Abstract
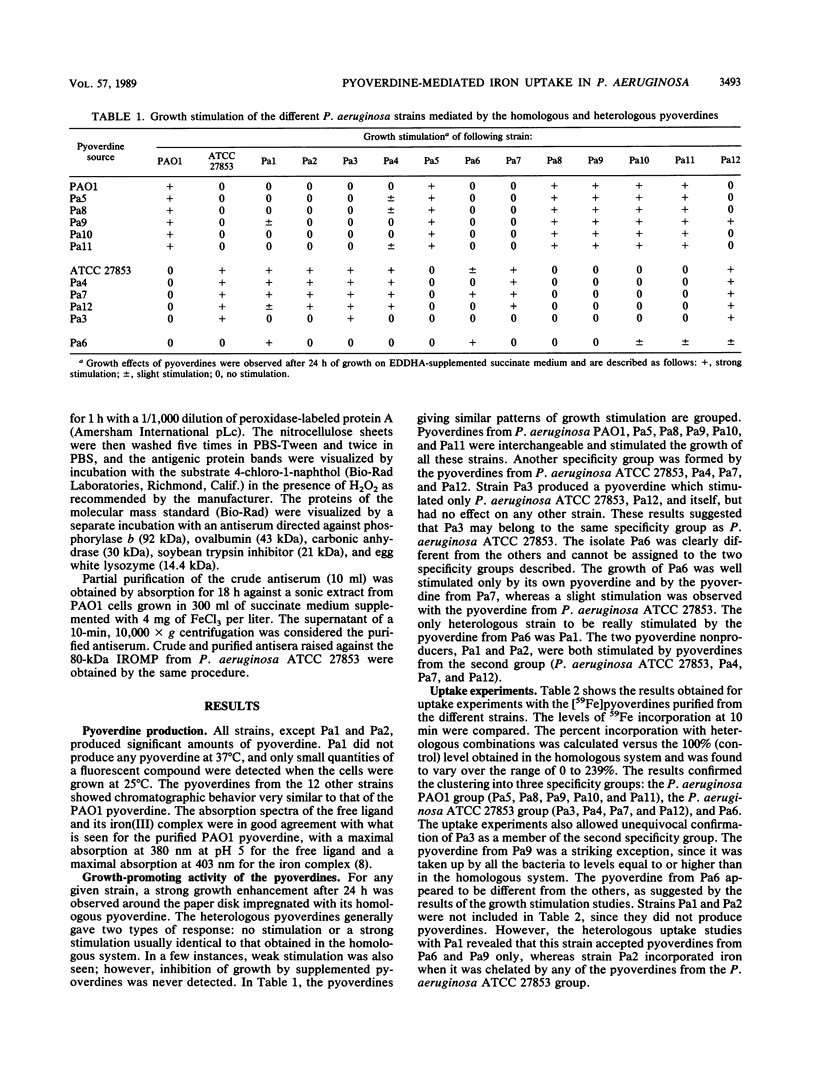
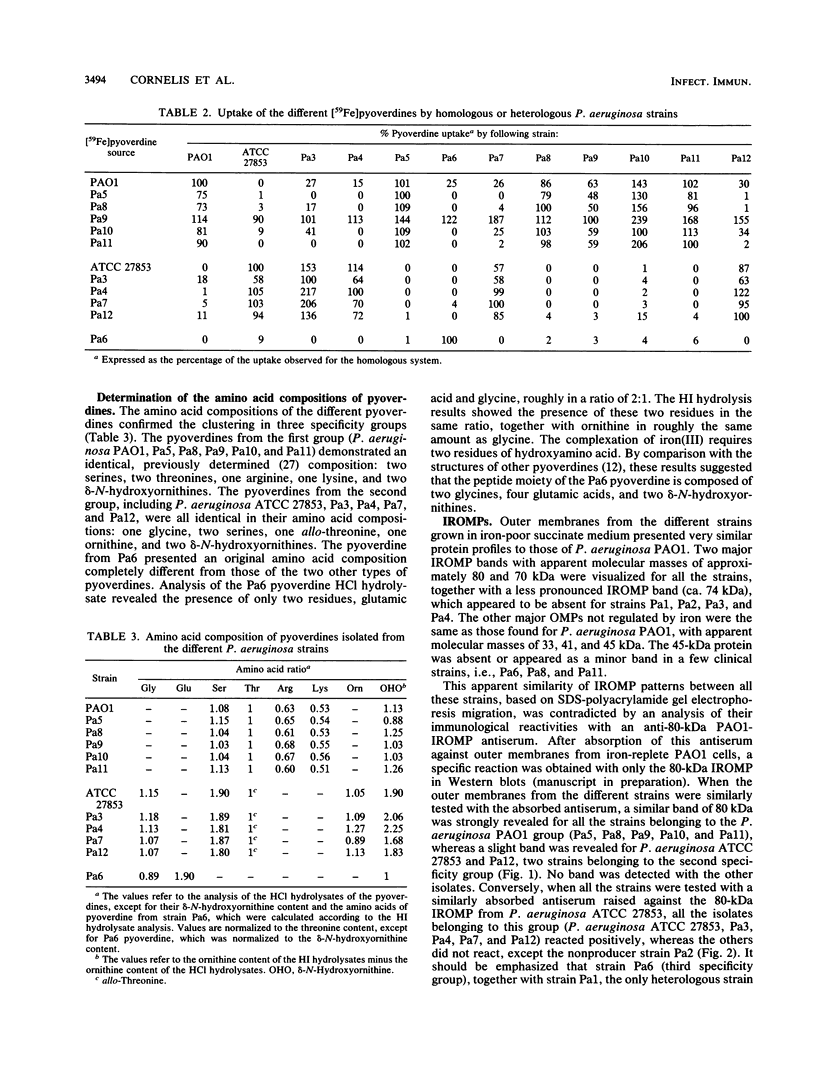
Fourteen strains of Pseudomonas aeruginosa (P. aeruginosa ATCC 15692, P. aeruginosa ATCC 27853, and 12 clinical isolates) were checked for the production of pyoverdine and for pyoverdine-mediated iron uptake. Under iron restriction, two isolates produced undetectable amounts of pyoverdine, but all the other strains produced a compound with physicochemical properties identical or close to those of the pyoverdine of P. aeruginosa ATCC 15692 (strain PAO1). The pyoverdines were purified and tested for their growth-promoting activity and for their ability to facilitate 59Fe uptake in homologous experiments involving each pyoverdine and its producing strain, as well as in heterologous systems involving all the other strains. The results of both types of experiments suggested the existence of three specificity groups. This was confirmed by analysis of the amino acid composition of the pyoverdines, which differed for each group. A partially purified polyclonal antiserum raised against a major 80-kilodalton (kDa) iron-regulated outer membrane protein (IROMP) of P. aeruginosa PAO1 recognized the 80-kDa IROMPs from P. aeruginosa PAO1 and the clinical isolates belonging to the same group, whereas the IROMPs from the strains belonging to the two other groups were not detected. A second antiserum raised against the P. aeruginosa ATCC 27853 80-kDa IROMP gave similar results by reacting specifically with the 80-kDa IROMP from the strains belonging to this group. Thus, together with the already known pyoverdine from P. aeruginosa PAO1, two new types of pyoverdines produced by strains belonging to this species were characterized.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Listowsky I. Iron transport and storage proteins. Annu Rev Biochem. 1980;49:357–393. doi: 10.1146/annurev.bi.49.070180.002041. [DOI] [PubMed] [Google Scholar]
- Ankenbauer R., Sriyosachati S., Cox C. D. Effects of siderophores on the growth of Pseudomonas aeruginosa in human serum and transferrin. Infect Immun. 1985 Jul;49(1):132–140. doi: 10.1128/iai.49.1.132-140.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagg A., Neilands J. B. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol Rev. 1987 Dec;51(4):509–518. doi: 10.1128/mr.51.4.509-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskot G., Taraz K., Budzikiewicz H. Siderophore vom Pyoverdin-Typ aus Pseudomonas aeruginosa. Z Naturforsch C. 1986 May-Jun;41(5-6):497–506. [PubMed] [Google Scholar]
- Buyer J. S., Leong J. Iron transport-mediated antagonism between plant growth-promoting and plant-deleterious Pseudomonas strains. J Biol Chem. 1986 Jan 15;261(2):791–794. [PubMed] [Google Scholar]
- Cornelis P., Moguilevsky N., Jacques J. F., Masson P. L. Study of the siderophores and receptors in different clinical isolates of Pseudomonas aeruginosa. Antibiot Chemother (1971) 1987;39:290–306. doi: 10.1159/000414354. [DOI] [PubMed] [Google Scholar]
- Döring G., Pfestorf M., Botzenhart K., Abdallah M. A. Impact of proteases on iron uptake of Pseudomonas aeruginosa pyoverdin from transferrin and lactoferrin. Infect Immun. 1988 Jan;56(1):291–293. doi: 10.1128/iai.56.1.291-293.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohnadel D., Meyer J. M. Specificity of pyoverdine-mediated iron uptake among fluorescent Pseudomonas strains. J Bacteriol. 1988 Oct;170(10):4865–4873. doi: 10.1128/jb.170.10.4865-4873.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu P. V., Shokrani F. Biological activities of pyochelins: iron-chelating agents of Pseudomonas aeruginosa. Infect Immun. 1978 Dec;22(3):878–890. doi: 10.1128/iai.22.3.878-890.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magazin M. D., Moores J. C., Leong J. Cloning of the gene coding for the outer membrane receptor protein for ferric pseudobactin, a siderophore from a plant growth-promoting Pseudomonas strain. J Biol Chem. 1986 Jan 15;261(2):795–799. [PubMed] [Google Scholar]
- Marugg J. D., de Weger L. A., Nielander H. B., Oorthuizen M., Recourt K., Lugtenberg B., van der Hofstad G. A., Weisbeek P. J. Cloning and characterization of a gene encoding an outer membrane protein required for siderophore-mediated uptake of Fe3+ in Pseudomonas putida WCS358. J Bacteriol. 1989 May;171(5):2819–2826. doi: 10.1128/jb.171.5.2819-2826.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer B., Müller A., Keller-Schierlein W., Zähner H. Stoffwechselprodukte von Mikroorganismen. 61. Ferribactin, ein Siderochrom aus Pseudomonas fluorescens Migula. Arch Mikrobiol. 1968;60(4):326–339. [PubMed] [Google Scholar]
- Mizuno T., Kageyama M. Separation and characterization of the outer membrane of Pseudomonas aeruginosa. J Biochem. 1978 Jul;84(1):179–191. doi: 10.1093/oxfordjournals.jbchem.a132106. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- Rogers H. J. Iron-Binding Catechols and Virulence in Escherichia coli. Infect Immun. 1973 Mar;7(3):445–456. doi: 10.1128/iai.7.3.445-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron withholding: a defense against infection and neoplasia. Physiol Rev. 1984 Jan;64(1):65–102. doi: 10.1152/physrev.1984.64.1.65. [DOI] [PubMed] [Google Scholar]
- de Weger L. A., van Boxtel R., van der Burg B., Gruters R. A., Geels F. P., Schippers B., Lugtenberg B. Siderophores and outer membrane proteins of antagonistic, plant-growth-stimulating, root-colonizing Pseudomonas spp. J Bacteriol. 1986 Feb;165(2):585–594. doi: 10.1128/jb.165.2.585-594.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]




