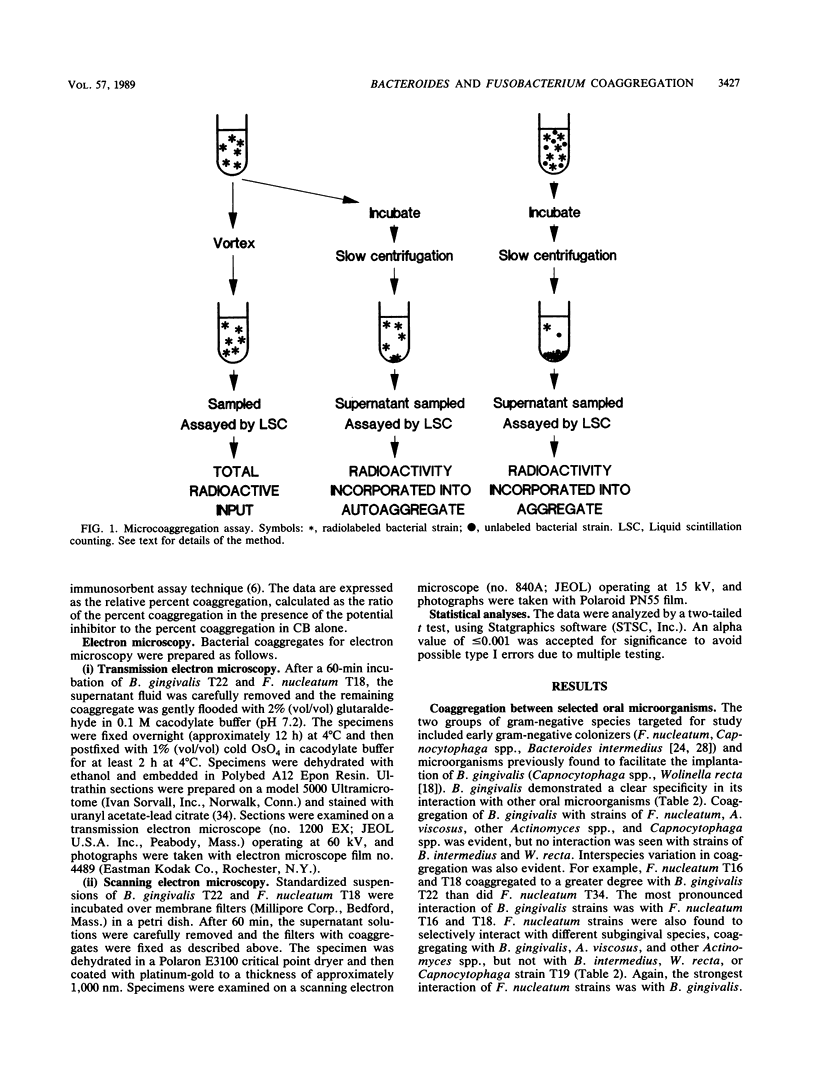
Abstract
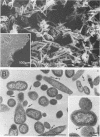
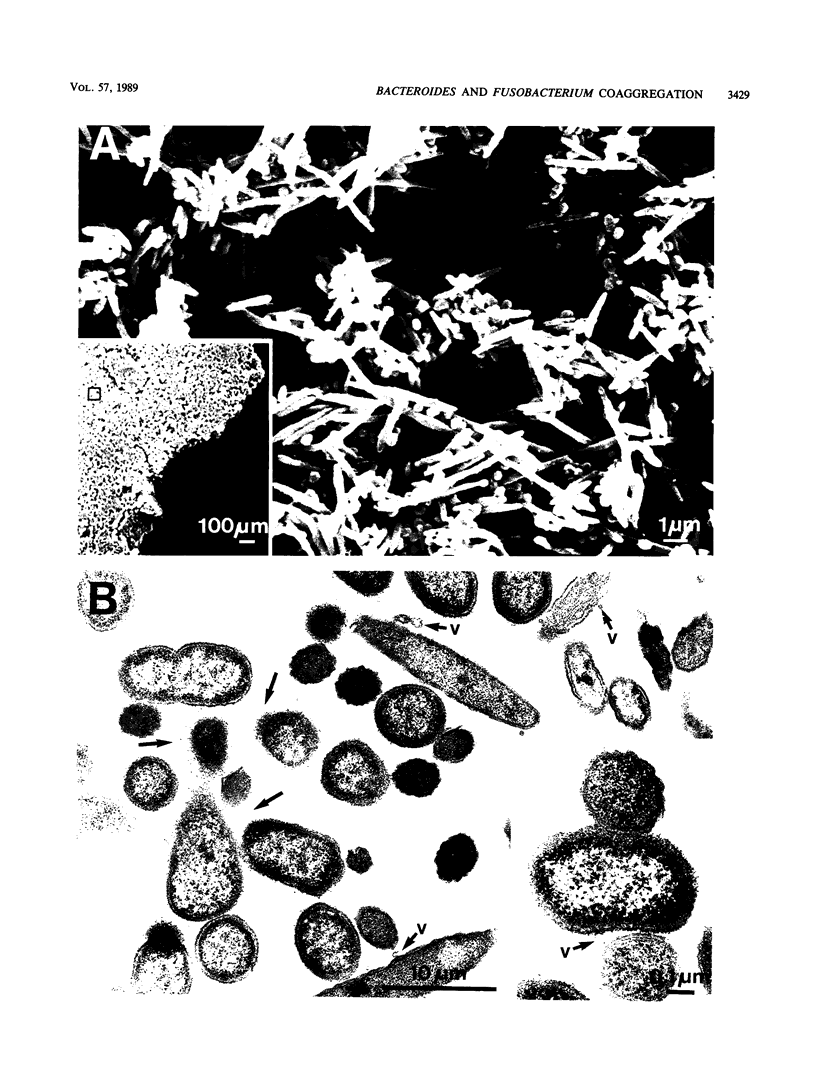
Bacterial adherence is a key factor in the colonization of the oral ecosystem, yet little is known about the mechanisms by which the pathogen Bacteroides gingivalis adheres in the periodontal environment. We examined the ability of strains of B. gingivalis to coaggregate with selected microorganisms isolated from the subgingival microbiota of the cynomolgus monkey. A strong interaction was demonstrated between strains of B. gingivalis and Fusobacterium nucleatum, whereas less pronounced or no interaction was observed with other oral isolates. Electron microscopic examination of coaggregates revealed large masses of bacteria, in which the fusiform F. nucleatum T18 and coccobacillary B. gingivalis T22 cells formed a woven pattern. To investigate this interaction and the nature of the bacterial cell surface molecules involved, we used a microcoaggregation assay. Galactose and galactose-related sugars blocked coaggregation, in contrast with the lack of effect of glucose or glucose-related sugars. The ability of F. nucleatum T18 cells to coaggregate was diminished by pretreatment with pronase. Pretreatment of B. gingivalis T22 cells with pronase resulted in an inhibition of coaggregation, whereas pretreatment with sodium metaperiodate completely abolished coaggregation. These data suggest that the coaggregation between B. gingivalis T22 and F. nucleatum T18 represents a carbohydrate-lectin interaction, mediated by a galactose-containing carbohydrate on B. gingivalis T22 and a protein on F. nucleatum T18.
Full text
PDF

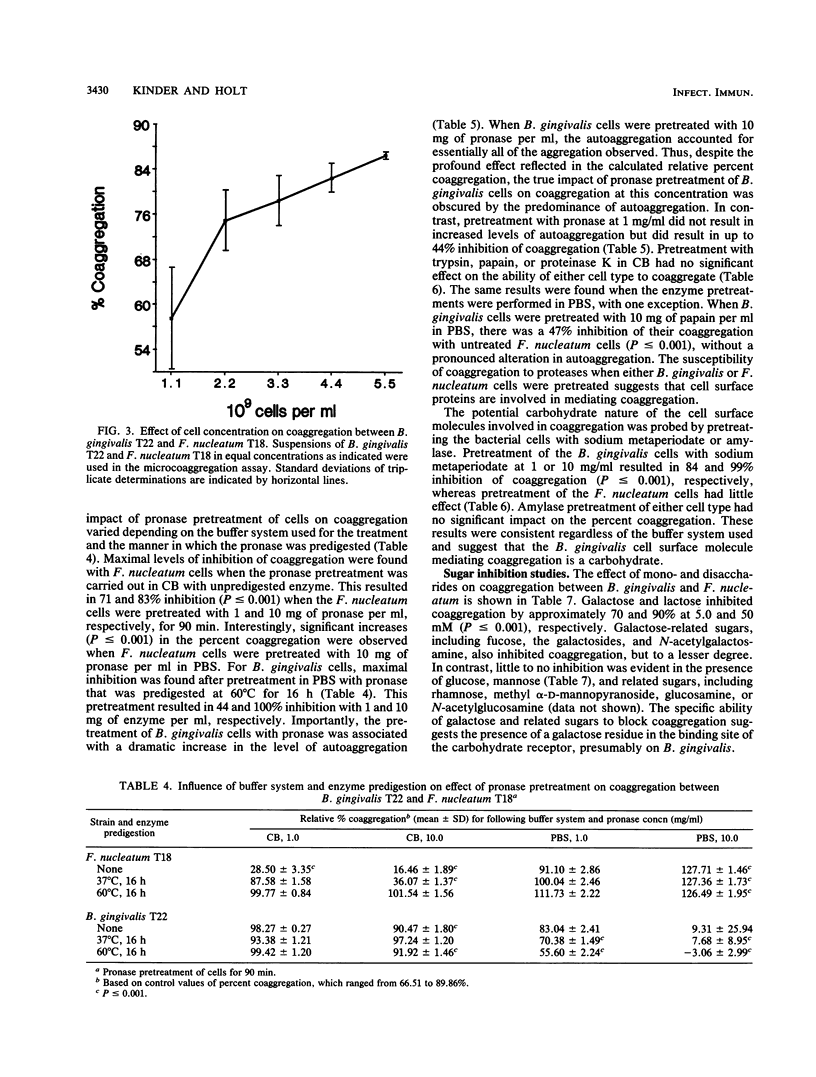
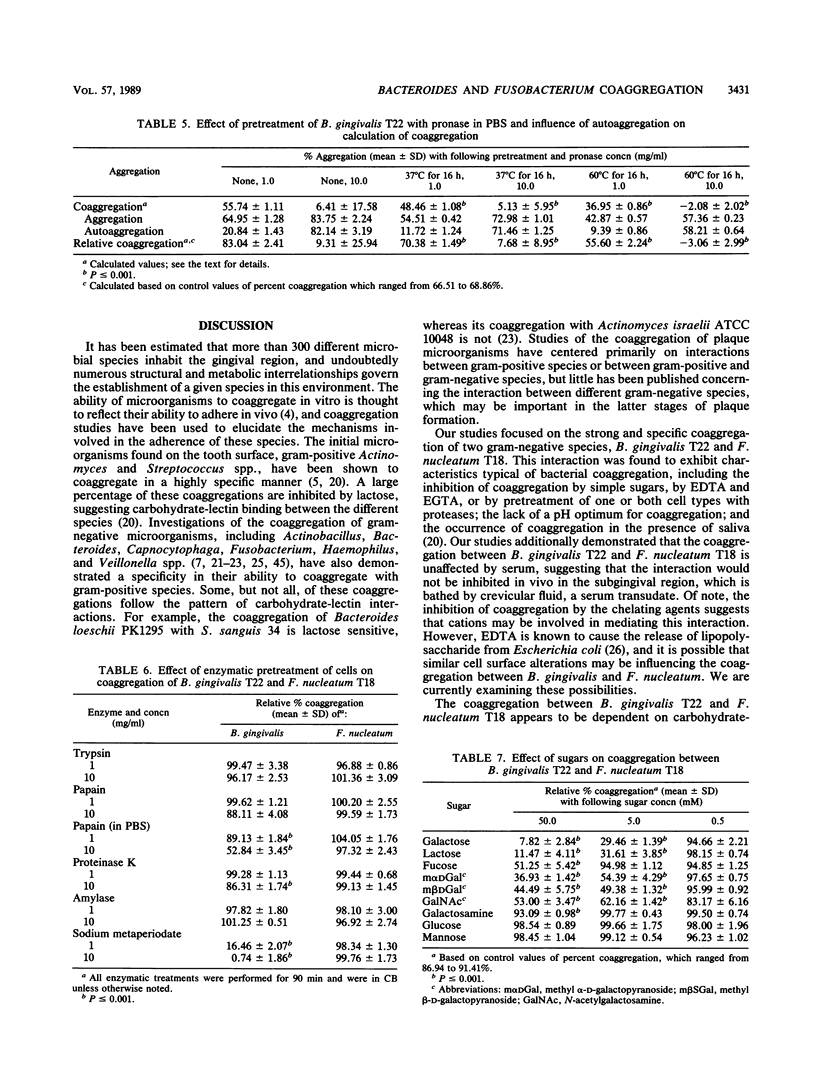


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H., Courtney H. S. Bacterial adherence: the attachment of group A streptococci to mucosal surfaces. Rev Infect Dis. 1987 Sep-Oct;9 (Suppl 5):S475–S481. doi: 10.1093/clinids/9.supplement_5.s475. [DOI] [PubMed] [Google Scholar]
- Britigan B. E., Cohen M. S., Sparling P. F. Gonococcal infection: a model of molecular pathogenesis. N Engl J Med. 1985 Jun 27;312(26):1683–1694. doi: 10.1056/NEJM198506273122606. [DOI] [PubMed] [Google Scholar]
- Cimasoni G., Song M., McBride B. C. Effect of crevicular fluid and lysosomal enzymes on the adherence of streptococci and bacteroides to hydroxyapatite. Infect Immun. 1987 Jun;55(6):1484–1489. doi: 10.1128/iai.55.6.1484-1489.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar J. O., Kolenbrander P. E., McIntire F. C. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979 Jun;24(3):742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersole J. L., Frey D. E., Taubman M. A., Smith D. J. An ELISA for measuring serum antibodies to Actinobacillus actinomycetemcomitans. J Periodontal Res. 1980 Nov;15(6):621–632. doi: 10.1111/j.1600-0765.1980.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Eke P. I., Rotimi V. O., Laughon B. E. Coaggregation of black-pigmented Bacteroides species with other oral bacteria. J Med Microbiol. 1989 Jan;28(1):1–4. doi: 10.1099/00222615-28-1-1. [DOI] [PubMed] [Google Scholar]
- Ellen R. P., Schwarz-Faulkner S., Grove D. A. Coaggregation among periodontal pathogens, emphasizing Bacteroides gingivalis--Actinomyces viscosus cohesion on a saliva-coated mineral surface. Can J Microbiol. 1988 Mar;34(3):299–306. doi: 10.1139/m88-055. [DOI] [PubMed] [Google Scholar]
- Falkler W. A., Jr, Burger B. W. Microbial surface interactions: reduction of the haemagglutination activity of the oral bacterium Fusobacterium nucleatum by absorption with Streptococcus and Bacteroides. Arch Oral Biol. 1981;26(12):1015–1025. doi: 10.1016/0003-9969(81)90112-6. [DOI] [PubMed] [Google Scholar]
- Falkler W. A., Jr, Hawley C. E. Hemagglutinating activity of Fusobacterium nucleatum. Infect Immun. 1977 Jan;15(1):230–238. doi: 10.1128/iai.15.1.230-238.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkler W. A., Jr, Smoot C. N., Mongiello J. R. Attachment of cell fragments of Fusobacterium nucleatum to oral epithelial cells, gingival fibroblasts and white blood cells. Arch Oral Biol. 1982;27(7):553–559. doi: 10.1016/0003-9969(82)90069-3. [DOI] [PubMed] [Google Scholar]
- Fröman G., Switalski L. M., Faris A., Wadström T., Hök M. Binding of Escherichia coli to fibronectin. A mechanism of tissue adherence. J Biol Chem. 1984 Dec 10;259(23):14899–14905. [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Interbacterial aggregation of plaque bacteria. Arch Oral Biol. 1970 Dec;15(12):1397–1400. doi: 10.1016/0003-9969(70)90031-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. On the formation of dental plaques. J Periodontol. 1973 Jun;44(6):347–360. doi: 10.1902/jop.1973.44.6.347. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Selective bacterial adherence to oral epithelial surfaces and its role as an ecological determinant. Infect Immun. 1971 Apr;3(4):567–573. doi: 10.1128/iai.3.4.567-573.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C., Ebersole J., Felton J., Brunsvold M., Kornman K. S. Implantation of Bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science. 1988 Jan 1;239(4835):55–57. doi: 10.1126/science.3336774. [DOI] [PubMed] [Google Scholar]
- Kaufman J., DiRienzo J. M. Isolation of a corncob (coaggregation) receptor polypeptide from Fusobacterium nucleatum. Infect Immun. 1989 Feb;57(2):331–337. doi: 10.1128/iai.57.2.331-337.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel R. A., Kornman K. S., Robertson P. B. Clinical and microbiological effects of localized ligature-induced periodontitis on non-ligated sites in the cynomolgus monkey. J Periodontal Res. 1983 Mar;18(2):200–211. doi: 10.1111/j.1600-0765.1983.tb00353.x. [DOI] [PubMed] [Google Scholar]
- Kinder S. A., Holt S. C., Korman K. S. Penicillin resistance in the subgingival microbiota associated with adult periodontitis. J Clin Microbiol. 1986 Jun;23(6):1127–1133. doi: 10.1128/jcm.23.6.1127-1133.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Andersen R. N. Cell to cell interactions of Capnocytophaga and Bacteroides species with other oral bacteria and their potential role in development of plaque. J Periodontal Res. 1984 Nov;19(6):564–569. doi: 10.1111/j.1600-0765.1984.tb01315.x. [DOI] [PubMed] [Google Scholar]
- Kolenbrander P. E., Andersen R. N., Holdeman L. V. Coaggregation of oral Bacteroides species with other bacteria: central role in coaggregation bridges and competitions. Infect Immun. 1985 Jun;48(3):741–746. doi: 10.1128/iai.48.3.741-746.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Andersen R. N. Multigeneric aggregations among oral bacteria: a network of independent cell-to-cell interactions. J Bacteriol. 1986 Nov;168(2):851–859. doi: 10.1128/jb.168.2.851-859.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E. Intergeneric coaggregation among human oral bacteria and ecology of dental plaque. Annu Rev Microbiol. 1988;42:627–656. doi: 10.1146/annurev.mi.42.100188.003211. [DOI] [PubMed] [Google Scholar]
- Kornman K. S., Holt S. C., Robertson P. B. The microbiology of ligature-induced periodontitis in the cynomolgus monkey. J Periodontal Res. 1981 Jul;16(4):363–371. doi: 10.1111/j.1600-0765.1981.tb00987.x. [DOI] [PubMed] [Google Scholar]
- LOE H., THEILADE E., JENSEN S. B. EXPERIMENTAL GINGIVITIS IN MAN. J Periodontol. 1965 May-Jun;36:177–187. doi: 10.1902/jop.1965.36.3.177. [DOI] [PubMed] [Google Scholar]
- Lancy P., Jr, Dirienzo J. M., Appelbaum B., Rosan B., Holt S. C. Corncob formation between Fusobacterium nucleatum and Streptococcus sanguis. Infect Immun. 1983 Apr;40(1):303–309. doi: 10.1128/iai.40.1.303-309.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem Biophys Res Commun. 1965 Nov 22;21(4):290–296. doi: 10.1016/0006-291x(65)90191-9. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Syed S. A. Bacteriology of human experimental gingivitis: effect of plaque and gingivitis score. Infect Immun. 1978 Sep;21(3):830–839. doi: 10.1128/iai.21.3.830-839.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrand D., Holt S. C. Biology of asaccharolytic black-pigmented Bacteroides species. Microbiol Rev. 1988 Mar;52(1):134–152. doi: 10.1128/mr.52.1.134-152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middeldorp J. M., Witholt B. K88-mediated binding of Escherichia coli outer membrane fragments to porcine intestinal epithelial cell brush borders. Infect Immun. 1981 Jan;31(1):42–51. doi: 10.1128/iai.31.1.42-51.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongiello J. R., Falkler W. A., Jr Sugar inhibition of oral Fusobacterium nucleatum haemagglutination and cell binding. Arch Oral Biol. 1979;24(7):539–545. doi: 10.1016/0003-9969(79)90133-x. [DOI] [PubMed] [Google Scholar]
- Murray P. A., Kern D. G., Winkler J. R. Identification of a galactose-binding lectin on Fusobacterium nucleatum FN-2. Infect Immun. 1988 May;56(5):1314–1319. doi: 10.1128/iai.56.5.1314-1319.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakobphol A., Murray P. A., Fisher S. J. Bacterial adherence on replicas of sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1987 Jul;164(1):5–11. doi: 10.1016/0003-2697(87)90359-9. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterwhite T. K., Evans D. G., DuPont H. L., Evans D. J., Jr Role of Escherichia coli colonisation factor antigen in acute diarrhoea. Lancet. 1978 Jul 22;2(8082):181–184. doi: 10.1016/s0140-6736(78)91921-9. [DOI] [PubMed] [Google Scholar]
- Schwarz S., Ellen R. P., Grove D. A. Bacteroides gingivalis-Actinomyces viscosus cohesive interactions as measured by a quantitative binding assay. Infect Immun. 1987 Oct;55(10):2391–2397. doi: 10.1128/iai.55.10.2391-2397.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Gibbons R. J. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect Immun. 1978 Jan;19(1):254–264. doi: 10.1128/iai.19.1.254-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoot C. N., Falkler W. A., Jr Attachment of serum non-antibody glycoproteins to the Fusobacterium nucleatum found in periodontal disease. Arch Oral Biol. 1981;26(11):859–864. doi: 10.1016/0003-9969(81)90143-6. [DOI] [PubMed] [Google Scholar]
- Socransky S. S., Dzink J. L., Smith C. M. Chemically defined medium for oral microorganisms. J Clin Microbiol. 1985 Aug;22(2):303–305. doi: 10.1128/jcm.22.2.303-305.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky S. S., Manganiello A. D., Propas D., Oram V., van Houte J. Bacteriological studies of developing supragingival dental plaque. J Periodontal Res. 1977 Mar;12(2):90–106. doi: 10.1111/j.1600-0765.1977.tb00112.x. [DOI] [PubMed] [Google Scholar]
- Svanborg Edén C., Hagberg L., Hanson L. A., Hull S., Hull R., Jodal U., Leffler H., Lomberg H., Straube E. Bacterial adherence--a pathogenetic mechanism in urinary tract infections caused by Escherichia coli. Prog Allergy. 1983;33:175–188. [PubMed] [Google Scholar]
- Syed S. A., Loesche W. J. Bacteriology of human experimental gingivitis: effect of plaque age. Infect Immun. 1978 Sep;21(3):821–829. doi: 10.1128/iai.21.3.821-829.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed S. A., Svanberg M., Svanberg G. The predominant cultivable dental plaque flora of beagle dogs with gingivitis. J Periodontal Res. 1980 Mar;15(2):123–136. doi: 10.1111/j.1600-0765.1980.tb00266.x. [DOI] [PubMed] [Google Scholar]
- Theilade E., Wright W. H., Jensen S. B., Löe H. Experimental gingivitis in man. II. A longitudinal clinical and bacteriological investigation. J Periodontal Res. 1966;1:1–13. doi: 10.1111/j.1600-0765.1966.tb01842.x. [DOI] [PubMed] [Google Scholar]
- Weiss E. I., London J., Kolenbrander P. E., Kagermeier A. S., Andersen R. N. Characterization of lectinlike surface components on Capnocytophaga ochracea ATCC 33596 that mediate coaggregation with gram-positive oral bacteria. Infect Immun. 1987 May;55(5):1198–1202. doi: 10.1128/iai.55.5.1198-1202.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]