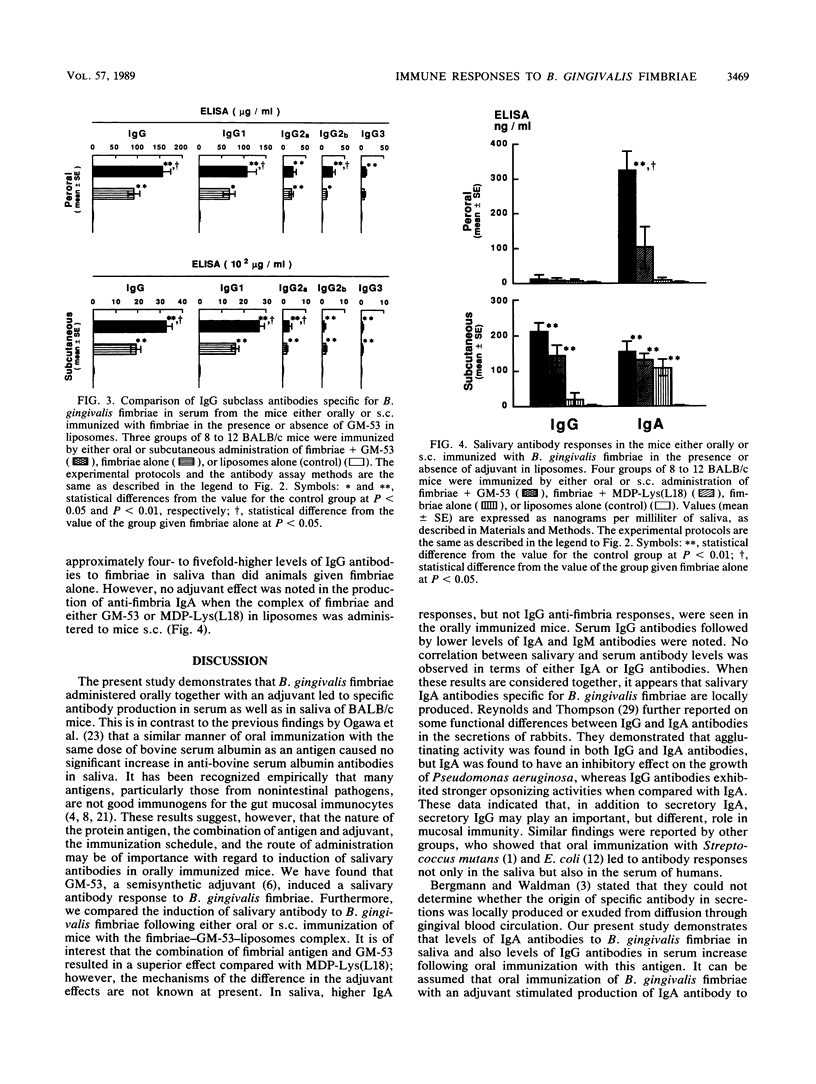
Abstract
A 41,000-molecular-weight fimbrial protein was isolated from freshly cultivated whole cells of Bacteroides gingivalis 381 and purified chromatographically. Salivary and serum antibody responses to the fimbriae, which had been orally administered in the presence of an acyl derivative of muramylpeptides, i.e., either N2-[(N-acetylmuramyl)-L-alanyl-D-isoglutaminyl]-N6-stearoyl-L-lysine [MDP-Lys(L18)] or sodium beta-N-acetyl-glucosaminyl-(1----4)-N-acetylmuramyl-L-alanyl-D-isoglu tam inyl- (L)-stearoyl-(D)-meso-2,6-diaminopimelic acid-(D)-amine-D-alanine (GM-53), or in the absence of adjuvant, were examined in BALB/c mice when administered by gastric intubation on days 0 and 1 as primary immunizations and on days 27 and 28 as booster immunizations. Gastric intubation of the fimbriae with an adjuvant significantly enhanced the production of anti-fimbria immunoglobulin A (IgA) in saliva. Subcutaneous injection of fimbriae along with an adjuvant also raised anti-fimbria IgA levels, as well as IgG levels, in saliva. Both immunization procedures enhanced the levels of anti-fimbria IgG, IgA, and IgM in serum, and the major class of fimbria-specific antibody was IgG, followed by IgA and IgM. However, subcutaneous injection was more effective than gastric intubation to enhance the production of serum antibody in mice. The subclasses of IgG antibody specific for fimbriae in serum were mainly IgG1, followed by IgG2a, IgG2b, and IgG3. These results demonstrated that the combined use of B. gingivalis fimbrial antigen and either GM-53 or MDP-Lys(L18) resulted in a sharply increased IgA antibody response in saliva and a predominantly stimulated IgG antibody response in serum, respectively. Both antibodies were found to be specific for the fimbriae used for immunization.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. G., Gregoriadis G. Liposomes as immunological adjuvants. Nature. 1974 Nov 15;252(5480):252–252. doi: 10.1038/252252a0. [DOI] [PubMed] [Google Scholar]
- Bergmann K. C., Waldman R. H. Stimulation of secretory antibody following oral administration of antigen. Rev Infect Dis. 1988 Sep-Oct;10(5):939–950. doi: 10.1093/clinids/10.5.939. [DOI] [PubMed] [Google Scholar]
- Clancy R., Pucci A. Sensitisation of gut-associated lymphoid tissue during oral immunisation. Aust J Exp Biol Med Sci. 1978 Jun;56(3):337–340. doi: 10.1038/icb.1978.36. [DOI] [PubMed] [Google Scholar]
- Erwin A. L., Kenny G. E., Smith A. L., Stull T. L. Human antibody response to outer membrane proteins and fimbriae of Haemophilus influenzae type b. Can J Microbiol. 1988 Jun;34(6):723–729. doi: 10.1139/m88-123. [DOI] [PubMed] [Google Scholar]
- Grenier D., Mayrand D. Etudes d'infections mixtes anaérobies comportant Bacteroides gingivalis. Can J Microbiol. 1983 May;29(5):612–618. [PubMed] [Google Scholar]
- Hanson D. G., Vaz N. M., Maia L. C., Lynch J. M. Inhibition of specific immune responses by feeding protein antigens. III. Evidence against maintenance of tolerance to ovalbumin by orally induced antibodies. J Immunol. 1979 Nov;123(5):2337–2343. [PubMed] [Google Scholar]
- Holt S. C., Ebersole J., Felton J., Brunsvold M., Kornman K. S. Implantation of Bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science. 1988 Jan 1;239(4835):55–57. doi: 10.1126/science.3336774. [DOI] [PubMed] [Google Scholar]
- Inoue K. Permeability properties of liposomes prepared from dipalmitoyllecithin, dimyristoyllecithin, egg lecithin, rat liver lecithin and beef brain sphingomyelin. Biochim Biophys Acta. 1974 Mar 29;339(3):390–402. doi: 10.1016/0005-2736(74)90166-7. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Anderson P., Rosen F. S., Smith D. H. Characterization of human antibody to polyribophosphate, the capsular antigen of Hemophilus influenzae, type B. Clin Immunol Immunopathol. 1973 Jan;1(2):234–240. doi: 10.1016/0090-1229(73)90024-x. [DOI] [PubMed] [Google Scholar]
- Kaijser B. Peroral immunization of healthy adults with live Escherichia coli O4K12 bacteria. Antibody response as measured in serum and secretions. Int Arch Allergy Appl Immunol. 1983;70(2):164–168. doi: 10.1159/000233316. [DOI] [PubMed] [Google Scholar]
- Kastelein P., van Steenbergen T. J., Bras J. M., de Graaff J. An experimentally induced phlegmonous abscess by a strain of Bacteroides gingivalis in guinea pigs and mice. Antonie Van Leeuwenhoek. 1981 Mar;47(1):1–9. doi: 10.1007/BF00399062. [DOI] [PubMed] [Google Scholar]
- Lambden P. R., Heckels J. E., Watt P. J. Effect of anti-pilus antibodies on survival of gonococci within guinea pig subcutaneous chambers. Infect Immun. 1982 Oct;38(1):27–30. doi: 10.1128/iai.38.1.27-30.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter L., Wilhelm J. A., Angehrn W., Skvaril F., Schopfer K. Selective antibody deficiency and recurrent pneumococcal bacteremia in a patient with Sjögren's syndrome, hyperimmunoglobulinemia G, and deficiencies of IgG2 and IgG4. N Engl J Med. 1985 Apr 18;312(16):1039–1042. doi: 10.1056/NEJM198504183121607. [DOI] [PubMed] [Google Scholar]
- Mayrand D., McBride B. C. Exological relationships of bacteria involved in a simple, mixed anaerobic infection. Infect Immun. 1980 Jan;27(1):44–50. doi: 10.1128/iai.27.1.44-50.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestecky J., McGhee J. R. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- Morrow C. D., Dorey F., Stevens R. H. Isoelectric focusing of human anti-diphtheria toxoid antibodies: identical spectrotypes of anti-fragment A antibodies with the same IgG subclass and light chain constant regions are expressed in multiple individuals. J Immunol. 1983 Feb;130(2):818–823. [PubMed] [Google Scholar]
- Mouton C., Hammond P. G., Slots J., Genco R. J. Serum antibodies to oral Bacteroides asaccharolyticus (Bacteroides gingivalis): relationship to age and periondontal disease. Infect Immun. 1981 Jan;31(1):182–192. doi: 10.1128/iai.31.1.182-192.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedrud J. G., Liang X. P., Hague N., Lamm M. E. Combined oral/nasal immunization protects mice from Sendai virus infection. J Immunol. 1987 Nov 15;139(10):3484–3492. [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Ogawa T., Kotani S., Shimauchi H. Enhancement of serum antibody production in mice by oral administration of lipophilic derivatives of muramylpeptides and bacterial lipopolysaccharides with bovine serum albumin. Methods Find Exp Clin Pharmacol. 1986 Jan;8(1):19–26. [PubMed] [Google Scholar]
- Ogawa T., McGhee M. L., Moldoveanu Z., Hamada S., Mestecky J., McGhee J. R., Kiyono H. Bacteroides-specific IgG and IgA subclass antibody-secreting cells isolated from chronically inflamed gingival tissues. Clin Exp Immunol. 1989 Apr;76(1):103–110. [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Tarkowski A., McGhee M. L., Moldoveanu Z., Mestecky J., Hirsch H. Z., Koopman W. J., Hamada S., McGhee J. R., Kiyono H. Analysis of human IgG and IgA subclass antibody-secreting cells from localized chronic inflammatory tissue. J Immunol. 1989 Feb 15;142(4):1150–1158. [PubMed] [Google Scholar]
- Okuda K., Kato T., Naito Y., Takazoe I., Kikuchi Y., Nakamura T., Kiyoshige T., Sasaki S. Protective efficacy of active and passive immunizations against experimental infection with Bacteroides gingivalis in ligated hamsters. J Dent Res. 1988 May;67(5):807–811. doi: 10.1177/00220345880670050201. [DOI] [PubMed] [Google Scholar]
- Pichichero M. E., Loeb M., Anderson, Smith D. H. Do pili play a role in pathogenicity of Haemophilus influenzae type B? Lancet. 1982 Oct 30;2(8305):960–962. doi: 10.1016/s0140-6736(82)90161-1. [DOI] [PubMed] [Google Scholar]
- Reynolds H. Y., Thompson R. E. Pulmonary host defenses. II. Interaction of respiratory antibodies with Pseudomonas aeruginosa and alveolar macrophages. J Immunol. 1973 Aug;111(2):369–380. [PubMed] [Google Scholar]
- Rodbard D., Bridson W., Rayford P. L. Rapid calculation of radioimmunoassay results. J Lab Clin Med. 1969 Nov;74(5):770–781. [PubMed] [Google Scholar]
- Rowland R. N., Woodley J. F. The stability of liposomes in vitro to pH, bile salts and pancreatic lipase. Biochim Biophys Acta. 1980 Dec 5;620(3):400–409. doi: 10.1016/0005-2760(80)90131-9. [DOI] [PubMed] [Google Scholar]
- Schonfeld S. E., Kagan J. M. Specificity of gingival plasma cells for bacterial somatic antigens. J Periodontal Res. 1982 Jan;17(1):60–69. doi: 10.1111/j.1600-0765.1982.tb01131.x. [DOI] [PubMed] [Google Scholar]
- Seppälä I. J., Routonen N., Sarnesto A., Mattila P. A., Mäkelä O. The percentages of six immunoglobulin isotypes in human antibodies to tetanus toxoid: standardization of isotype-specific second antibodies in solid-phase assay. Eur J Immunol. 1984 Sep;14(9):868–875. doi: 10.1002/eji.1830140918. [DOI] [PubMed] [Google Scholar]
- Slots J., Listgarten M. A. Bacteroides gingivalis, Bacteroides intermedius and Actinobacillus actinomycetemcomitans in human periodontal diseases. J Clin Periodontol. 1988 Feb;15(2):85–93. doi: 10.1111/j.1600-051x.1988.tb00999.x. [DOI] [PubMed] [Google Scholar]
- Smith H. W. The nature of the protective effect of antisera against Escherichia coli diarrhoea in piglets. J Med Microbiol. 1972 Aug;5(3):345–353. doi: 10.1099/00222615-5-3-345. [DOI] [PubMed] [Google Scholar]
- Stewart D. J., Clark B. L., Emery D. L., Peterson J. E., Jarrett R. G., O'Donnell I. J. Cross-protection from Bacteroides nodosus vaccines and the interaction of pili and adjuvants. Aust Vet J. 1986 Apr;63(4):101–106. doi: 10.1111/j.1751-0813.1986.tb07674.x. [DOI] [PubMed] [Google Scholar]
- Tramont E. C., Sadoff J. C., Boslego J. W., Ciak J., McChesney D., Brinton C. C., Wood S., Takafuji E. Gonococcal pilus vaccine. Studies of antigenicity and inhibition of attachment. J Clin Invest. 1981 Oct;68(4):881–888. doi: 10.1172/JCI110343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D., Mayrand D. Association of oral Bacteroides with gingivitis and adult periodontitis. J Periodontal Res. 1981 May;16(3):259–265. doi: 10.1111/j.1600-0765.1981.tb00974.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Takahashi K., Nodasaka Y., Suzuki T. Purification and characterization of a novel type of fimbriae from the oral anaerobe Bacteroides gingivalis. J Bacteriol. 1984 Dec;160(3):949–957. doi: 10.1128/jb.160.3.949-957.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yount W. J., Dorner M. M., Kunkel H. G., Kabat E. A. Studies on human antibodies. VI. Selective variations in subgroup composition and genetic markers. J Exp Med. 1968 Mar 1;127(3):633–646. doi: 10.1084/jem.127.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambon J. J., Reynolds H. S., Slots J. Black-pigmented Bacteroides spp. in the human oral cavity. Infect Immun. 1981 Apr;32(1):198–203. doi: 10.1128/iai.32.1.198-203.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooijen N., van Nieuwmegen R. Liposomes in immunology: the immune response against antigen-containing liposomes. Immunol Commun. 1977;6(5):489–498. doi: 10.3109/08820137709094148. [DOI] [PubMed] [Google Scholar]



