Abstract
Objective
To determine the efficacy of biomedical risk assessment (eg, exhaled carbon monoxide (CO), or genetic susceptibility to lung cancer) as an aid for smoking cessation.
Data sources
Cochrane Tobacco Addiction Group Specialized Register, Cochrane Central Register of Controlled Trials, Medline (1966–2004) and EMBASE (1980–2004).
Study selection
Randomised controlled smoking cessation interventions using biomedical tests with at least 6 months follow‐up.
Data extraction
Two reviewers independently screened all search results (titles and abstracts) for possible inclusion. Each reviewer then extracted data from the selected studies, and assessed their methodological quality based on the CONSORT (Consolidated Standards of Reporting Trials) statement criteria.
Data synthesis
Of 4049 retrieved references, eight trials were retained for data extraction and analysis. Three trials isolated the effect of exhaled CO on smoking cessation rates resulting in the following ORs and 95% CIs: 0.73 (0.38 to 1.39), 0.93 (0.62 to 1.41) and 1.18 (0.84 to 1.64). Measurement of exhaled CO and spirometry were used together in three trials, resulting in the following ORs (95% CI): 0.60 (0.25 to 1.46), 2.45 (0.73 to 8.25) and 3.50 (0.88 to 13.92). Spirometry results alone were used in one other trial with an OR (95% CI) of 1.21 (0.60 to 2.42). Ultrasonography of carotid and femoral arteries performed on light smokers gave an OR (95% CI) of 3.15 (1.06 to 9.31).
Conclusions
Scarcity and limited quality of the current evidence does not support the hypothesis that biomedical risk assessment increases smoking cessation as compared with the standard treatment.
Despite increasing scientific knowledge about health hazards due to cigarette consumption, there is, in many countries, an increase in the prevalence of smoking among young people.1,2 The gap between knowledge and smoking cessation has been attributed, partly, to smokers' underestimation of their personal risks of smoking‐related illness.3,4
A possible strategy for increasing quit rates might be to provide a personalised feedback on the physical effects of smoking by physiological measurements. We can distinguish three different types of feedback: the first one explores biomarkers of smoking exposure (cotinine and carbon monoxide (CO)); the second one gives information on smoking‐related disease risk (eg, lung cancer susceptibility according to CYP2D6 genotyping)5; and the third one depicts smoking‐related harm (eg, atherosclerotic plaque and impaired lung functions).6 The rationale for such interventions is to promote risk awareness and motivation to accelerate changes in smoking‐behaviour.7,8
Individual studies have provided conflicting data on the effect of physiological feedback.9,10,11,12,13,14,15,16,17 We aimed to review the data on smoking cessation rates from controlled trials using feedback on the physiological effects of smoking or on the genetic susceptibility to smoking‐related diseases. This article is a shortened version of our Cochrane review.18
Methods
We carried out a systematic review of the current evidence to determine the efficacy of providing smokers with personal feedback, indicating the effects of smoking or susceptibility to smoking‐related illness to help them to quit. We included randomised controlled trials in which a physical measurement, such as exhaled CO measurement, spirometry or genetic testing, was used to increase the motivation to quit. We excluded trials in which the effect of biological measurements was confounded by other components (eg, intensive counselling) in the active intervention. We used the most conservative measure of quitting (biochemically validated smoking cessation, when available) at the longest follow‐up (at least 6 months), and considered the participants lost to follow‐up as continuing smokers.
We searched the Cochrane Tobacco Addiction Group Specialized Register, which includes searches of electronic databases including Medline, EMBASE, PsycINFO and Science Citation Index, and abstracts from the Society for Research on Nicotine and Tobacco and World Tobacco or Health conferences. We conducted additional searches of the Central Register of Controlled Trials, Medline (1996–2004) and EMBASE (1980–2004) for any of the keywords related to the following topics in titles, abstracts or indexing fields: patient education, patient compliance, persuasive communication, spirometry, respiratory function, bronchospirometry, carbon monoxide, forced expiratory flow rates, obstructive lung diseases, genetic testing and genetic susceptibility. Generic terms like “counselling”, “biomarker” or “feedback” were also used to be inclusive of any type of biomedical risk assessment. This search was combined with smoking‐related terms and trial design terms.
Two reviewers independently screened all search results (titles and abstracts) for possible inclusion or to use as useful background. They selected studies for full‐text assessment if retained by at least one of the reviewers. Each reviewer then extracted data from the selected studies, and assessed their methodological quality (eg, adequacy of the randomisation process or concealment of allocation) based on the CONSORT (Consolidated Standards of Reporting Trials) statement criteria.19 We converted the study results into odds ratios (ORs) with 95% CIs. An OR >1 favours the intervention group. If it seemed appropriate, the results were pooled using a Maentel–Haenszel fixed‐effects model.
Results
We identified 22 trials for possible inclusion out of 4049 references. Eleven studies were excluded because the effect of biomedical risk assessment could not be isolated,20,21,22,23,24,25,26,27,28,29,30 one because smoking cessation was not considered as an outcome,31 one because the biomedical risk assessment was not carried out on the smoker himself but on his or her children32 and one because the full‐text article could not be found.33 One of the excluded trials28 generated two reports.28,34
We therefore analysed data from eight trials (table 1). One of them5 tested two interventions (CO measurement and the combination of the latter with feedback about genetic susceptibility), giving rise to three possible comparisons of effectiveness. Three trials tested the effect of exhaled CO measurements alone,5,35,36 three trials tested the combination of exhaled CO measurement and spirometry,37,38,39 one trial tested the effect of CO and feedback about genetic susceptibility,5 one trial tested spirometry alone,40 one trial tested the effect of undergoing an ultrasonography of carotid and femoral arteries with photographic demonstration of atherosclerotic plaques when present41 and one trial tested feedback about genetic susceptibility to lung cancer.5 The mean number of cigarettes smoked per day varied between 11.9 and 29.2 and was highest in the trials set in a “smoking clinic”.39
Table 1 Characteristics of included studies.
| Study | Methods | Participants | Interventions | Outcomes | Notes | Allocation concealment |
|---|---|---|---|---|---|---|
| Audrain et al, 19975 | Setting: smoking clinic, USA | 550 smokers (defined as ⩾5 cpd for ⩾1 year) out of 1104 eligible | Intervention 1: exposure biomarker feedback (CO) and 60 min quit‐smoking consultation | Definition of abstinence: 30‐day point prevalence | Per protocol analysis. Distribution of baseline 550 participants among the three groups not reported | Unclear |
| Design: randomised controlled trial, two intervention and one control groups | Intervention 2: susceptibility biomarker feedback (CYP2D6), exposure biomarker feedback (CO) and 60 min quit‐smoking consultation | Duration of follow‐up: 12 months | ||||
| Recruitment: lay press | Mean age 44 years | Control: 60 min Quit‐smoking consultation (quit plan, gaining support) | Biochemical validation of non‐smokers: none | |||
| Selected: advertisement: free smoking‐cessation study | 62.8% women | |||||
| 83.9% white | ||||||
| Randomisation: not detailed | Mean cpd: 22.7 | |||||
| SoC: preparation stage: 37.5% | ||||||
| Mean Fagerström score: 5.4 | ||||||
| Therapist: trained health educator | ||||||
| Bovet et al, 200241 | Setting: Seychelles Heart Study II | 155 smokers (defined as ⩾1 cpd during previous week) | Intervention: ultrasonography of carotid and femoral arteries. Smokers with ⩾1 plaque given two photographs of their plaque and explanation along with quit‐smoking counselling | Definition of abstinence: 7‐day point prevalence | Two participants lost to follow‐up not included in analysis | Unclear |
| Design: randomised controlled trial | Mean age 46 years | Control: quit‐smoking counselling | Duration of follow up: 6 months. | |||
| Recruitment: age‐ and sex‐stratified sample drawn from general population of Mahé, invited by letter to a cardiovascular risk factor survey | 15% female | Biochemical validation of non‐smokers: none | ||||
| Selected: last 155 participants to the Seychelles Heart Study II | Mean cpd: 11.9 | |||||
| Randomisation: pre‐established random sequences of numbers matched to rank of arrival. Assessors blinded | Therapist: physician | |||||
| Jamrozik et al, 198435 | Setting: six general practices, UK | 2110 smoker (defined as a person admitting to smoking cigarettes) out of 6052 screened | Intervention: demonstration of patient exhaled CO, verbal advice and booklet | Definition of abstinence: point prevalence without mention of duration | OR based on unvalidated data | Inadequate |
| Design: randomised controlled trial | 61% female | Control: verbal advice and booklet | Duration of follow‐up: 12 months | |||
| Recruitment: clinic, first visit | No detailed patient characteristics given. Significant difference of social classes between groups | Biochemical validation of non‐smokers: urinary cotinine in a sample (41%) of self‐reported non‐smokers | ||||
| Selected: outpatients | Therapist: physician | |||||
| Randomisation: according to the day of attendance, balanced over 4 weeks | ||||||
| Risser and Belcher 199037 | Setting: US Veterans Administration Demonstration Project. | 90 smokers (not defined) | Intervention: spirometry, exhaled CO, discussion of pulmonary symptoms and control intervention | Definition of abstinence: point prevalence without mention of duration | Unclear | |
| Design: randomised controlled trial. | Mean age 53.7 years (55.5 vs 51.7 years) | Control: 50 min educational intervention, review of self‐help manual, invitation to a nine‐session one‐to‐one counselling programme | Duration of follow‐up: 12 months | |||
| Recruitment: veterans attending a health promotion clinic | 4% female | Biochemical validation of non‐smokers: exhaled CO⩽10 ppm | ||||
| Selected: responding to mailed invitations for health promotion. Some second visit | Mean cpd: 23.5 | |||||
| Randomisation: not detailed | Mean pack‐year: 60.4 | |||||
| Assessors blinded | Initial cessation intent 51% vs 44% | |||||
| Therapist: nurse‐practitioner | ||||||
| Sanders et al, 198936 | Setting: 11 UK general practices | 751 participants out of 4330 identified smokers (self‐defined) | Intervention: exhaled CO measure, discussion of significance and control intervention | Definition of abstinence: point prevalence without mention of duration | Inadequate | |
| Design: randomised controlled trial | Mean age 38.5 years. | Control: counselling by practice nurse, written material given and offer of a follow‐up appointment | Duration of follow‐up: 12 months | |||
| Recruitment: screening of all outpatients | Other characteristics not mentionned | Biochemical validation of non‐smokers: urinary cotinine. Cut‐off not reported | ||||
| Selected: outpatients and who made appointment for health check | Therapist: practice nurse | |||||
| Randomisation: by day of attendance on a 1:2 basis. Desktop card reminding doctors of right allocation. 120 wrongly allocated patients, excluded from further analysis | ||||||
| Segnan et al, 199140 | Setting: 44 general practices, Italy | 923 included out of 1009 screened. Smoker definition not given | Intervention: spirometry prescription and control intervention | Definition of abstinence: 7‐day point prevalence | In the intervention group, 124 subjects out of 292 reported to have actually had a spirometry test | Adequate |
| Design: randomised controlled trial | Age: 20.1% <31 years; 28.0% 31–40 years; 26.8% 41–50 years; 25.0% >50 years | Control: repeated counselling with reinforcement sessions | Duration of follow‐up: 12 months | |||
| Recruitment: screening of outpatients on specific days. Selected: outpatients | 38% female | (two other groups not used in our comparison: minimal intervention and repeated counselling and nicotine gum) | Biochemical validation of non‐smokers: urinary cotinine <100 ng/mg | |||
| Randomisation: sequence of random numbers, sealed envelopes | cpd: 16.7% ⩽10 cpd; 55.2% 11–20 cpd; 28.1% >20 cpd | |||||
| 51% reporting symptoms | ||||||
| Therapist: physician | ||||||
| Sippel et al, 1999.38 | Setting: two primary care clinics, USA | 205 included out of 360 smokers (self‐defined) | Intervention: spirometry and exhaled CO and control intervention | Definition of abstinence: sustained from quit date to the time of follow‐up | Inadequate | |
| Design: randomised controlled trial with formal estimation of sample size | Mean age 38.5 years | Control: counselling according to transtheoretical model of change, written material and NRT encouraged if prepared to stop | Duration of follow‐up: 9 months | |||
| Recruitment: all smokers among outpatients | 62.5% female | Biochemical validation of non‐smokers: none | ||||
| Selected: outpatients | Mean cpd: 20.0 | |||||
| Randomisation: questionnaires numbered consecutively (time of check‐in). Odd‐numbered = intervention | Mean pack‐years: 28.9 | |||||
| Assessors blinded | SoC: 36% in preparation stage | |||||
| Therapist: study staff | ||||||
| Walker and Franzini 198539 | Setting: stop‐smoking clinic, USA | 64 out of 141 eligible (smoker, self‐defined) | Intervention 1: exhaled CO and spirometry feedback, and taste satiation | Definition of abstinence: “smoking not >1 cigarette in the past 10 days” | Unclear | |
| Design: 2×2×2 randomised controlled trial | Mean age: 35.5 years | Intervention 2: exhaled CO and spirometry feedback and focused smoking | Duration of follow‐up: 6 months | |||
| Recruitment: public service announcement and media advertising | 59% female | Booster sessions for half of each intervention group | Biochemical validation of non‐smokers: exhaled CO <8ppm | |||
| Selected: those responding to advertising, paying US$45 | Mean cpd: 29.2 | Control 1: taste satiation | ||||
| Randomisation: not detailed | Mean 3.4 previous quit attempts | Control 2: focused smoking | ||||
| Therapist: first author | Booster sessions for half of each control group |
CO, carbon monoxide; cpd, cigarettes per day; NRT, nicotine replacement therapy; ppm, parts per million; SoC, stage of change.
Only one of the eight trials reported an adequate randomisation procedure.40 Only three studies explicitly mentioned that assessors were blinded to allocation at the time of outcome determination.37,38,41 Only one study proposed a formal estimation of sample size before recruitment.38 Biochemical validation of smoking cessation was adequately used in four studies.36,37,39,40 Participation rates (ie, the proportion of those approached who agreed to take part in the trial) were seldom recorded. In two studies,5,39 it was not possible to determine the initial allocation of the participants who were subsequently lost to follow‐up, and analysis had to be performed per protocol.
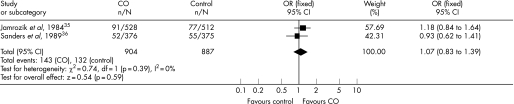
Figure 1 shows the ORs and 95% CIs from the two trials using exhaled CO in a primary care setting as a way to motivate smokers to quit.35,36 These two studies were similar enough in terms of recruitment, intervention and setting to allow the pooling of data. χ2 test did not show evidence for significant heterogeneity. There was no evidence of a significant benefit from these pooled studies (Mantel–Haenszel fixed‐effect OR 1.07, 95% CI 0.83 to 1.39).
Figure 1 Individual and pooled ORs and 95% CIs from the two trials using exhaled carbon monoxide (CO) in a primary care setting.
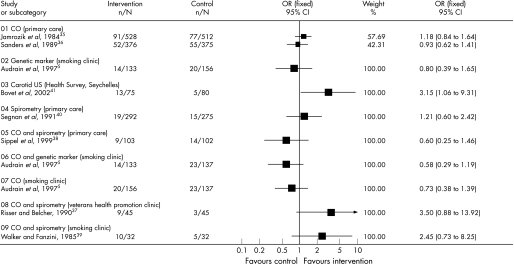
Figure 2 shows the individual ORs and 95% CIs from all the included interventions. Three studies isolated the effect of exhaled CO measurement on smoking cessation rate5,35,36 with ORs (95% CI) of 0.73 ( 0.38 to 1.39), 1.18 (0.84 to 1.64) and 0.93 (0.62 to 1.41), respectively. Exhaled CO measurement and spirometry were used together in three trials37,38,39 with ORs (95% CI) of 3.50 (0.88 to 13.92), 0.60 (0.25 to 1.46) and 2.45 (0.73 to 8.25), respectively. We did not pool these studies because of heterogeneous settings that would preclude the drawing of clinically relevant conclusions. Spirometry results were used in one primary care‐based trial40 with an OR (95% CI) of 1.21 (0.60 to 2.42). One trial5 used both genetic susceptibility to lung cancer alone with an OR (95% CI) of 0.80 (0.39 to 1.65), as well as genetic susceptibility to lung cancer combined with exhaled CO measurement with an OR (95% CI) of 0.58 (0.29 to 1.19). Finally, ultrasonography of carotid and femoral arteries was used in one trial41 with an OR (95% CI) of 3.15 (1.06 to 9.31). This study was conducted among light smokers (average 10–12 cigarettes a day).
Figure 2 Individual ORs and 95% CIs from all included interventions.
Discussion
Owing to the scarcity of evidence of sufficient quality, we could make no definitive statements about the effectiveness of biomedical risk assessment as an aid for smoking cessation. Existing evidence of lower quality does not, however, support the hypothesis that biomedical risk assessment increases smoking cessation as compared with the standard treatment.
Only two studies were similar enough in terms of recruitment, setting and intervention to allow pooling of data and meta‐analysis. Their combined results further tended towards the null hypothesis. The external validity of the only study with a statistically significant positive OR41 can be questioned as the sample was made up predominantly of male light smokers (average 10–12 cigarettes a day).
Other studies identified by our search strategy did not isolate the specific effect of biomedical feedback.20,21,22,23,24,25,26,27,28,29,30 Two of these studies27,28 demonstrated an OR significantly favouring the intervention group rather than the control group. Demonstration of smokers' child's exposure to environmental tobacco smoke by measuring the child's urinary cotinine level was used in another trial32 with an OR (95% CI) of 0.15 ( 0.01 to 2.89). We excluded this study from our analysis, because, it seemed to us that providing biomarker feedback about someone else's health (even one's own children) would act differently and may not contribute to counteracting the hypothesised personal optimistic bias.3,4 Smoking cessation was, moreover, documented as a secondary outcome in this study, as the primary outcome was a smoking ban in the home. In any event, this trial did not show a positive effect; the study had low power to detect an effect and its quality was limited. One study identified by McClure42 as “in press” seems never to have been published,43 and several attempts to contact the authors failed to provide us with more detailed information.
An earlier non‐systematic review was conducted on the use of biomarkers in smoking cessation.42 The aim of this work was to review the theoretical rationale and the empirical evidence regarding this practice. Focus was, therefore, not specifically directed at the assessment of the efficacy of biomarker feedback as a way to increase smoking cessation. Therefore, the review included non‐randomised trials,13,44,45,46 trials providing multicomponent interventions that precluded the isolation of the specific effect of biomarkers feedback,9,11,34 trials comparing the effect of abnormal test results versus normal test results rather than test versus no tests,12 and trials reporting outcomes other than smoking cessation. Four studies mentioned by McClure were also retained in our review.5,35,37,39 We identified four more trials for our review.36,38,40,41 When focusing on efficacy data, McClure concluded that biomarkers feedback may enhance the likelihood of cessation, because a trend for increased abstinence was found in three randomised trials.37,39,43 The fact that two of these trials37,39 are subject to major methodological limitations (small samples, inadequate randomisation procedures), and that the report of Hoffman et al43 remains unpublished, calls for great caution in drawing such conclusions.
In most of the studies included in the current review, the biomedical testing component was added to intensive quit‐smoking sessions, with counselling lasting up to 60 min and completed by written material and reinforcement sessions or follow‐up telephone calls. The incremental effect of biomedical risk assessment might have been diluted by the high intensity of the standard care used. It is also possible that the changes in motivational stages induced by biomedical risk assessment are too subtle to be characterised as directly leading to a successful quit attempt.47 Another possible explanation for the absence of effectiveness of biomedical risk assessment provided in addition to counselling could be the potentially counterproductive effect of communicating normal results to smokers. Only two included studies provided some insight about smoking cessation rates according to test results. Sippel et al38 did not find any correlation between smoking cessation and abnormal spirometry results, whereas Bovet et al41 found a non‐significant lower smoking cessation rate among participants without plaques at ultrasonography compared with participants who did not undergo ultrasonography. Similarly, whether the presence of smoking‐related symptoms may modify the effect of biomedical feedback is unknown. These particular questions, and the way to communicate normal test results should be explored in future trials.
What is already known on this topic
Feedback on biomedical characteristics indicating effects of smoking, or susceptibility to smoking‐related illness, has been advocated to help smokers to quit.
What this study adds
Due to the scarcity of evidence of sufficient quality, we could make no definitive statements about the effectiveness of biomedical risk assessment as an aid for smoking cessation.
Existing evidence of lower quality does not, however, support the hypothesis that biomedical risk assessment increases smoking cessation as compared with the standard treatment.
The methodological quality of trials exploring this research question needs to improve substantially.
Acknowledgements
We thank Olivier Terraz of the Institute for Social and Preventive Medicine, University of Lausanne, for his assistance in retrieving and selecting references identified by our search strategy, and Alvine Bissery of the same institution for her statistical expertise. We also thank Jon Britton and Jonathan Foulds for their helpful suggestions on the protocol, and Andy McEwen and Lion Shahab for constructive comments on the earlier drafts of this review.
Abbreviations
CO - carbon monoxide
Footnotes
Funding: This study was funded by the Clinical Epidemiology Center (CEPIC), University of Lausanne, Switzerland
Competing interests: JC was the coauthor of one of the studies included in the review (Bovet P, Perret F, Cornuz J, et al. Prev Med 2002;34:215–20.)
The results of a Cochrane Review can be interpreted differently, depending on people's perspectives and circumstances. Please consider the conclusions presented carefully. They are the opinions of review authors, and are not necessarily shared by The Cochrane Collaboration.
References
- 1.Gmel G. Prevalence of tobacco use in Switzerland in the 1990's: estimation of consumption trends based on 2 methods. Soz Praventivmed 20004564–72. [DOI] [PubMed] [Google Scholar]
- 2.Lewit E M, Hyland A, Kerrebrock N.et al Price, public policy, and smoking in young people. Tob Control 19976(Suppl 2)S17–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lerman C, Orleans C T, Engstrom P F. Biological markers in smoking cessation treatment. Semin Oncol 199320359–367. [PubMed] [Google Scholar]
- 4.Romer D, Jamieson P. Do adolescents appreciate the risks of smoking? Evidence from a national survey. J Adolesc Health 20012912–21. [DOI] [PubMed] [Google Scholar]
- 5.Audrain J, Boyd N R, Roth J.et al Genetic susceptibility testing in smoking‐cessation treatment: one‐year outcomes of a randomized trial. Addict Behav 199722741–751. [DOI] [PubMed] [Google Scholar]
- 6.Buist A S. Guidelines for the management of chronic obstructive pulmonary disease. Respir Med 200296(Suppl C)S11–S16. [DOI] [PubMed] [Google Scholar]
- 7.Curry S J. Self‐help interventions for smoking cessation. J Consult Clin Psychol 199361790–803. [DOI] [PubMed] [Google Scholar]
- 8.Miller W R, Rollnick S.Motivational interviewing: preparing people to change addictive behavior. New York: Guilford, 1991
- 9.Bauman K E, Bryan E S, Dent C W.et al The influence of observing carbon monoxide level on cigarette smoking by public prenatal patients. Am J Public Health 1983731089–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hepper N G, Drage C W, Davies S F.et al Chronic obstructive pulmonary disease: a community‐oriented program including professional education and screening by a voluntary health agency. Am Rev Respir Dis 198012197–104. [DOI] [PubMed] [Google Scholar]
- 11.Lerman C, Gold K, Audrain J.et al Incorporating biomarkers of exposure and genetic susceptibility into smoking cessation treatment: effects on smoking‐related cognitions, emotions, and behavior change. Health Psychol 19971687–99. [DOI] [PubMed] [Google Scholar]
- 12.Li V C, Kim Y J, Ewart C K.et al Effects of physician counseling on the smoking behavior of asbestos‐exposed workers. Prev Med 198413462–476. [DOI] [PubMed] [Google Scholar]
- 13.Loss R W, Hall W J, Speers D M. Evaluation of early airway disease in smokers: cost effectiveness of pulmonary function testing. Am J Med Sci 197927827–37. [DOI] [PubMed] [Google Scholar]
- 14.McBride C M, Halabi S, Bepler G.et al Maximizing the motivational impact of feedback of lung cancer susceptibility on smokers' desire to quit. J Health Commun 20005229–241. [DOI] [PubMed] [Google Scholar]
- 15.Petty T L, Pierson D J, Dick N P.et al Follow‐up evaluation of a prevalence study for chronic bronchitis and chronic airway obstruction. Am Rev Respir Dis 1976114881–890. [DOI] [PubMed] [Google Scholar]
- 16.Stitzer M L, Bigelow G E. Contingent reinforcement for reduced carbon monoxide levels in cigarette smokers. Addict Behav 19827403–412. [DOI] [PubMed] [Google Scholar]
- 17.Weinberger M, Greene J Y, Mamlin J J.et al Health beliefs and smoking behavior. Am J Public Health 1981711253–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bize R, Burnand B, Mueller Y.et al Biomedical risk assessment as an aid for smoking cessation. Cochrane Database Syst Rev 2005(4)CD004705. [DOI] [PubMed]
- 19.Moher D, Schulz K F, Altman D G.et al The CONSORT statement: revised recommendations for improving the quality of reports of parallel group randomized trials. BMC Med Res Methodol 200112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anthonisen N R, Connett J E, Kiley J P.et al Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA 19942721497–1505. [PubMed] [Google Scholar]
- 21.Borrelli B, McQuaid E L, Becker B.et al Motivating parents of kids with asthma to quit smoking: the PAQS project. Health Educ Res 200217659–669. [DOI] [PubMed] [Google Scholar]
- 22.Hajek P, West R, Lee A.et al Randomized controlled trial of a midwife‐delivered brief smoking cessation intervention in pregnancy. Addiction 200196485–494. [DOI] [PubMed] [Google Scholar]
- 23.Hajek P, Taylor T Z, Mills P. Brief intervention during hospital admission to help patients to give up smoking after myocardial infarction and bypass surgery: randomised controlled trial. BMJ 200232487–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Humerfelt S, Eide G E, Kvale G.et al Effectiveness of postal smoking cessation advice: a randomized controlled trial in young men with reduced FEV1 and asbestos exposure. Eur Respir J 199811284–290. [DOI] [PubMed] [Google Scholar]
- 25.Kanner R E. Early intervention in chronic obstructive pulmonary disease. A review of the Lung Health Study results. Med Clin North Am 199680523–547. [DOI] [PubMed] [Google Scholar]
- 26.Kanner R E, Connett J E, Williams D E.et al Effects of randomized assignment to a smoking cessation intervention and changes in smoking habits on respiratory symptoms in smokers with early chronic obstructive pulmonary disease: the Lung Health Study. Am J Med 1999106410–416. [DOI] [PubMed] [Google Scholar]
- 27.McBride C M, Bepler G, Lipkus I M.et al Incorporating genetic susceptibility feedback into a smoking cessation program for African‐American smokers with low income. Cancer Epidemiol Biomarkers Prev 200211521–528. [PubMed] [Google Scholar]
- 28.Richmond R L, Austin A, Webster I W. Three year evaluation of a programme by general practitioners to help patients to stop smoking. BMJ (Clin Res Ed) 1986292803–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shoptaw S, Rotheram‐Fuller E, Yang X.et al Smoking cessation in methadone maintenance. Addiction 2002971317–1328. [DOI] [PubMed] [Google Scholar]
- 30.Terazawa T, Mamiya T, Masui S.et al The effect of smoking cessation counseling at health checkup. Sangyo Eiseigaku Zasshi 200143207–213. [DOI] [PubMed] [Google Scholar]
- 31.McIntosh N A, Clark N M, Howatt W F. Reducing tobacco smoke in the environment of the child with asthma: a cotinine‐assisted, minimal‐contact intervention. J Asthma 199431453–462. [DOI] [PubMed] [Google Scholar]
- 32.Wakefield M, Banham D, McCaul K.et al Effect of feedback regarding urinary cotinine and brief tailored advice on home smoking restrictions among low‐income parents of children with asthma: a controlled trial. Prev Med 20023458–65. [DOI] [PubMed] [Google Scholar]
- 33.Frank J E, Hoffman D S, Flanagan V. Use of repeated cotinine determinations as a motivational and educational tool in smoking cessation counseling for pregnant women. Pediatr Res 1999451153 [Google Scholar]
- 34.Richmond R L, Webster I W. A smoking cessation programme for use in general practice. Med J Aust 1985142190–194. [PubMed] [Google Scholar]
- 35.Jamrozik K, Vessey M, Fowler G.et al Controlled trial of three different antismoking interventions in general practice. BMJ (Clin Res Ed) 19842881499–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanders D, Fowler G, Mant D.et al Randomized controlled trial of anti‐smoking advice by nurses in general practice. J R Coll Gen Pract 198939273–276. [PMC free article] [PubMed] [Google Scholar]
- 37.Risser N L, Belcher D W. Adding spirometry, carbon monoxide, and pulmonary symptom results to smoking cessation counseling: a randomized trial. J Gen Intern Med 1990516–22. [DOI] [PubMed] [Google Scholar]
- 38.Sippel J M, Osborne M L, Bjornson W.et al Smoking cessation in primary care clinics. J Gen Intern Med 199914670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker W B, Franzini L R. Low‐aversive group treatments, physiological feedback, and booster sessions for smoking cessation. Behav Ther 198516263–274. [Google Scholar]
- 40.Segnan N, Ponti A, Battista R N.et al A randomized trial of smoking cessation interventions in general practice in Italy. Cancer Causes Control 19912239–246. [DOI] [PubMed] [Google Scholar]
- 41.Bovet P, Perret F, Cornuz J.et al Improved smoking cessation in smokers given ultrasound photographs of their own atherosclerotic plaques. Prev Med 200234215–220. [DOI] [PubMed] [Google Scholar]
- 42.McClure J B. Are biomarkers a useful aid in smoking cessation? A review and analysis of the literature. Behav Med 20012737–47. [DOI] [PubMed] [Google Scholar]
- 43.Hoffman D W, Flanagan V A, Frank J E. Use of repeated cotinine determinations as a motivational and educational tool in smoking cessation counseling for pregnant women. Princeton, NJ: Robert Wood Johnson Foundation, 1998, Unpublished final grant report
- 44.Haddow J E, Knight G J, Kloza E M.et al Cotinine‐assisted intervention in pregnancy to reduce smoking and low birthweight delivery. Br J Obstet Gynaecol 199198859–865. [DOI] [PubMed] [Google Scholar]
- 45.Kilburn K H, Warshaw R H. Effects of individually motivating smoking cessation in male blue collar workers. Am J Public Health 1990801334–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scott R R, Mayer J A, Denier C A.et al Long‐term smoking status of cardiac patients following symptom‐specific cessation advice. Addict Behav 199015549–552. [DOI] [PubMed] [Google Scholar]
- 47.Prochaska J O, Velicer W F. The transtheoretical model of health behavior change. Am J Health Promot 19971238–48. [DOI] [PubMed] [Google Scholar]