Abstract
Genital herpes simplex virus type 2 (HSV2) is highly prevalent worldwide and an increasingly important cause of genital ulcer disease (GUD). Continued HSV2 transmission is facilitated by the large number of undiagnosed cases, the frequency of atypical disease and the occurrence of asymptomatic shedding. The lack of easy, affordable diagnostic methods and specific antiviral treatment in countries with low and middle income is of great concern, given the ability of GUD to enhance HIV transmission and acquisition. With rising HSV2 prevalence contributing to an increase in the proportion of GUD attributed to genital herpes in high‐HIV prevalence settings, a safe and effective HSV vaccine is urgently needed. Meanwhile, multifaceted interventions are required to improve recognition of genital herpes, to prevent its spread and also to prevent its potential to promote HIV transmission in developing countries.
Genital herpes infection is the primary cause of genital ulcer disease (GUD) worldwide.1 It is mainly caused by herpes virus simplex (HSV) type 2 (HSV2) but can also be caused by HSV1. HSV1 has been estimated to cause 30% of cases of primary genital herpes in the US.2 In developing countries, the proportion of genital herpes caused by HSV1 is unknown, although it is assumed to be low. Recent attention has focused on the role of HSV2 as a cofactor for HIV infection and, hence, HSV2 treatment may have a role as an HIV prevention strategy. The World Health Organization (WHO) has recently issued new guidelines for the syndromic management of genital ulcers, which recommend antiviral treatment for herpes in settings with high HSV2 prevalence.3 Aciclovir is inexpensive, well tolerated and has an acceptable profile for widespread treatment; however, it is not widely available in countries with low and middle income. While an effective HSV vaccine is available, other treatment and control strategies need to be evaluated and, if effective, implemented.
This paper reviews the epidemiology of HSV2 in developing countries, the importance of HSV2 as a cause of GUD, the available evidence on the interaction between HSV2 and HIV, the diagnostic options in developing countries and the role of antiherpes treatment in the management of GUD.
Search methods
To identify relevant published literature, we comprehensively searched the Medline database covering studies from 1985 to 2005. The websites of the WHO, US Centers for Disease Control and Prevention, UK Public Health Laboratory Service and UK Medical Society for the Study of Venereal Diseases were also searched for relevant publications and abstracts. Search terms used were “genital herpes”, “STI (sexually transmitted infection)” and “developing countries, genital ulcer, HSV, HSV‐2, herpes”. For the review of GUD aetiology in developing countries, we included all papers that reported laboratory methods used, and we excluded those that provided only clinical diagnosis.
HSV2 epidemiology
Few countries have population‐based national estimates of HSV2 that allow the estimation of seroprevalence trends. In the US, a representative national sample of the population aged ⩾12 years indicated that age‐adjusted HSV2 prevalence has increased from 16.4% to 21.7% of adults from 1976 to 1994.4 European cross‐sectional surveys conducted between 1989 and 2000 found that age‐standardised HSV2 seroprevalence ranged from 4% in England and Wales to 24% in Bulgaria.5
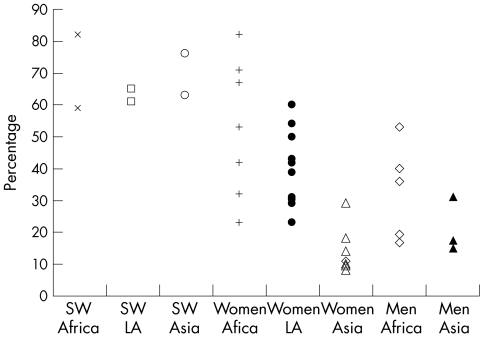
Compared with developing countries, substantially higher rates of HSV2 have been observed in sub‐Saharan Africa, with age‐adjusted prevalences in adults ranging from 30% to 80% in women and 10% to 50% in men (fig 1). In South America, available data are mainly for women, in whom HSV2 prevalence ranges from 20% to 40%. Prevalence in the general population in Asian countries shows lower values, from 10% to 30%.6
Figure 1 Prevalence of herpes simplex virus type 2 (HSV2) among sex workers, women not at high risk, and men from Africa, Latin America and Asia. SW, sex worker, LA, Latin America. Data abstracted from tables 1–3 from Weiss.6 Studies providing only pooled estimates for women and men were not included; for sex workers in Africa, Latin America and Asia, only two studies were available per continent; seven studies were included for women not at high risk in Africa; eleven for women in Latin America, seven for women in Asia; six for men in Africa; three for men in Asia, and no studies were available for men in Latin America.
The changing epidemiology of GUD
The prevalence and aetiology of GUD in different geographical regions and populations in Africa seem to vary widely, and are also changing over time. Studies on GUD in developing countries published before 1995 identified syphilis and chancroid as the main causes of GUD (table 1).7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35 In resource‐poor nations, the spread of HIV and factors such as enhanced case identification and treatment for bacterial GUD since 1990 seem to have resulted in altered epidemic patterns of sexually transmitted infections (STIs). Among studies conducted during the past 10 years, HSV2 has been reported as an important cause of GUD in Africa, South East Asia and Latin America. In at least seven locations in Africa where longitudinal data are available, there is evidence of a shift in the aetiology of GUD towards HSV2 (table 1). For example, cross‐sectional studies among miners in South Africa reported that the proportion of ulcers due to herpes increased from 3% in 1986 to 17% in 1994 and to 36% in 1998.19,20 Paz‐Bailey et al7 reported similar findings from Botswana, where 24% of GUD among patients with STIs was due to HSV2 in 1993, increasing to 60% in 2002. In Botswana, the increases in HSV2 were still marked after adjusting the prevalence for the differences in sensitivity and specificity of diagnostic tests used in 1993 and 2002.
Table 1 Review of aetiology of genital ulcer disease in Africa, Asia and Latin America.
| City, country, reference | Year | Population | Aetiology of genital ulcer disease, n (%) | Serology, n (%) | Laboratory techniques | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TP | HD | HSV | LGV | Mixed aetiology | No detection | HSV2 | Syphilis | HIV | ||||
| Africa | ||||||||||||
| Gaborone, Botswana7 | 1993 | 108 M, F | 1 (1) | 27 (25) | 25 (23) | 2 (2) | 5 (5) | 58 (54) | NR | 56 (52) | 40 (37) | TP dark field; HD, CT and HSV culture; syphilis serology RPR+TPHA; CT antibodies MIF; HIV ELISA |
| Gaborone, Francistown and Selebi Phikwe, Botswana7 | 2002 | 137 M, F | 2 (2) | 1 (1) | 80 (58) | NR | 0 (0) | 54 (39) | NR | 7 (5) | 72 (74) | TP, HD and HSV MPCR; syphilis serology RPR+TPHA; HIV ELISA |
| Nairobi, Kenya8 | 1985 | 89 F | 11 (12) | 43 (48) | 2 (2) | NR | 3 (3) | 31 (35) | NR | 24 (27) | NR | TP dark field; HD and HSV culture; syphilis serology RPR+MHA‐TP |
| Nairobi, Kenya9 | 1985 | 115 M | 3 (3) | 81 (70) | 10 (9) | NR | NR | 21 (18) | 37 (32) | 17 (15) | 19 (17) | TP dark field; HD and HSV culture; syphilis serology RPR+FTA‐abs; HSV2 serology western blot; HIV ELISA+western blot |
| Nairobi, Kenya10 | 1990–1 | 168 M, F | NR | 56 (33) | NR | NR | NR | NR | NR | 24 (14) | NR | HD culture; syphilis serology RPR+TPPA |
| Nairobi, Kenya11 | 1995–7 | 245 M, F | 89 (36) | 111 (45) | 49 (20) | NR | 39 (16) | 36 (15) | NR | 69 (28) | 88 (36) | TP, HD and HSV MPCR; syphilis serology RPR+TPHA; HIV EIA |
| Maseru, Lesotho12 | 1993–4 | 105 M, F | 23 (23) | 56 (56) | 26 (26) | 7 (7) | 17 (17) | 6 (6) | 54 (54) | 25 (25) | 37 (36) | TP, HD and HSV MPCR; syphilis serology RPR+FTA‐abs; CT antibodies MIF; HIV ELISA+western blot; HSV2 serology western blot |
| Blantyre, Malawi13 | 1993 | 778 M | 136 (20) | 204 (26) | NR | NR | NR | NR | NR | 129 (17) | 445 (57) | TP direct IF; HD culture; syphilis serology RPR+MHA‐TP; HIV ELISA |
| Lilongwe, Malawi14 | 1998–9 | 137 M, F | 5 (4) | 41 (30) | 47 (34) | NR | 2 (1) | 46 (34) | 107 (80) | 12 (9) | NR | TP, HD and HSV MPCR; syphilis serology RPR+TPPA; HSV2 serology focus |
| Antananarivo, Madagascar15 | 1997 | 196 M, F | 56 (29) | 64 (33) | 19 (10) | NR | 6 (3.1) | 62 (32) | NR | 59 (33) | NR | P, HD and HSV MPCR; syphilis serology RPR+TPHA; CT antibodies MIF |
| Kigali, Rwanda16 | 1986 | 210 M, F | 63 (30) | 36 (34) | 37 (18) | 29 (14) | 29 (14) | 75 (36) | NR | 81 (39) | 124 (59) | HD, LGV and HSV culture; primary syphilis defined as RPR>1:2 and TPHA or FTA‐abs positive; syphilis serology RPR+TPHA or FTA‐abs; HIV ELISA |
| Kigali, Rwanda17 | 1990–2 | 395 M, F | 110 (28) | 115 (29) | 89 (23) | NR | 46 (12) | 127 (32) | NR | NR | 289 (73) | HD, LGV and HSV culture; primary syphilis defined as RPR>1:2 and TPHA or FTA‐abs positive; syphilis serology RPR+TPHA or FTA‐abs; HIV ELISA |
| Dakar, Senegal18 | 1992 | 39 M, F | 6 (15) | 22 (56) | 5 (13) | NR | 4 (10) | 10 (26) | NR | NR | NR | TP, HD, HSV PCR |
| Carletonville, South Africa19* | NR, 1990 publication date | 240 M | 25 (10) | 147 (61) | 7 (3) | 15 (6) | 14 (6) | 29 (12) | NR | 52 (47) | NR | TP dark field; HD, CT and HSV culture; syphilis serology RPR+FTA‐abs; CT antibodies MIF; donovanosis Giemsa stain |
| Carletonville, South Africa20 | 1993–4 | 232 M | 24 (10) | 161 (69) | 40 (17) | NR | 17 (7) | 25 (11) | NR | 110 (48) | 126 (54) | TP, HD and HSV MPCR; syphilis serology RPR+FTA‐abs; HIV ELISA |
| Carletonville, South Africa20 | 1998 | 186 M | 23 (12) | 94 (51) | 67 (36) | NR | 31 (17) | 39 (21) | NR | 74 (40) | 108 (59) | TP, HD and HSV MPCR; syphilis serology RPR+FTA‐abs; HIV ELISA |
| Cape Town, South Africa21 | 1993–4 | 180 M | 59 (33) | 31 (17) | 43 (24) | NR | 6 (3) | 54 (30) | 86 (49) | 61 (47) | 24 (13) | TP, HD and HSV MPCR; syphilis serology RPR+FTA‐abs; HSV2 serology western blot; HIV ELISA+western blot |
| Johannesburg, South Africa21 | 1993–4 | 159 M | 19 (10) | 34 (21) | 78 (49) | NR | 6 (4) | 34 (21) | 78 (50) | 58 (46) | 76 (48) | TP, HD and HSV MPCR; syphilis serology RPR+FTA‐abs; HSV2 serology western blot; HIV ELISA+western blot |
| Durban, South Africa22 | 1984 | 100 M | 23 (23) | 40 (40) | 9 (9) | NR | 11 (11) | 41 (41) | 97 (97) | 35 (35) | NR | TP dark field; culture HD and HSV; HSV2 serology ELISA; syphilis serology RPR+FTA‐abs |
| Durban, South Africa23* | 1988–9 | 100 M | 42 (42) | 34 (34) | 10 (10) | 6 (6) | 14 (14) | 24 (24) | NR | 44 (44) | 5 (5) | TP dark field; culture HD and HSV; syphilis serology RPR+TPHA; CT antibodies MIF; HIV ELISA; donovanosis Giemsa stain |
| Durban, South Africa24* | 1988–9 | 100 F | 40 (40) | 14 (14) | 18 (18) | 6 (6) | 13 (13) | 20 (20) | NR | 49 (49) | 3 (3) | TP dark field; culture HD and HSV; syphilis serology RPR+TPHA; CT antibodies MIF; HIV ELISA; donovanosis Giemsa stain |
| Durban, South Africa21 | 1993–4 | 199 M | 29 (15) | 106 (53) | 72 (36) | NR | 33 (17) | 29 (15) | 81 (49) | 39 (23) | 115 (58) | TP, HD, HSV MPCR; syphilis serology RPR+FTA‐abs; HIV ELISA+western blot |
| Durban, South Africa25* | 2000–1 | 587 M, F | 85 (14) | 57 (10) | 284 (48) | 63 (11) | 63 (11) | 156 (27) | NR | NR | 441 (75) | TP, HD, HSV MPCR; LGV PCR; primary syphilis PCR+ or FTA‐abs+EIA or FTA‐abs+RPR; HIV Determine+Capillus |
| Dar es Salaam and Mbeya, Tanzania26 | 1999 | 102 M, F | 1 (1) | 21 (21) | 59 (58) | NR | 9 (9) | 39 (38) | NR | 15 (15) | 50 (48) | TP, HD, HSV MPCR, syphilis serology RPR+TPPA, HIV Determine+ELISA |
| Dar es Salaam, Tanzania27 | NR | 69 M, F | NR | NR | 45 (64) | NR | NR | n | 55 (80) | NR | 29 (42) | TP, HD and HSV MPCR; HSV2 serology ELISA; HIV ELISA |
| Fajara, The Gambia28 | 1980 | 104 M, F | 5 (5) | 54 (52) | 4 (4) | 7 (7) | NR | 28 (27) | NR | 22 (21) | NR | TP dark field TP; HD, CT and HSV culture; syphilis VDRL+TPHA; CT serology MIF |
| Kampala, Uganda29 | 1990–1 | 98 M, F | 2 (2) | 0 (0) | 35 (36) | 0 (0) | 2 (2) | 61 (61) | NR | 11 (12) | 58 (65) | TP dark field; HSV immunofluorescence; HIV EIA; syphilis RPR+MHA‐TP; CT serology MIF |
| Rakai, Uganda30 | 1994–8 | 207 M, F | 8 (4) | 6 (3) | 89 (43) | NR | NR | 103 (51) | NR | NR | 0 | TP, HD, HSV MPCR; syphilis serology TRUST+TPHA; HIV ELISA+western blot |
| Asia | ||||||||||||
| Pune, India31 | 1994 | 302 M, F | 29 (10) | 69 (23) | 79 (26) | NR | 21 (7) | 104 (34) | NR | NR | 67 (22) | TP, HD, HSV MPCR; syphilis serology VDRL+FTA‐abs; HIV ELISA+western blot |
| Kuala Lumpur, Malaysia32 | 1989–90 | 249 M, F | 18 (7) | 22 (9) | 48 (19) | 4 (2) | 3 (1) | 153 (62) | NR | 41 (17) | NR | TP dark field TP; HD, CT and HSV culture; syphilis serology RPR+TPHA |
| Chiang Mai, Thailand33 | 1995–6 | 38 M, F | 1 (3) | 0 (0) | 32 (84) | NR | 1 | 6 (16) | NR | NR | 11 (46) | TP, HD, HSV MPCR; HIV test NR |
| Latin America | ||||||||||||
| Santo Domingo, Dominican Republic34 | 1995–6 | 81 M | 4 (5) | 21 (26) | 35 (43) | NR | 0 | 21 (26) | NR | 6 (7) | 11 (14) | TP, HD, HSV MPCR; syphilis serology VDRL+FTA‐abs; HIV ELISA+western blot |
| Kingston, Jamaica35 | 1996 | 304 M, F | 31 (10) | 72 (24) | 158 (52) | NR | 22 (7) | 67 (22) | NR | 64 (21) | 67 (22) | TP, HD, HSV MPCR; syphilis serology TRUST+MHA‐TP; HIV ELISA+western blot |
| Lima, Peru34 | 1994–5 | 63 M | 6 (10) | 3 (5) | 27 (43) | NR | 3 (5) | 30 (48) | NR | 5 (8) | 2 (3) | TP, HD, HSV MPCR; syphilis serology RPR+MHA‐TP; HIV ELISA+western blot |
CT, Chlamydia trachomatis; EIA, enzyme immunoassay; F, female; FTA‐abs, fluorescent treponemal antibody absorption; HD, Haemophilus ducreyi; HSV, herpes simplex virus; IF, immunofluorescence; LGV, Lymphogranuloma venereum; M, male; MHA‐TP, microhaemagglutination assay for Treponema pallidum; MIF, microimmunofluorescence; MPCR, multiplex polymerase chain reaction; NR, not reported; PCR, polymerase chain reaction; RPR, rapid plasma reagin; TP, Treponema pallidum; TPHA, Treponema pallidum haemagglutination assay; TPPA, Treponema pallidum particle agglutination assay; TRUST, toluidine red unheated serum test; VDRL, venereal disease research laboratory.
*Donovanosis reported only in three studies from South Africa: 1 (0.4%) case from Carletonville in 1990,15 11 (11%) among women and 16 (16%) among men in 1989 in Durban19,20 and 8 (1%) in 2001 in Durban.21
Number of cases and percentage for each organism include cases of mixed etiology; therefore percentages will not add up to 100%.
Possible explanations for the changes in GUD aetiology include changes in laboratory techniques, HIV‐attributable mortality among high‐risk groups, behavioural change, improved syndromic management and increase in antibiotic use. Changes in laboratory techniques, including availability of more sensitive tests such as polymerase chain reaction (PCR), may explain some of the differences but cannot account for all the observed changes in prevalence.7 HIV‐attributable mortality might disproportionately affect the prevalence of chancroid, which heavily relies on core groups to persist. In contrast, owing to its longer duration of infection, HSV2 does not rely on people at high risk to spread and is less influenced by the differential mortality associated with AIDS.36,37 Syndromic management was implemented in several countries in Africa in the early 1990s, and widespread use of antibiotics, together with mandatory serological testing for syphilis, may have contributed to a decrease in the prevalence of syphilis and other STIs.
HSV2 and HIV
An increasing body of literature suggests that HIV and HSV2 manifest bidirectional interactions. Clinical, biological and epidemiological studies support the hypothesis that HSV2 increases the efficiency of HIV acquisition38,39,40,41,42,43 and transmission.30,44,45,46 Similarly, HIV may increase susceptibility to HSV2, HSV2 shedding47,48,49 and, at low CD4 counts, severity of clinical manifestations.50
In terms of HIV acquisition, HSV2 can cause ulcers and microulcerations, and GUD has been associated with a 7–11‐fold risk of HIV acquisition in cohort studies.51,52,53 In a meta‐analysis of the epidemiological literature of HSV2 and the risk of HIV infection, cohort and nested case–control studies showed an increased risk of acquiring HIV in HSV2‐seropositive heterosexual men (risk ratio (RR) 2.7, 95% confidence interval (CI) 1.9 to 3.9), men who have sex with men (RR 1.7, 95% CI 1.2 to 2.4), and women from the general population (RR 3.1, 95% CI 1.7 to 5.6). Among high‐risk women, RR values ranged widely, and the summary estimate did not show a significant association (RR 1.0, 95% CI 0.5 to 2.0).38 HIV acquisition risk is highest among those with recent HSV2 infection,39,40,41,42 which may reflect the higher frequency and severity of herpetic ulcers during the early stages of HSV2.
HSV2 infection may enhance HIV and HSV2 transmission among people with coinfection. HIV RNA has been isolated from herpetic lesions, and clinically recurrent herpes and asymptomatic shedding is associated with transient increases in HIV load in plasma and genital secretions.44,45,46 These findings suggest that HSV2 reactivation may increase HIV infectiousness. Gray et al30 studied HIV transmission rates per sex act among HIV‐discordant monogamous couples in Rakai, Uganda. The two most important risk factors for HIV transmission were the presence of genital ulcers and higher HIV plasma load.30 Most GUDs in Rakai (87% of all ulcers with known aetiology) were due to HSV2.
Genital herpes in people with HIV infection
Although clinical observations suggest that patients with HIV have more frequent and prolonged episodes,54 few studies have examined the effect of HIV on the natural history of HSV2 infection. The influence of immunodeficiency on the clinical expression of HSV2 infection is complex, and although impaired T cell function is expected to increase HSV2 re‐activation rates and shedding, it may also reduce the host imflammatory response, and therefore the clinical expression of HSV2 infection. Consistent with this hypothesis, HSV2 infection has been included as a possible manifestation of the immunoreconstitution syndrome induced by highly active antiretroviral therapy.55 Available data from cohort studies among people with dual infections do not show more symptomatic herpes manifestations among HIV‐positive people but do show increased HSV2 shedding. Schacker et al47 showed that HIV status had only a modest effect on the rate and duration of clinical recurrences (0.34 v 0.23 recurrences per month among HIV‐negative people); however, the rate of subclinical shedding was markedly higher among HIV‐positive people. Ramaswamy et al56 followed up 549 HIV/HSV2‐infected people and reported that only 21% had a clinical diagnosis of genital herpes over a median 5 years of follow‐up.
Laboratory diagnosis of genital herpes: options for resource‐limited settings
Virus detection in genital swabs
Virus isolation in cell culture has long been considered the diagnostic gold standard for HSV. Specificity is virtually 100%, but levels of virus shedding, specimen quality and transport conditions influence sensitivity. Test performance declines with time since the onset of lesions and is on average 52–93% for vesicles, 41–72% for ulcers and 19–27% for crusted lesions.57 PCR increases the sensitivity of culture by 11–75%,58 and it has recently been recommended as the new diagnostic gold standard for genital herpes.59 Additional methods include detection of viral antigen by direct immunofluorescence assay using fluorescein‐labelled monoclonal antibodies on smears, or by enzyme immunoassay on swabs. Immunofluorescence is expensive and shows lower sensitivity (74%) and specificity (85%) than virus culture.60 Antigen enzyme immunoassay shows ⩾95% specificity and 62–100% sensitivity relative to virus culture.61
HSV2‐specific serology
Commercially available assays to detect antibodies to HSV2‐specific glycoprotein gG‐2 have markedly improved serological diagnosis of HSV2 infection over the past 10 years. These tests have been evaluated in industrialised countries, and their sensitivity against western blot ranged from 93% to 100%, and the specificity ranged from 95% to 100%.59 Recent evaluations of these tests have raised concerns about lower specificity with African sera.62,63,64 These findings could be due to problems of cross‐reactivity, or reflect the fact that some tests detect HSV2 seroconversions earlier than others, including western blot. It has been recommended that the threshold for calculating positive results should be increased as a way of improving specificity, although this may also reduce sensitivity in early infection.63
The role of routine laboratory investigations
The cost:benefit ratio of adopting virus detection and HSV2‐specific serology for routine management of patients attending STI clinics in developing countries remains currently uncertain. Given that the clinical diagnosis of genital herpes is typically unreliable, a laboratory confirmation is desirable. Antigen enzyme immunoassay may be an option for resource‐limited settings, as assays are commercially available and easy to perform, but the test is expensive and its sensitivity is suboptimal. Although PCR allows less stringent conditions for sample storage and transport than virus culture and can be highly automated, it requires sophisticated laboratory equipment, high‐level technical expertise and strict quality control. These conditions combined make PCR a difficult diagnostic option in most resource‐limited settings. Centralised laboratory facilities may be one method of providing testing. Although results may not be available for immediate patient management, important information is gained on local epidemiology of GUD and the validity of syndromic approaches to GUD management, thus assisting programme managers in evidence‐based decision making.
Similar considerations apply to the use of HSV‐specific serology for screening purposes, given that the tests are expensive and in many settings infection with HSV2 is nearly universal among patients with GUD. The strongest argument in favour of screening is that HSV2‐seropositive people can be counselled about the increased risk of HIV acquisition.64 Thus, the scope for using HSV2 serology to detect infected people may grow in developing countries should evidence from ongoing studies support the use of antiviral treatment against HSV2 as a strategy for HIV prevention. In this case, serological screening could be considered to identify people coinfected with both viruses who could benefit from suppressive anti‐HSV2 treatment. This might be especially important for couples with discordant HSV2/HIV serostatus.
Current treatment strategies
Antiviral treatment
For bacterial STI, treatment is usually short and aimed at both reducing the duration and severity of symptoms and eliminating the aetiological agent. For viral STI such as herpes, treatment can be either short and episodic, which will reduce the severity and duration of symptomatic recurrences, or longer‐term supressive treatment, which will reduce both the number and severity of symptomatic episodes and asymptomatic shedding. Episodic treatment in recurrent episodes reduces pain, decreases time to ulcer healing by 1–2 days and reduces HSV2 shedding by 1–2 days, although it has no effect on recurrence rates.65,66 Suppressive treatment with aciclovir can prevent or delay 80% of recurrences, and reduce clinical and subclinical shedding by >90%.67 Aciclovir's pharmaceutical patent has recently expired and the drug is now available in generic form from nine manufacturers in seven countries worldwide for <US$0.20 per tablet.68
Addition of aciclovir to the syndromic management of GUD
Previous WHO guidelines for the treatment of GUD were concerned with bacterial aetiologies and provided no suggestions for effective treatment for genital herpes. Owing to the observed increases in the worldwide prevalence of HSV2 infection, the WHO now recommends giving antiherpes treatment in places where the relative prevalence of HSV2 infection is >30%.3 Botswana is the first country in Africa to incorporate aciclovir treatment for GUD management, and the government is closely monitoring its implementation.7 However, some questions about the incorporation of antiherpes treatment into syndromic management still remain. Firstly, evidence on the effect of episodic aciclovir treatment on ulcer duration suggests that it is primarily effective when given early, ideally at ulcer onset. Under field conditions, patients attending primary healthcare facilities are likely to have a delay in symptom recognition, and thus there is a delay in initiating treatment. Treatment strategies will have to be accompanied by successful campaigns targeting early symptom recognition and prompt treatment. Secondly, unnecessary overtreatment might become more common, as syphilis and chancroid are becoming rare, and herpes is responsible for most cases of GUD. Treatment for these bacterial infections will probably have to continue for several years until syphilis and chancroid are eliminated. The WHO is currently assembling a 5‐year control initiative for genital ulcers, with the goal of eliminating chancroid and reducing the prevalence of syphilis.69 Thirdly, it is still unclear how partner notification for herpes contacts will be implemented. Although many patients are infected by asymptomatic partners, some may be taught to recognise signs and symptoms of genital herpes that were previously unrecognised. Counselling would provide an opportunity for health education, condom promotion, HIV testing and reinforcing abstention from sexual intercourse while lesions are present.70 Finally, further adjustment to algorithms for GUD management will be needed in areas with a high prevalence of HIV where delayed healing of ulcers and multiple recurrences are already an increasing problem. These patients would greatly benefit from suppressive treatment.
Trials are currently under way in South Africa, Ghana and Malawi to evaluate the incorporation of aciclovir into the syndromic management of GUD and its effect on reduction of pain, ulcer healing and HIV shedding from ulcers (table 2). The cost effectiveness of this strategy needs to be determined.
Table 2 Research Initiatives Evaluating HSV‐2 and HIV‐1 Interactions and the Use of Anti‐Herpes Therapy to Reduce Acquisition and Transmission of HIV‐1.
| Intervention | Population | Outcome | Status |
|---|---|---|---|
| HSV‐2 suppressive treatment | |||
| Acyclovir treatment to prevent HIV‐1 acquisition | 1600 HIV‐1‐negative HSV‐2‐positive women, in Zimbabwe, Zambia and Johannesburg; and, 1600 high‐risk, HIV‐1‐negative HSV‐2‐positive men who have sex with men in Peru and United States | HIV‐1 incidence | Ongoing until the end of 2006 |
| Acyclovir treatment to prevent HIV‐1 acquisition and reduce HIV‐1 vaginal shedding | 1300 HSV‐2‐positive high risk women, both HIV‐1‐positive and negative in Tanzania | HIV‐1 incidence among initially HIV‐, and HSV‐2 & HIV‐1 vaginal shedding among HIV‐1‐positive | Ongoing until 2007 |
| Acyclovir treatment to prevent HIV‐1 transmission | 3646 HIV‐1 discordant couples (HIV‐1‐positive partner is also HSV‐2‐positive and has a CD4 count of 250 cells/mm3) in Botswana, Kenya, Zambia, Rwanda, Tanzania and Uganda | HIV‐1 incidence in HIV‐1‐negative partner | Ongoing until 2007 |
| Valacyclovir treatment to reduce HIV‐1 shedding | 140 HIV‐1‐positive HSV‐2‐positive women in Burkina Faso | HIV‐1 cervico‐vaginal and plasma viral loads | Finished recruitment October 2005 |
| Acyclovir treatment to reduce HIV‐1 shedding | 300 HIV‐1‐positive HSV‐2‐positive women in South Africa | HIV‐1 and HSV‐2 cervico‐vaginal and plasma viral loads | Finished recruitment in May 2006 |
| Acyclovir treatment to reduce HIV‐1 shedding | 67 HIV‐1 and HSV‐2 infected women, Chiang Rai, Thailand | HIV‐1 and HSV‐2 cervico‐vaginal shedding | Finished recruitment in 2006 |
| Acyclovir treatment to reduce HIV‐1 shedding | 380 HIV‐1/HSV‐2 positive high‐risk women in Tanzania (part of the second study listed above) | HIV‐1 and HSV‐2 cervico‐vaginal and plasma viral loads | Finished follow‐up in October 2005 |
| HSV‐2 episodic treatment as part of syndromic management of genital ulcer disease | |||
|---|---|---|---|
| Acyclovir treatment for 5 days | 600 men HIV‐1‐positive and negative with genital ulcers in South Africa | Ulcer healing, HIV‐1 ulcer shedding | Ongoing until the end of 2006 |
| Acyclovir treatment for 5 days | 410 women HIV‐1 positive and negative with genital ulcers in Ghana and Central African Republic | Ulcer healing, HIV‐1 cervico‐vaginal and ulcer shedding | Finished recruitment in October 2005 |
| Acyclovir treatment for 5 days | 400 men and women HIV‐1 positive and negative with genital ulcers in Malawi | Ulcer healing, HIV‐1 vaginal, ulcer and semen shedding | Ongoing until July 2006 |
Treatment of genital herpes for the prevention of acquisition and transmission of HIV
As antiviral treatment can reduce virus shedding and viral loads, it has been hypothesised that they also reduce transmission of HSV2 and HIV infections. The first trial to assess the effect of antiviral treatment on the heterosexual transmission of a viral infection has been completed recently. Provision of suppressive treatment was shown to reduce HSV2 transmission. A multicentre study among monogamous, heterosexual, HSV2‐discordant couples found that a once‐daily suppressive dose of valaciclovir reduced the incidence of symptomatic genital herpes among the uninfected by 77% and the overall risk of transmission by 50%.71
Several trials are under way to evaluate the effect of antiherpes treatment in reducing HIV transmission and acquisition. These trials are considering the following public health issues:
Reducing the enhancing effect of HSV2 on HIV, by episodic treatment of genital herpes in populations with high risk of transmission or acquisition of HIV (ie patients with STI)
Supression of HSV2, to reduce susceptibility to HIV among HIV‐negative, HSV2‐seropositive people
Suppression of HSV2, to reduce infectiousness of HIV among people with HIV/HSV2 coinfection.64
Encouraging data were recently presented by Nagot et al72 showing that suppressive treatment with valaciclovir reduced HIV vaginal shedding and HIV plasma load among women with dual infections.
Other herpes interventions
Condom use
Few studies have evaluated the protection provided by condoms against genital herpes. Most cross‐sectional studies have failed to show an association and even suggest increased risk among men using condoms,73 probably because condom use is a marker of high‐risk relationships. One prospective study reported protection from condom use only among women.74 A more recent study showed that participants reporting more frequent condom use (condom use for >75% of sexual acts) were at a lower risk of acquiring HSV2 (hazard ratio 0.7, 95% CI 0.6 to 0.9).75
HSV2 vaccines
Although animal studies on vaccination strategies to prevent genital and neonatal herpes may be promising, clinical trials of HSV2 vaccines in humans have failed to prove efficacy.1 In a recent study, an HSV2 glycoprotein D vaccine using alum morpholine as adjuvant induced protection from clinical disease (73%) and overall HSV2 transmission (about 40%).76 By contrast, no protective efficacy was observed with a similar vaccine based on the viral glycoproteins B and D and using MF59 as adjuvant.77 The protective effect of the MPL vaccine was seen only in women who were HSV1 and HSV2 seronegative, and there was no protection among men or among HSV1‐seropositive women.76 The partial efficacy was shown to be related to the induction of T cell immunity with a T helper 1 profile. The vaccine is currently being evaluated in a large multicentre trial targeting pre‐pubertal girls.
Discussion
HSV2 is highly prevalent in most regions, especially those experiencing HIV epidemics, and it has become the most important cause of GUD worldwide. In addition to the suffering and expense incurred by people with HSV2 infections, substantial data support an important role of HSV2 in facilitating HIV transmission and acquisition.
As with all STIs, the most cost‐effective intervention is primary prevention. However, STI control has been successful only in a limited number of settings, and primary prevention programmes have obstacles of acceptance and effectiveness among populations who are both vulnerable and at risk. Given the epidemiology of HSV2 acquisition as primarily an incident infection among young people, we should undoubtedly be focusing primary prevention efforts on this age group, especially in resource‐poor settings where incidence and prevalence remain highest. Primary prevention should include delaying sexual debut among adolescents and reducing the number of sexual partners. Male condoms protect against HSV2 less effectively than against HIV, but still have some efficacy.74 Given the strong efficacy of condoms in protecting against HIV and other STIs, promotion of condom use should always be included in any STI prevention effort.
Once infected with HSV2, a lifelong viral infection requires different management strategies compared with managing “one‐off” bacterial infections. Although conventional wisdom may have mitigated against the cost effectiveness of trying to control a recurrent infection in resource‐poor settings, increasing evidence of the interaction between HSV2 infection and the risk of HIV transmission, and the increasing contribution of HSV2 to the burden of GUD, has raised the salience of HSV2. Whether providing suppressive or episodic antiviral treatment, more attention should be focused on ensuring that people infected with HSV2 have access to appropriate and effective care. Services should be part of a prevention package including HIV testing and counselling to all patients with STI, counselling to improve recognition of herpes symptoms, early initiation of episodic aciclovir treatment and counselling on condom use, and abstaining from sexual intercourse during herpes outbreaks.
Prevention and treatment strategies should be first targeted at people with genital herpes lesions. Although these may be a small proportion of those who are HSV2 seropositive, people with lesions are at highest risk of transmitting the virus. Questions on the effect of antiherpes treatment in preventing HIV transmission and acquisition remain unanswered. Ongoing trials will assist in filling these knowledge gaps and will provide the evidence required to design new interventions for herpes. In countries in sub‐Saharan Africa, where the prevalence of HSV2 is extremely high, the provision of aciclovir for people with genital ulcers who are being treated syndromically might represent the most cost‐effective and easiest means to implement a strategy for HIV prevention.
While the inclusion of herpes treatment in syndromic management is further evaluated on the basis of current WHO and US STI management recommendations,2,72 aciclovir should be made available for patients with primary herpes (although hard to identify in developing countries), those with severe episodes, immunosuppressed patients and patients with frequent recurrences. Developing countries should also consider adding aciclovir to the essential drug list. Further, with the help of WHO, aciclovir should be made available and affordable in developing countries.
Abbreviations
GUD - genital ulcer disease
HSV - herpes simplex virus
PCR - polymerase chain reaction
STI - sexually transmitted infection
WHO - World Health Organization
Footnotes
Competing interests: None declared.
References
- 1.World Health Organization Herpes simplex virus type 2 programmatic and research priorities in developing countries. WHO/HIV_AIDS/2001.05. Report of a WHO/UNAIDS/LSHTM Workshop, 14–16 February 2001, London
- 2.Centers for Disease Control and Prevention Sexually Transmitted Diseases Treatment Guidelines. 2002;51 [Google Scholar]
- 3.World Health Organization Guidelines for the management of sexually transmitted infections. Revised version. WHO/RHI/01.10 2003. Geneva: WHO, 2003
- 4.Fleming D T, McQuillan G M, Johnson R E.et al Herpes simplex virus type 2 in the United States, 1976 to 1994. N Engl J Med 19973371105–1111. [DOI] [PubMed] [Google Scholar]
- 5.Pebody R G, Andrews N, Brown D.et al The seroepidemiology of herpes simplex virus type 1 and 2 in Europe. Sex Transm Infect 200480185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss H. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes 200411A24–A35. [PubMed] [Google Scholar]
- 7.Paz‐Bailey G, Rahman M, Chen C.et al Changes in the etiology of sexually transmitted diseases in Botswana between 1993 and 2002: implications for the clinical management of genital ulcer disease. Clin Infect Dis 2005411304–1312. [DOI] [PubMed] [Google Scholar]
- 8.Plummer F A, D'Costa L J, Nsanze H.et al Clinical and microbiologic studies of genital ulcers in Kenyan women. Sex Transm Dis 198512193–197. [DOI] [PubMed] [Google Scholar]
- 9.Greenblatt R M, Lukehart S A, Plummer F A.et al Genital ulceration as a risk factor for human immunodeficiency virus infection. AIDS 1988247–50. [DOI] [PubMed] [Google Scholar]
- 10.Ndinya‐Achola J O, Kihara A N, Fisher L D.et al Presumptive specific clinical diagnosis of genital ulcer disease in a primary health care setting in Nairobi. Int J STD AIDS 19967201–205. [DOI] [PubMed] [Google Scholar]
- 11.Malonza I M, Tyndall M W, Ndinya‐Achola J O.et al A randomized, double‐blind, placebo‐controlled trial of single‐dose ciprofloxacin versus erythromycin for the treatment of chancroid in Nairobi, Kenya. J Infect Dis 19991801886–1893. [DOI] [PubMed] [Google Scholar]
- 12.Morse S A, Trees D L, Htun Y.et al Comparison of clinical diagnosis and standard laboratory and molecular methods for the diagnosis of genital ulcer disease in Lesotho: association with human immunodeficiency virus infection. J Infect Dis 1997175583–589. [DOI] [PubMed] [Google Scholar]
- 13.Behets F M, Liomba G, Lule G.et al Sexually transmitted diseases and human immunodeficiency virus control in Malawi: a field study of genital ulcer disease. J Infect Dis 1995171451–455. [DOI] [PubMed] [Google Scholar]
- 14.Hoyo C, Hoffman I, Moser B K.et al Improving the accuracy of syndromic diagnosis of genital ulcer disease in Malawi. Sex Transm Dis 200532231–237. [DOI] [PubMed] [Google Scholar]
- 15.Behets F M, Andriamiadana J, Randrianasolo D.et al Chancroid, primary syphilis, genital herpes, and lymphogranuloma venereum in Antananarivo, Madagascar. J Infect Dis 19991801382–1385. [DOI] [PubMed] [Google Scholar]
- 16.Bogaerts J, Ricart C A, Van Dyck E.et al The etiology of genital ulceration in Rwanda. Sex Transm Dis 198916123–126. [DOI] [PubMed] [Google Scholar]
- 17.Bogaerts J, Kestens L, van Dyck E.et al Genital ulcers in a primary health clinic in Rwanda: impact of HIV infection on diagnosis and ulcer healing (1986–1992). Int J STD AIDS 19989706–710. [DOI] [PubMed] [Google Scholar]
- 18.Totten P A, Kuypers J M, Chen C Y.et al Etiology of genital ulcer disease in Dakar, Senegal, and comparison of PCR and serologic assays for detection of Haemophilus ducreyi. J Clin Microbiol 200038268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dangor Y, Ballard R C, da L Exposto F.et al Accuracy of clinical diagnosis of genital ulcer disease. Sex Transm Dis 199017184–189. [DOI] [PubMed] [Google Scholar]
- 20.Lai W, Chen C Y, Morse S A.et al Increasing relative prevalence of HSV2 infection among men with genital ulcers from a mining community in South Africa. Sex Transm Infect 200379202–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen C Y, Ballard R C, Beck‐Sague C M.et al Human immunodeficiency virus infection and genital ulcer disease in South Africa: the herpetic connection. Sex Transm Dis 20002721–29. [DOI] [PubMed] [Google Scholar]
- 22.Coovadia Y M, Kharsany A, Hoosen A. The microbial aetiology of genital ulcers in black men in Durban, South Africa. Genitourin Med 198561266–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Farrell N, Hoosen A A, Coetzee K D.et al Genital ulcer disease in men in Durban, South Africa. Genitourin Med 199167327–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Farrell N, Hoosen A A, Coetzee K D.et al Genital ulcer disease in women in Durban, South Africa. Genitourin Med 199167322–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moodley P, Sturm P D, Connolly C.et al Identification of women at high STD risk among STD clinic attendees: implications for STD programmes. Int J STD AIDS 200314526–531. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed H J, Mbwana J, Gunnarsson E.et al Etiology of genital ulcer disease and association with human immunodeficiency virus infection in two tanzanian cities. Sex Transm Dis 200330114–119. [DOI] [PubMed] [Google Scholar]
- 27.Mwansasu A, Mwakagile D, Haarr L.et al Detection of HSV‐2 in genital ulcers from STD patients in Dar es Salaam, Tanzania. J Clin Virol 200224183–192. [DOI] [PubMed] [Google Scholar]
- 28.Mabey D C, Wall R A, Bello C S. Aetiology of genital ulceration in the Gambia. Genitourin Med 198763312–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamya M R, Nsubuga P, Grant R M.et al The high prevalence of genital herpes among patients with genital ulcer disease in Uganda. Sex Transm Dis 199522351–354. [DOI] [PubMed] [Google Scholar]
- 30.Gray R H, Wawer M J, Sewankambo N K.et al Relative risks and population attributable fraction of incident HIV associated with symptoms of sexually transmitted diseases and treatable symptomatic sexually transmitted diseases in Rakai District, Uganda. Rakai Project Team. AIDS 1999132113–2123. [DOI] [PubMed] [Google Scholar]
- 31.Risbud A, Chan‐Tack K, Gadkari D.et al The etiology of genital ulcer disease by multiplex polymerase chain reaction and relationship to HIV infection among patients attending sexually transmitted disease clinics in Pune, India. Sex Transm Dis 19992655–62. [DOI] [PubMed] [Google Scholar]
- 32.Zainah S, Cheong Y M, Sinniah M.et al A microbiological study of genital ulcers in Kuala Lumpur. Med J Malaysia 199146274–282. [PubMed] [Google Scholar]
- 33.Beyrer C, Jitwatcharanan K, Natpratan C.et al Molecular methods for the diagnosis of genital ulcer disease in a sexually transmitted disease clinic population in northern Thailand: predominance of herpes simplex virus infection. J Infect Dis 1998178243–246. [DOI] [PubMed] [Google Scholar]
- 34.Sanchez J, Volquez C, Totten P A.et al The etiology and management of genital ulcers in the Dominican Republic and Peru. Sex Transm Dis 200229559–567. [DOI] [PubMed] [Google Scholar]
- 35.Behets F M, Brathwaite A R, Hylton‐Kong T.et al Genital ulcers: etiology, clinical diagnosis, and associated human immunodeficiency virus infection in Kingston, Jamaica. Clin Infect Dis 1999281086–1090. [DOI] [PubMed] [Google Scholar]
- 36.Korenromp E L, Bakker R, De Vlas S J.et al Can behavior change explain increases in the proportion of genital ulcers attributable to herpes in sub‐Saharan Africa? A simulation modeling study. Sex Transm Dis 200229228–238. [DOI] [PubMed] [Google Scholar]
- 37.Boily M C, Lowndes C M, Gregson S. Population‐level risk factors for HIV transmission and “the 4 Cities Study”: temporal dynamics and the significance of sexual mixing patterns. AIDS 2002162101–2102. [DOI] [PubMed] [Google Scholar]
- 38.Freeman E E, Weiss H A, Glynn J R.et al Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta‐analysis of longitudinal studies. AIDS 20062073–83. [DOI] [PubMed] [Google Scholar]
- 39.del Mar Pujades Rodriguez M, Obasi A, Mosha F.et al Herpes simplex virus type 2 infection increases HIV incidence: a prospective study in rural Tanzania. AIDS 200216451–462. [DOI] [PubMed] [Google Scholar]
- 40.Ramjee G, Williams B, Gouws E.et al The impact of incident and prevalent herpes simplex virus‐2 infection on the incidence of HIV‐1 infection among commercial sex workers in South Africa. J Acquir Immune Defic Syndr 200539333–339. [DOI] [PubMed] [Google Scholar]
- 41.Reynolds S J, Risbud A R, Shepherd M E.et al Recent herpes simplex virus type 2 infection and the risk of human immunodeficiency virus type 1 acquisition in India. J Infect Dis 20031871513–1521. [DOI] [PubMed] [Google Scholar]
- 42.McFarland W, Gwanzura L, Bassett M T.et al Prevalence and incidence of herpes simplex virus type 2 infection among male Zimbabwean factory workers. J Infect Dis 19991801459–1465. [DOI] [PubMed] [Google Scholar]
- 43.Holmberg S D, Stewart J A, Gerber A R.et al Prior herpes simplex virus type 2 infection as a risk factor for HIV infection. JAMA 19882591048–1050. [PubMed] [Google Scholar]
- 44.Schacker T, Ryncarz A J, Goddard J.et al Frequent recovery of HIV‐1 from genital herpes simplex virus lesions in HIV‐1‐infected men. JAMA 199828061–66. [DOI] [PubMed] [Google Scholar]
- 45.Mole L, Ripich S, Margolis D.et al The impact of active herpes simplex virus infection on human immunodeficiency virus load. J Infect Dis 1997176766–770. [DOI] [PubMed] [Google Scholar]
- 46.Schacker T, Zeh J, Hu H.et al Changes in plasma human immunodeficiency virus type 1 RNA associated with herpes simplex virus reactivation and suppression. J Infect Dis 20021861718–1725. [DOI] [PubMed] [Google Scholar]
- 47.Schacker T, Zeh J, Hu H L.et al Frequency of symptomatic and asymptomatic herpes simplex virus type 2 reactivations among human immunodeficiency virus‐infected men. J Infect Dis 19981781616–1622. [DOI] [PubMed] [Google Scholar]
- 48.Wright P W, Hoesley C J, Squires K E.et al A prospective study of genital herpes simplex virus type 2 infection in human immunodeficiency virus type 1 (HIV‐1)‐seropositive women: correlations with CD4 cell count and plasma HIV‐1 RNA level. Clin Infect Dis 200336207–211. [DOI] [PubMed] [Google Scholar]
- 49.Mbopi‐Keou F X, Gresenguet G, Mayaud P.et al Interactions between herpes simplex virus type 2 and human immunodeficiency virus type 1 infection in African women: opportunities for intervention. J Infect Dis 20001821090–1096. [DOI] [PubMed] [Google Scholar]
- 50.Siegal F P, Lopez C, Hammer G S.et al Acquired immunodeficiency in male homosexuals manifested by chronic perianal ulcerative herpes simplex lesions. N Engl J Med 19813051439–1444. [DOI] [PubMed] [Google Scholar]
- 51.Royce R A, Sena A, Cates W., Jret al Sexual transmission of HIV. N Engl J Med 19973361072–1078. [DOI] [PubMed] [Google Scholar]
- 52.Cameron D W, Simonsen J N, D'Costa L J.et al Female to male transmission of human immunodeficiency virus type 1: risk factors for seroconversion in men. Lancet 19892403–407. [DOI] [PubMed] [Google Scholar]
- 53.Mehendale S M, Rodrigues J J, Brookmeyer R S. Incidence and predictors of human immunodeficiency virus type 1 seroconversion in patients attending sexually transmitted disease clinics in India. J Infect Dis 19951721486–1491. [DOI] [PubMed] [Google Scholar]
- 54.Krause P, Straus S. The treatment, management and prevention of genital herpes. In: Stanberry L, ed. Genital and neonatal herpes. 1st edn. Sussex, UK: Wiley, 1996
- 55.Puthanakit T, Oberdorfer P, Akarathum N.et al Immune reconstitution syndrome after HAART in human immunodeficiency virus‐infected Thai children. Pediatr Infect Dis J 20062553–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramaswamy M, Sabin C, McDonald C.et al Herpes simplex virus type 2 (HSV‐2) seroprevalence at the time of HIV‐1‐1. Diagnosis and seroincidence after HIV‐1‐1. Diagnosis in an ethnically diverse cohort of HIV‐1‐1‐infected persons. Sex Transm Dis 20063396–101. [DOI] [PubMed] [Google Scholar]
- 57.Scoular A, Gillespie G, Carman W F. Polymerase chain reaction for diagnosis of genital herpes in a genitourinary medicine clinic. Sex Transm Infect 20027821–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramaswamy M, McDonald C, Smith M.et al Diagnosis of genital herpes by real time PCR in routine clinical practice. Sex Transm Infect 200480406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Geretti A M, Brown D W. National survey of diagnostic services for genital herpes. Sex Transm Infect 200581316–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lafferty W E, Krofft S, Remington M.et al Diagnosis of herpes simplex virus by direct immunofluorescence and viral isolation from samples of external genital lesions in a high‐prevalence population. J Clin Microbiol 198725323–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Slomka M J, Emery L, Munday P E.et al A comparison of PCR with virus isolation and direct antigen detection for diagnosis and typing of genital herpes. J Med Virol 199855177–183. [PubMed] [Google Scholar]
- 62.Laeyendecker O, Henson C, Gray R H.et al Performance of a commercial, type‐specific enzyme‐linked immunosorbent assay for detection of herpes simplex virus type 2‐specific antibodies in Ugandans. J Clin Microbiol 2004421794–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Dyck E, Buve A, Weiss H A.et al Performance of commercially available enzyme immunoassays for detection of antibodies against herpes simplex virus type 2 in African populations. J Clin Microbiol 2004422961–2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Celum C L, Robinson N J, Cohen M S. Potential effect of HIV type 1 antiretroviral and herpes simplex virus type 2 antiviral therapy on transmission and acquisition of HIV type 1 infection. J Infect Dis 2005191S107–S114. [DOI] [PubMed] [Google Scholar]
- 65.Nilsen A E, Aasen T, Halsos A M.et al Efficacy of oral acyclovir in the treatment of initial and recurrent genital herpes. Lancet 19822571–573. [DOI] [PubMed] [Google Scholar]
- 66.Reichman R C, Badger G J, Mertz G J.et al Treatment of recurrent genital herpes simplex infections with oral acyclovir. A controlled trial. JAMA 19842512103–2107. [PubMed] [Google Scholar]
- 67.Wald A, Corey L. Acyclovir depresses subclinical shedding of herpes simplex virus. Ann Intern Med 1996125776–777. [DOI] [PubMed] [Google Scholar]
- 68.World Health Organization Sources and price of selected drugs and diagnostics for people living with HIV/AIDS. Geneva: WHO, 2002
- 69.Schmid G, Steen R, N'Dowa F. Control of bacterial sexually transmitted diseases in the developing world is possible. Clin Infect Dis 2005411313–1315. [DOI] [PubMed] [Google Scholar]
- 70.O'Farrell N. Increasing prevalence of genital herpes in developing countries: implications for heterosexual HIV transmission and STD control programmes. Sex Transm Infect 199975377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Corey L, Wald A, Patel R.et al Once daily valaciclovir to reduce the risk of transmission of genital herpes. N Engl J Med 200435011–20. [DOI] [PubMed] [Google Scholar]
- 72.Nagot N, Ouedraogo A, Mayaud P.et al Effect of HSV‐2 suppressive therapy on HIV‐1 genital shedding and plasma viral load: a proof of concept randomized double‐blind placebo controlled trial (ANRS 1285 Trial) [abstract 33LB]. 13th Conference on Retroviruses and Opportunistic Infections, 5–8 February 2006, Denver, Colorado
- 73.National Institute of Allergy and Infectious Diseases, National Institutes of Health and Department of Health and Human Services Workshop summary: scientific evidence on condom effectiveness for sexually transmitted disease (STD) prevention. Herndon, VA: 12–13 June 2000. http://niaid.nih.gov/dmid/stds/condomreport (accessed 10 Oct 2006)
- 74.Wald A, Langenberg A G, Link K.et al Effect of condoms on reducing the transmission of herpes simplex virus type 2 from men to women. JAMA 20012853100–3106. [DOI] [PubMed] [Google Scholar]
- 75.Wald A, Langenberg A G, Krantz E.et al The relationship between condom use and herpes simplex virus acquisition. Ann Intern Med 2005143707–713. [DOI] [PubMed] [Google Scholar]
- 76.Stanberry L R, Spruance S L, Cunningham A L.et al Glycoprotein‐D‐adjuvant vaccine to prevent genital herpes. N Engl J Med 20023471652–1661. [DOI] [PubMed] [Google Scholar]
- 77.Corey L, Langenberg A G, Ashley R.et al Recombinant glycoprotein vaccine for the prevention of genital HSV‐2 infection; two randomized controlled trials. JAMA 1999281331–340. [DOI] [PubMed] [Google Scholar]