Abstract
Objective
To determine whether providing corrective health information can reduce the tendency of consumers to believe that the implied marketing message that two “potentially reduced exposure products” (PREPs) are safer than regular cigarettes.
Design
Face‐to‐face interviews with smokers assigned to one of four conditions, which varied in terms of the presence or absence of health information that qualified claims made in advertising for two PREPs.
Subjects
A convenience sample of 177 smokers in Boston area.
Interventions
Health information detailed the extent to which exposure to toxins and health risks of the brands were unknown.
Main outcome measures
Respondents' assessments of the health risks and toxicity of the two combustible PREPs, Advance and Eclipse.
Results
The health information had a modest but significant effect on ratings of health risk, and reduced perceptions that switching to the new brands would lower a smoker's risk of cancer (OR 0.75; p<0.05). The health information had no effect on perceptions of toxicity.
Conclusions
A small dose of corrective information was effective in tempering smokers' perceptions. A higher dose of public health campaigns would be needed to affect misperceptions likely to follow a full‐scale tobacco marketing effort.
Tobacco companies are developing a new generation of tobacco products that are reputed to be less harmful. The new tobacco products, dubbed “potentially reduced exposure products” or “PREPs” by the Institute of Medicine,1 are designed to result in lower levels of particular carcinogens such as tobacco‐specific nitrosamines and polycyclic aromatic hydrocarbons.2
There is considerable controversy about the appropriate public health approach to tobacco modification as a strategy for harm reduction. On one hand, the Institute of Medicine stated that products which involve less combustion than regular cigarettes or lower levels of particular toxins, such as tobacco‐specific nitrosamines, could potentially lower tobacco‐related morbidity and mortality.1 On the other hand, the research community is sceptical of the likelihood that modified combustible products would lower individual risk, and even if they did, they might have the detrimental effect of increasing smoking initiation, promoting relapse or undermining cessation by holding out the hope of a less dangerous tobacco product.3 Unfortunately, the research required to clarify the uncertainties regarding PREPs will take a long time, and in the meantime more and more of these products are being introduced. The public health community, which failed to counteract the marketing of light cigarettes as a less harmful tobacco alternative, is loath to let the tobacco companies control the message again.
To date, there has been little marketing and promotion of PREPs in the US.4 Awareness of these products in the population at large is low.5 However, as new products enter the market (such as Camel Snus and Taboka, new low‐nitrosamine smokeless tobacco products by RJ Reynolds and Philip Morris, respectively), awareness is likely to grow. As that happens, it will be important to have public health communications that will provide accurate information to consumers about what is known and what is unknown about the relative harmfulness of these products.
Research on perceptions of light cigarettes shows that consumers tend to believe the implicit and explicit health claims in tobacco advertising,6,7 and there is a high likelihood of over‐interpreting the marketing claims of PREPs in the face of minimal scientific data.8 Several studies have assessed perceptions of PREPs by exposing respondents to actual advertisements or by reading descriptions of the products over the phone. These studies indicate that substantial proportions of respondents accept advertised claims that the product entails lower exposure to toxins in relation to regular cigarettes and that smoking them would result in lower risks to health.9,10,11 It is particularly worrisome that those most interested in trying these products are smokers who are more, rather than less, motivated to quit smoking altogether,10 reinforcing the concern that wide availability of these products will reduce the cessation rate.
Some evidence suggests that counter‐advertising can modify erroneous beliefs about light cigarettes.12,13,14 The task is more difficult for PREPs, however, because the research on relative harmfulness of PREPs is more incomplete and ambiguous. The purpose of this study was to determine whether providing additional information outlining the bases for uncertainty about these products would temper the tendency to accept the implied message that these two combustible PREPs are less harmful than ordinary cigarettes.
Providing accurate health information
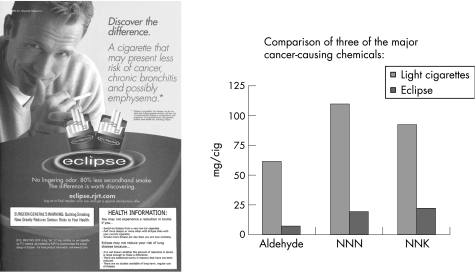
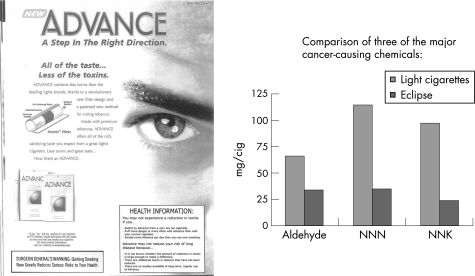
The main goals of the current study were to help consumers understand that levels of exposure to toxins from two combustible PREPs were variable depending on individual smoking behaviour, and to distinguish the concept of reduced exposure from the concept of reduced disease risk. The information was formatted as a health information box and inserted into advertisements for two PREP brands, Eclipse and Advance (fig 1).
Figure 1 Health information box.
The first statement in the box, about switching from a low‐tar cigarette, emerged from research showing increases or small decreases in a number of smoke carcinogens between Eclipse and two low‐tar cigarettes (NOW and Carlton).15 The authors concluded that Eclipse was not less toxic than already available conventional cigarettes. The second statement comes from tobacco industry research showing that Eclipse smokers take more puffs, puff more deeply and wait less time between puffs, for an overall increase in puff volume and duration compared with their usual cigarette. The third statement was derived from research showing that when smokers did not feel satisfied by their light cigarettes, they altered how they smoked, including increasing the amount they smoked in order to feel satisfied.16,17,18
The remaining statements are based on the fact that, currently, there is no evidence to suggest that the extent of toxin reduction with the existing combustible PREPs would lead to a significant reduction in disease risk, nor is there knowledge of the extent of toxin exposure reduction necessary to result in reduction of disease.8 Furthermore, there are no studies of long‐term PREP use that could document the consequences for diseases that emerge after many years of exposure to the product.
Methods
Sample design
A convenience sample of adults was recruited through door‐to‐door canvassing. Professional interviewers from UMass Boston's Center for Survey Research, Boston, Massachusetts, USA, were assigned to recruit adults in 20 towns in the Boston metropolitan area estimated to have relatively high rates of smoking. Interviewers recruited up to five respondents per block in assigned towns and filled a quota based on smoking status (current vs former smokers) and age (<45 and >45 years). Participants were offered US$20 (£10.13, €14.99) to respond to a 30 min survey about “what kind of information is helpful to people when evaluating advertisements for tobacco products.” For the current analyses, the sample included 177 current smokers.
Experimental design
Each participant was shown marketing materials for two combustible PREPs: Advance and Eclipse. The materials for each brand consisted of an actual full‐page colour magazine advertisement for the product and a second page consisting of a bar graph that showed levels of three major carcinogens in the PREPs in relation to the levels in a standard light cigarette (figs 2 and 3). The information about the levels of the carcinogens was based on information on either a package onsert or the manufacturer's website. One of the two colour advertisements seen by each participant was altered so that it contained a 2×3 inch box, described above, which was labelled “health information”. For each brand, there was a second advertisement that was identical to the one in the figure except for the absence of the health information box.
Figure 2 Eclipse stimulus materials. NNK, 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanone; NNN, N‐nitrosonornicotine.
Figure 3 Advance stimulus materials. NNK, 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanone; NNN, N‐nitrosonornicotine.
The presentation of materials for the two PREPs was counterbalanced for order of brand, presence of the health information box and whether the health information box appeared on the first or the second brand shown, creating four conditions:
Eclipse with health information box, followed by Advance without health information box;
Eclipse without health information box, followed by Advance with health information box;
Advance with health information box, followed by Eclipse without health information box; and
Advance without health information box, followed by Eclipse with health information box.
One‐quarter of the participants were pre‐assigned to each condition, permitting examination of the main effects on product perception of the health information box (present vs absent), controlling for whether it appeared on the first or the second advertisement seen and controlling for whether the health information box appeared on the Eclipse or the Advance materials. The following hypotheses were tested:
PREPs advertised with the health information box will be seen as higher in toxins and higher in health risk than PREPs advertised without the health information box.
When the health information box is presented for the first PREP, perceptions of toxins and health risks for both PREPs will be higher than when the health information box is presented for the second PREP.
Procedures
After collecting general information about smoking history, interviewers had respondents rate “light cigarettes, like Marlboro Lights or Camel Lights” on two 0–10 scales illustrated on show cards: one scale for “the amount of chemicals or toxins that cause cancer” and the other scale for “overall health risk”. In each case, the highest rating, 10, was labelled as the amount of toxins or health risk associated with “a regular, full‐flavoured cigarette like Marlboro, Salem or Camel” and 0 was labelled “contains no cancer‐causing chemicals or toxins” on one scale and “no health risk” on the other. These ratings of light cigarettes help provide a context for understanding respondents' perceptions of PREPs. Following Shiffman et al,10 the PREPs were described as containing “far less of many of the chemical compounds or toxins found in cigarettes that are believed to contribute to the risk of cancer”. Then respondents were shown an advertisement for brand 1, and were given up to 2 min to read it. They were then shown the accompanying chart and were read the following statement: “This chart shows the results of chemical testing of (Advance/Eclipse) in comparison to light cigarettes. These three chemicals—aldehyde, NNN, and NNK—are three of the major cancer‐causing toxins in cigarettes. You can see that in the testing that has been carried out, (Advance/Eclipse) is much lower in these three chemicals than light cigarettes.” Respondents were given about 1 min to look at the chart. They were then asked to respond to a series of questions assessing their perceptions of the product. When the questions about the first product were answered, participants were shown the materials for the second product and their perceptions of the second product were assessed. These procedures were approved by the University of Massachusetts Institutional Review Board.
Measures
Perceived toxicity of the product was assessed with three measures. Participants rated each product on the 0–10 scale of toxicity described above. A second dichotomous measure asked whether smokers who switched from their regular brand to the new product “would be exposed to fewer cancer‐causing chemicals”. The third question asked “... how many of the cancer‐causing chemicals found in regular cigarettes have been reduced? All, most, some or none?”. Perceived health risk was measured with two questions: the 0–10 scale of health risk described above and a dichotomous question asking whether switching from their regular brand to the PREP would reduce smokers' chance of cancer.
Analysis plan
Preliminary analyses were carried out to examine perceptions of light cigarettes and of the two PREPs regardless of brand, presence of the health information box or order of presentation. Since each respondent reported perceptions of two PREPs, we used SPSS V.14.0 complex sample procedures, which take account of clustering within individuals in a manner similar to a generalised estimating equation analysis. Then multivariate analyses were carried out—linear regression or logistic regression depending on whether the outcome was continuous or dichotomous—examining the impact of the health information box on perceptions of toxicity of PREPs and health risks. These analyses controlled for the order of the advertisement (first or second), whether the health information box was present and the brand. Additional covariates included respondents' age, gender, education level (high‐school diploma or less vs more than high‐school diploma) and race/ethnicity (non‐Hispanic white vs minority).
Results
Sample characteristics
By design, this convenience sample was evenly split between men and women, and those aged >45 and <45 years. Because of the relationship between smoking and low socioeconomic status, it was not surprising that these participants, recruited in towns with high smoking rates, were more likely to be less educated (61% reported ⩽12 years of education vs 48% among smokers in the state as a whole) and to have a lower income (61% reported an annual income of ⩽US$30 000 (£15 198.92, €22 498.20) vs 23% of smokers in the state as a whole) than a population‐based sample of adult smokers in Massachusetts.19 In all, 31% of the participants were members of a racial or ethnic minority group, about twice the rate of minorities in Massachusetts. They also reported higher levels of nicotine dependency: 41% (vs 35% in a representative sample) reported smoking ⩾20 cigarettes per day and smoking their first cigarette within 30 min of waking.
Perceptions of combustible PREPs versus conventional light cigarettes
All respondents rated the two combustible PREPs, Advance and Eclipse, as well as “light cigarettes like Marlboro Lights or Camel Lights” on toxicity and overall health risk on a 0–10 scale, on which 10 was equated with the toxicity and health risk of regular full‐flavour cigarettes. Results are shown in the left‐hand columns of table 1. Conventional light cigarettes were clearly perceived as significantly less toxic (7.7; 95% CI 7.39 to 8.09) and entailing significantly less health risk (8.26; 95% CI 7.93 to 8.58) than full‐flavoured cigarettes, which were designated as 10 on the scale. The two PREPs, on average, were rated even lower on the same scale (5.73 for toxins and 6.40 for health risk), and paired Student's t tests demonstrated that they were seen as significantly less toxic and less risky than conventional light cigarettes.
Table 1 Perceptions of toxicity and health risks of conventional light cigarettes and two combustible potentially reduced exposure products (n = 177).
| Conventional light cigarettes vs Advance and Eclipse | Advance and Eclipse by presence of health information box | |||||
|---|---|---|---|---|---|---|
| Conventional light cigarettes | Advance and Eclipse | p Value | Health information box | No health information box | p Value | |
| Perceived toxicity | ||||||
| Mean level of toxins (0–10)* | 7.7 | 5.73 | 0 | 5.8 | 5.6 | NS |
| Switching to PREPs would reduce exposure (%)† | NA | NA | NA | 63.7 | 62.7 | NS |
| All or most toxins reduced (%)† | NA | NA | NA | 26.1 | 33.0 | 0.059 |
| Perceived health risk | ||||||
| Mean level of health risk (0–10)* | 8.26 | 6.40 | 0 | 6.6 | 6.2 | 0.006 |
| Switching to PREPs would reduce risk of cancer (%)† | NA | NA | NA | 30.8 | 36.8 | 0.040 |
NA, not applicable; NS, not significant; PREP, potentially reduced exposure product.
*Differences tested with paired Student's t test.
†Differences tested with χ2 test.
Impact of health information box on perceptions of PREPs
The right‐hand columns of table 1 show the simple differences in perceptions of toxin levels and health risks of Advance and Eclipse as a function of whether or not the health information box appeared on the advertisement. The analyses indicate that ignoring brand and order of presentation, the health information box did not have a significant impact on perceptions of the amount of toxins in the PREPs, or the belief that switching to Advance or Eclipse from a conventional cigarette would reduce a smoker's exposure to toxins. The health information box tended to reduce the likelihood of saying that “all” or “most” of the toxins were reduced (from 33% to 26%), but this did not reach conventional levels of significance. The health information box had a small but statistically significant effect on respondents' perceptions of the level of health risk from smoking the PREPs. With the box present, the mean rating on health risks rose from 6.2 to 6.6. In addition, after viewing the advertisement with the health information box, significantly fewer respondents reported that switching to the PREP would decrease a smoker's chance of cancer (30.8% vs 36.8%).
Multivariate analyses examining the same outcome variables tended to reinforce the bivariate results. Table 2 shows the analyses of the impact of the health information box on variables assessing perceptions of exposure to toxins associated with using Advance and Eclipse, controlling for order of presentation, brand, respondent's age, gender, education level and race/ethnicity. As in the bivariate analyses, the health information box had no significant impact on the mean rating of the amount of toxins in the two PREPs, or on the belief that a smoker would reduce exposure to toxins by switching from their regular brand to one of the PREPs. There was, however, a marginally significant impact on the belief that the two PREPs reduced “all or most” of the toxins that appear in regular cigarettes when brand, order of presentation and demographics were controlled. Table 2 also replicates the findings from the bivariate analyses of health risk: with the health information box present, respondents rated the health risks incurred from smoking PREPs significantly higher and were significantly less likely to believe that switching to one of the PREPs would reduce one's risk of cancer.
Table 2 Multivariate analyses of perceived toxicity and perceived health risks of two combustible potentially reduced exposure products (n = 177 smokers).
| Predictor variable | Perceived toxicity of Advance and Eclipse | Perceived health risk of Advance and Eclipse | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Linear regression | Logistic regression | Linear regression | Logistic regression | |||||||
| Amount of toxins (0–10) | Switching would reduce exposure to toxin | All or most of toxins have been reduced | Amount of health risk (0–10) | Switching would reduce chance of cancer | ||||||
| Coefficient | p Value | OR | p Value | OR | p Value | Coefficient | p Value | OR | p Value | |
| Health information box | 0.128 | NS | 1.042 | NS | 0.688 | 0.054 | 0.444 | 0.006 | 0.756 | 0.039 |
| Advertisement order | 0.053 | NS | 1.421 | 0.032 | 0.736 | NS | −0.058 | NS | 1.030 | NS |
| Brand | −0.608 | <0 | 1.029 | NS | 1.215 | NS | −0.308 | 0.055 | 1.207 | NS |
| Gender | −0.088 | NS | 1.255 | NS | 1.688 | 0.080 | −0.241 | NS | 1.755 | 0.065 |
| Race/ethnicity | 0.044 | NS | 0.819 | NS | 1.739 | 0.075 | 0.034 | NS | 1.013 | NS |
| Education (1–4)* | −0.136 | NS | 0.996 | NS | 0.645 | 0.015 | −0.083 | NS | 0.858 | NS |
| Age* | 0.017 | NS | 0.996 | NS | 0.966 | <0 | 0.014 | NS | 0.983 | 0.055 |
NS, not significant.
*Education and age were modelled as continuous variables. Coding for dichotomous variables was as follows: health information box (0, no; 1, yes); advertisement order (0, first; 1, second); brand (0, Advance; 1, Eclipse); gender (0, female; 1, male); race/ethnicity (0, white; 1, minority).
Few consistent effects for the covariates were noticed. Older people were less likely to believe that all or most of the toxins in Advance and Eclipse had been reduced and less likely to believe that switching to either of the PREPs would reduce one's risk of cancer. Also, a significant effect of brand was noticed on the ratings of the amount of toxins in the cigarette, with Advance scoring higher than Eclipse (6.03 vs 5.41, not shown), and a marginally significant effect noticed in the same direction for ratings of health risks (6.55 vs 6.24, not shown). This is probably due to differences in the advertised tar and nicotine levels (Advance advertises 10 mg of tar and 0.8 mg of nicotine; Eclipse advertises 4 mg of tar and 0.1 mg of nicotine), the explicit discussion of reduced disease risk on the Eclipse advertisement and the graphs that show lower levels of aldehyde, N‐nitrosonornicotine and NNK, 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanone for Eclipse.
Impact of order of presentation of health information
Although the mean ratings on toxicity and health risks for both PREPs were slightly higher if the box appeared on the first brand presented, the differences between the first and second presentations were not statistically significant. Multivariate analyses tested this question by including an interaction term for order of advertisement and presence of box. None of the interaction terms was statistically significant.
What this paper adds
Previous research has demonstrated that because of misleading advertising, consumers tend to form beliefs, unsupported by scientific evidence, that new “reduced exposure” tobacco products (potentially reduced exposure products (PREPs)) are less harmful than ordinary cigarettes.
Similar misconceptions regarding light and ultralight cigarettes are widespread in the population. Although some evidence suggests that corrective advertising could modify erroneous beliefs about light cigarettes, to date, no published study has investigated whether advertising could reduce the misperception of the harmfulness of PREPs.
The task is more difficult for PREPs than for light cigarettes because the research on relative harmfulness of PREPs is more ambiguous.
This study demonstrates that research providing consumers with even a minimal dose of accurate information about the range of unknowns in harm reduction had a significant moderating effect on their perceptions.
Discussion
The findings reported here replicate results of previous research,5,9,10 demonstrating that promotional materials for cigarettes advertised as potentially less harmful are extremely effective in convincing smokers that they are lower in toxins and health risks than conventional cigarettes. The recent ruling in the US federal court that bans tobacco companies from using terms such as “low tar” and “light” cigarettes20 may well be followed by rulings prohibiting new PREPs from being described as they were in the advertisements used in this study: “A cigarette that may present less risk of cancer, chronic bronchitis and possibly emphysema” and “All of the taste … Less of the toxins.” However, even without such advertising, the media may disseminate the news that these products may be less risky than conventional cigarettes. For example, even though advertising for the new, lower‐nitrosamine smokeless tobacco products makes no health claims, a recent ABC news report was entitled, “Smokeless tobacco: no chewing, no spitting, and fewer cancer‐causing chemicals?”21 Given the lack of scientific evidence of reduced harm of combustible PREPs, there are appropriate concerns that the new products could increase, rather than decrease, the negative health effects of tobacco use by undermining smokers' motivation to quit and possibly luring former smokers back into tobacco use with a product that may be seen as relatively harmless. Corrective messages will certainly be needed. The good news in the findings of this study is that research providing consumers with even a minimal dose of accurate information about the range of unknowns in harm reduction had a significant moderating effect on their perceptions. The health information box explained in brief why Advance and Eclipse might not really reduce smokers' exposure to toxins, and why—even if some toxins were reduced—the products might not reduce their risk of disease. These are complex concepts that were presented to a sample of smokers of substantially lower education level than average, and without comment or explanation, along with the actual product advertisements.
The study does have a number of limitations in this regard. We did not have measures of respondents' reading ability, the reading level of the health information box or the respondents' comprehension of the information in the box. We made a concerted effort to make the contents of the information box as simple and straightforward as possible, separating complex concepts into smaller, simplified ideas. However, we did not want to ask direct questions about the health information box because we were attempting to simulate realistic conditions, as if respondents were reading a magazine advertisement, and letting them concentrate on whatever they were drawn to.
Another limitation of the study is its small sample size. As noted in the Methods section, we had only 177 respondents assigned to four conditions. Some of the non‐significant findings with regard to perceived toxicity could potentially become significant with a larger sample. While it is important to note that despite the small sample size, some of the results were statistically significant, it is equally important to acknowledge that the impact was small, with only minor shifts in perceived health risk in the presence of the box. This raises a central question as to the ways in which we might be able to increase the impact of the corrective messages. One change might be to increase the graphic interest and improve the verbal content of the health information box to make it more appealing and consumer friendly. In addition, it would be worthwhile to pretest the box content for comprehensibility, assuring that it is understandable by people of different levels of education.
Since the participants in this study were of lower education and income than the general population of smokers, the findings should be interpreted with some caution. The participants may have been somewhat more likely to believe that both PREPs and lights are less harmful than regular full‐flavoured cigarettes. However, since education level did not have a significant impact on four of the five outcome variables, there is reason to believe that these findings would apply to a more educated sample.
The fact that this low‐dose intervention could have a significant impact on perceptions gives reason to be confident that a high‐dose, intensive effort to educate consumers about PREPs could be even more effective. It is noteworthy that having the health information on the first PREP viewed did not significantly affect the perceptions of the second PREP. This indicates that respondents were not generalising the information to a different product. Any educational intervention would need to take into account the variety of products currently on the market and those likely to be introduced in the future. It may also be necessary to require by law that some items of information similar to the points made in the box appear on every PREP advertisement.
As more and more new tobacco products are introduced into the market, such as the lower‐nitrosamine smokeless products, designing, evaluating and mounting an effective public health education campaign on the concept of tobacco harm reduction is imperative.
Acknowledgements
Funding for this study was provided by the American Legacy Foundation, Grant # 1662. We thank Rebecca Reimer for her assistance during the data collection phase and preliminary statistical analyses, and Catherine Garrett for the final analyses of the data. The authors are grateful to Peter Shields and Dorothy Hatsukami for reviewing the information used in the Health Information Box.
Abbreviations
PREP - potentially reduced exposure product
Footnotes
Competing interests: None declared.
References
- 1.Stratton K, Shetty P, Wallace R.et al, eds. Clearing the smoke: assessing the science base for tobacco harm reduction Washington, DC: National Academy Press, 2001 [PubMed]
- 2.Hatsukami D K, Giovino G A, Eissenberg T.et al Methods to assess potential reduced exposure products. Nicotine Tob Res 20057827–844. [DOI] [PubMed] [Google Scholar]
- 3.Joseph A M, Hennrikus D, Thoele M J.et al Community tobacco control leaders' perceptions of harm reduction. Tob Control 200413108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hickman N, Klonoff E A, Landrine H.et al Preliminary investigation of the advertising and availability of PREPs, the new “safe” tobacco products. J Behav Med 200427413–424. [DOI] [PubMed] [Google Scholar]
- 5.O'Connor R J, Hyland A, Giovino G A.et al Smoker awareness of and beliefs about supposedly less‐harmful tobacco products. Am J Prev Med 20052985–90. [DOI] [PubMed] [Google Scholar]
- 6.Shiffman S, Pillitteri J L, Burton S L.et al Smokers' beliefs about “light” and “ultra light” cigarettes. Tob Control 200110(Suppl 1)17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kozlowski L T, Goldberg M, Yost B.et al Smokers' misperceptions of light and ultra‐light cigarettes may keep them smoking. Am J Prev Med 1998159–16. [DOI] [PubMed] [Google Scholar]
- 8.Hatsukami D, Hecht S S.Hope or hazard? What research tells us about “potentially reduced‐exposure” tobacco products. Minneapolis, MN: University of Minnesota Transdisciplinary Tobacco Use Research Center, 2005
- 9.Hamilton W L, Norton G D, Ouellette T K.et al Smokers' responses to advertisements for regular and light cigarettes and potential reduced‐exposure tobacco products. Nicotine Tob Res 20046(Suppl 3)353–362. [DOI] [PubMed] [Google Scholar]
- 10.Shiffman S, Pillitteri J L, Burton S L.et al Smoker and ex‐smoker reactions to cigarettes claiming reduced risk. Tob Control 20041378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shadel W G, Lerman C, Cappella J.et al Evaluating smokers' reactions to advertising for new lower nicotine Quest cigarettes. Psychol Addict Behav 20062080–84. [DOI] [PubMed] [Google Scholar]
- 12.Kozlowski L T, Yost B, Stine M M.et al Massachusetts' advertising against light cigarettes appears to change beliefs and behavior. Am J Prev Med 200118339–342. [DOI] [PubMed] [Google Scholar]
- 13.Kozlowski L T, Pillitteri J L. Beliefs about “light” and “ultra light” cigarettes and efforts to change those beliefs: an overview of early efforts and published research. Tob Control 200110(Suppl 1)i12–i16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiffman S, Burton S, Pillitteri J.et al Test of “light” cigarette counter‐advertising using a standard test of advertising effectiveness. Tob Control 200110(Suppl 1)i33–i40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slade J, Connolly G N, Lymperis D. Eclipse: does it live up to its health claims? Tob Control 200211(Suppl II)ii64–ii70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashley M J, Cohen J, Ferrence R. “Light” and “mild” cigarettes: Who smokes them? Are they being misled? Can J Public Health 200192407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benowitz N L. Compensatory smoking of low‐yield cigarettes. In: Shopland DR, Burns DM, Benowitz NL, Amacher RH, eds. Risks associated with smoking cigarettes with low machine‐measured yields of tar and nicotine. Monograph 13. Rockville, MD: US Department of Health and Human Services, National Institute of Health, 200139–64.
- 18.Hammond D, Collishaw N E, Callard C. Secret science: tobacco industry research on smoking behaviour and cigarette toxicity. Lancet 2006367781–787. [DOI] [PubMed] [Google Scholar]
- 19.Biener L. UMass Tobacco Study. Unpublished data. Center for Survey Research, University of Massachusetts Boston, Boston, Massachusetts, USA
- 20.United States of America vs Philip Morris USA I.et al Final Judgment and Remedial Order. Civil Action Number 99‐2496. Order #1015. US District Court for the District of Columbia 2006
- 21.Quraishi F. Smokeless tobacco: no chewing, no spitting, and fewer cancer‐causing chemicals? 21 June 2006. http://abcnews.go.com/Health/print?id = 2099026 (accessed 4 Apr 2007)