Abstract
Background
HIV‐positive patients treated for syphilis may be at increased risk for serological failure.
Objective
To compare follow‐up serologies and serological responses to treatment between HIV‐positive and HIV‐negative patients attending two sexually transmitted disease (STD) clinics.
Study design
Existing records were reviewed from HIV‐positive patients who were diagnosed and treated for syphilis at the public STD clinics in Baltimore, Maryland, USA, between 1992 and 2000. Results of their serological follow‐up were compared with those of HIV‐negative clinic patients at the time of syphilis treatment. Failure was defined as lack of a fourfold drop in rapid plasma reagin (RPR) titre by 400 days after treatment or a fourfold increased titre between 30 and 400 days.
Results
Of the 450 HIV‐positive patients with syphilis, 288 (64%) did not have documented follow‐up serologies and 129 (28.5%) met the inclusion criteria; 168 (17%) of 1000 known HIV‐negative patients were similarly eligible. There were 22 failures in the HIV‐positive group and 5 in the HIV‐negative group (p<0.001). The median times to successful serological responses in both groups were 278 (95% confidence interval (CI) 209 to 350) and 126 (95% CI 108 to 157) days, respectively (p<0.001). A multivariate Cox's proportional hazards model showed an increased risk of serological failure among the HIV‐positive patients (hazards ratio 6.0, 95% CI 1.5 to 23.9; p = 0.01).
Conclusion
HIV‐positive patients treated for syphilis may be at higher risk of serological failure. Despite recommendations for more frequent serological follow‐up, most patients did not have documentation of serological response after standard treatment for syphilis.
The interaction between syphilis and HIV is complex and not fully understood. The prevalence of syphilis is higher in HIV‐postive patients,1,2,3 HIV has been identified in primary syphilitic ulcers4 and HIV viral load has been shown to increase during primary and secondary syphilis,5,6 thus confirming the biological plausibility of epidemiological observations that syphilis facilitates the transmission and acquisition of HIV. Although dramatic and unusual presentations of syphilis have been reported among HIV‐coinfected patients,7,8 larger studies report no major differences in the presentation of syphilis and its response to treatment.9 A troubling observation in the largest treatment trial of early syphilis was higher serological failure rates among HIV‐positive patients than among HIV‐negative patients after treatment for primary or secondary syphilis, but the reverse after treatment for early‐latent syphilis, and an overall serological failure rate of 14% at 1 year.10 In a recent cohort study that compared syphilis serological titre responses after treatment in Lima, Perú,11 HIV status (n = 11) had no effect on the rate of serological response. Regardless of HIV status, 17% of patients required additional treatment. Recent data also indicate higher rates of neurosyphilis among HIV‐positive patients, but do not clarify whether this is a result of more rapid progression of syphilis in HIV or therapeutic failure.12 Syphilis treatment regimens at this time do not differ for HIV‐positive patients, but the Centers for Disease Control and Prevention recommends more aggressive follow‐up in those who are coinfected.13 Our goal was to compare the number of follow‐up serologies and serological responses to syphilis treatment between HIV‐positive and HIV‐negative patients in a clinical setting with a high prevalence of both infections.
Methods
Data collection and definitions
We analysed electronic data records from patients diagnosed with primary, secondary, early‐latent and late‐latent syphilis who attended the Baltimore sexually transmitted disease (STD) clinics between 1992 and 2000. Diagnoses were made by trained clinicians on the basis of published criteria.14 The rapid plasma reagin (RPR) test was used, and reactive specimens were confirmed using the fluorescence treponemal antibody absorption test. Primary syphilis with non‐reactive serologies at the time of treatment was excluded, as this study focused on serological responses. This study was approved by the institutional review boards of the participating institutions.
Information on demographics, behavioural risks, syphilis stage and treatment was collected on standardised clinical encounter forms, scanned on to a database, and linked to laboratory results. We also reviewed additional serological test results from a statewide syphilis registry that included results from public and private testing venues throughout Maryland.
Patients were HIV negative if they had a non‐reactive HIV test at the time of syphilis diagnosis or any visit thereafter, and they were HIV postive if a test was reactive at or before the time of syphilis diagnosis. Records from patients whose HIV serologies were undocumented were excluded. Records with no HIV result documented, no initial RPR titre or no follow‐up titre within 400 days of treatment were also excluded.
Serological failure was defined as a fourfold rise in RPR titres 30–400 days after treatment or lack of a fourfold drop in RPR titres at 270–400 days, and no evidence of reinfection according to records of disease intervention specialists (DISs). DISs are trained public health workers who find and interview patients who have positive syphilis serologies. The records include detailed behavioural information on the index patient and their sex partners. Those with low pretreatment baseline titres (⩽1/2) who did not serorevert and had no clinical evidence of failure were not considered serological failures. We used the 400‐day cut‐off based on preliminary data analysis indicating capture of most patients returning for their 12‐month follow‐up serologies. Similarly, although we were interested in serological failures occurring 1 year after treatment, records with follow‐up serologies obtained 270–400 days after treatment met our criteria. DIS records were reviewed on all patients who did not respond to treatment, and patients whose failure was deemed secondary to reinfection were excluded.
We compared the time to serological response between HIV‐postive and HIV‐negative patients. Time to serological response was defined as the earliest date after treatment documenting a fourfold drop in RPR titres. Patients whose titres had not dropped fourfold and whose last follow‐up titres were <270 days from the date of treatment were censored at the date of last follow‐up serology.
Data analysis
Time‐to‐event statistical models were used. We constructed Kaplan–Meier curves and used the non‐parametric log rank test to evaluate the equality of the “survival” functions. Cox's proportional hazards models were used after testing the proportional hazards assumption using both the re‐estimation and the Schoenfeld residuals methods. Variables were included in the final multivariate model if their univariate Wald's test p value was <0.2 or they were biologically relevant. Cox–Snell residuals were used to assess the overall model fit. The χ2 test was used to compare independent proportions, the K equality of medians test was used to compare independent median values, and the independent t test was used to compare mean values; p<0.05 was considered to be significant. Data were analysed using Stata/SE 8.2 for Windows.
Results
We found 3607 records documenting primary, secondary, early‐latent or late‐latent syphilis or syphilis of unknown duration between 2 January 1992 and 31 December 2000. We excluded 196 (5.4%) records documenting primary syphilis on the basis of negative serologies but positive dark‐field microscopy findings, and 1613 (44.7%) records without documentation of HIV serostatus, leaving records of 450 (12.5%) HIV‐positive patients and 1348 (37.4%) HIV‐negative patients. Of the HIV‐positive patients, 286 (63.6%) had no documented follow‐up serologies available and 35 (7.8%) had follow‐up serological titres obtained >400 days after treatment. Of the 1348 HIV‐negative patients, 1000 patients were randomly selected, of whom 768 (76.8%) did not have documented follow‐up serologies and 64 (6.4%) had follow‐up serological titres obtained >400 days after treatment. Thus, we included records from 129 HIV‐positive and 168 HIV‐negative patients.
Table 1 gives the baseline characteristics of eligible patients and those excluded. HIV‐positive patients were more likely to report having had >5 sex partners in the past month (p<0.001), to have late‐latent or unknown‐duration syphilis (p<0.001), and to have lower (<1/16) RPR titres (p<0.01). HIV‐negative patients were more likely to report known contact with a syphilis‐infected partner (p<0.001) and a history of syphilis infection (p<0.01). HIV‐positive patients had more follow‐up RPR serological values than HIV‐negative patients (2.1 v 1.5, p<0.001).
Table 1 Baseline characteristics of patients in the three groups: HIV‐positive, HIV‐negative and patients excluded from the analysis.
| HIV positive (n = 129) | HIV negative (n = 168) | p Value* | Excluded (n = 3310) | |
|---|---|---|---|---|
| Mean (range) number of follow‐up RPR | 2.12 (2–9) | 1.47 (1–5) | 0.001 | NA |
| Women (%) | 60 | 53 | NS | 43 |
| Median (IQR) age (years) | 34 (30–45) | 33 (27–40) | NS | 36 (27–42) |
| Race (%) | ||||
| Black | 97 | 98 | NS | 96 |
| Other | 3 | 2 | 4 | |
| Reason for clinic visit (%)* | ||||
| Symptoms | 22 | 29 | NS | 23 |
| Known contact with syphilis | 10 | 32 | <0.001 | 13 |
| Positive syphilis serology | 22 | 32 | NS | 29 |
| No of partners in the past month (%) | ||||
| 1 | 37 | 54 | <0.01 | 74 |
| 2–5 | 10 | 26 | <0.01 | 23 |
| >5 | 53 | 20 | <0.001 | 3 |
| Previous STI (%) | 93 | 81 | NS | 88 |
| History of syphilis (%) | 16 | 30 | <0.01 | 15 |
| Current stage of syphilis (%) | ||||
| Early | 28 | 92 | <0.001 | 45 |
| Late‐latent or unknown duration | 67 | 8 | <0.001 | 55 |
| RPR titres % | ||||
| <1/16 | 37 | 8 | <0.01 | 30 |
| 1/16–1/64 | 33 | 29 | NS | 33 |
| >1/64 | 30 | 62 | <0.01 | 37 |
| Treatment % | ||||
| BPG | 96 | 98 | NS | 98 |
| DOXY | 4 | 2 | 2 | |
*Multiple answers were possible; p values based on the χ2 test for all comparisons except mean age which was compared using independent t test.
BPG, benzathine penicillin G; DOXY, doxycycline; NA, not applicable; NS, not significant; IQR, interquartile range; STI, sexually transmitted infection; RPR, rapid plasma reagin test.
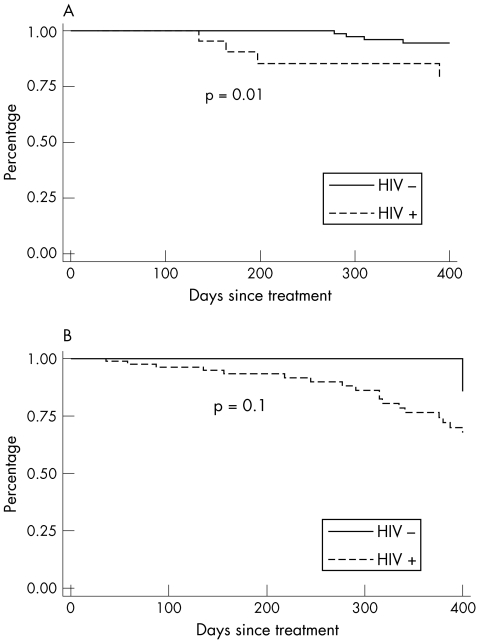
There were 29 serological failures among the HIV‐positive patients and seven failures among the HIV‐negative patients. After reviewing DIS records, seven failures among the HIV‐coinfected patients and two failures among the HIV‐negative patients were documented to be due to reinfection and were excluded from the analysis. Table 2 describes the remaining patients with serological failure. We found 22 (17%) failures among the HIV‐positive patients and 5 (3%) failures among the HIV‐negative patients (p<0.001). When stratified by syphilis stage, 4 (12.5%) of 32 HIV‐positive patients with early syphilis had serological failure compared with 4 (2.6%) of the 154 HIV‐negative (p<0.01); 18 (18.6%) of 97 HIV‐positive patients with late‐latent or unknown‐duration syphilis had evidence of serological failure compared with 1 (7.1%) of 14 HIV‐negative patients (p = 0.3).
Table 2 Characteristics of patients with serological failure among HIV‐positive and HIV‐negative patients.
| HIV positive (n = 22/129) | HIV negative (n = 5/168) | p Value | |
|---|---|---|---|
| Failures by syphilis stage, n (%) | |||
| Early | 4/32 (12.5) | 4/154 (2.6) | 0.01* |
| Late or unknown duration | 18/97 (18.6) | 1/14 (7.1) | 0.3* |
| Failure of fourfold drop, n (%) | 12/22 (55) | 3/5 (60) | NS* |
| Early | 1/4 (25) | 2/4 (50) | NS* |
| Late or unknown duration | 11/18 (61) | 1/1 (100) | NS* |
| Median initial RPR titre | 1/8 | 1/8 | NS‡ |
| Initial documented fourfold drop with subsequent fourfold increase (%) | 0 (0) | 0 (0) | NS* |
| Fourfold increase without drop (%) | 10/22 (45) | 2/5 (40) | NS* |
| Early | 3/4 (75) | 2/4 (50) | NS* |
| Late or unknown | 7/18 (39) | 0/1 (0) | NS* |
| Median initial RPR titre | 1/64 | 1/32 | NS† |
*χ2 test.
†K equality of medians test.
RPR, rapid plasma reagin; NS, not significant (p>0.05).
Among patients with early‐stage disease, 3.1% (1/32) of HIV‐positive and 1.3% (2/154) of HIV‐negative patients failed as a result of lack of a fourfold drop in titre (p = 0.5) and 9.4% (3/32) of HIV‐positive and 1.3% (2/154) of HIV‐negative patients failed as a result of fourfold increase in titre (p<0.05). Among patients with late‐latent disease, 11.3% (11/97) of HIV‐positive and 7.1% (1/14) of HIV‐negative patients failed as a result of lack of a fourfold drop in titre (p = 0.6) and 7.2% (7/97) of HIV‐positive and 0% (0/14) of HIV‐negative patients failed as a result of fourfold increase in titre (p = 0.4). None who failed showed evidence of an early interval drop in titres followed by a fourfold rise (relapsers).
Figure 1 shows the Kaplan–Meier curves comparing time to serological failure between HIV‐positive and HIV‐negative patients, stratified by syphilis stage. Time to failure was significantly longer in the HIV‐positive group with early syphilis (log rank p = 0.01); this difference was not seen in the patients with late‐latent infection. In a multivariable Cox's proportional hazards model adjusting for age, history of syphilis, baseline RPR titre, number of follow‐up RPR titres, type of treatment (doxycycline v benzathine penicillin G) and syphilis stage, the risk of serological failure was higher among the HIV‐positive patients (hazards ratio (HR) 6.0, 95% confidence interval (CI) 1.5 to 23.9) and lower among those whose baseline RPR titres were ⩾1/64 (HR 0.03, 95% CI 0.001 to 0.8).
Figure 1 Kaplan–Meier curves comparing time to serological failure between HIV‐positive and HIV‐negative patients with (A) early (primary, secondary or early‐latent) syphilis and (B) late‐latent syphilis or syphilis of unknown duration.
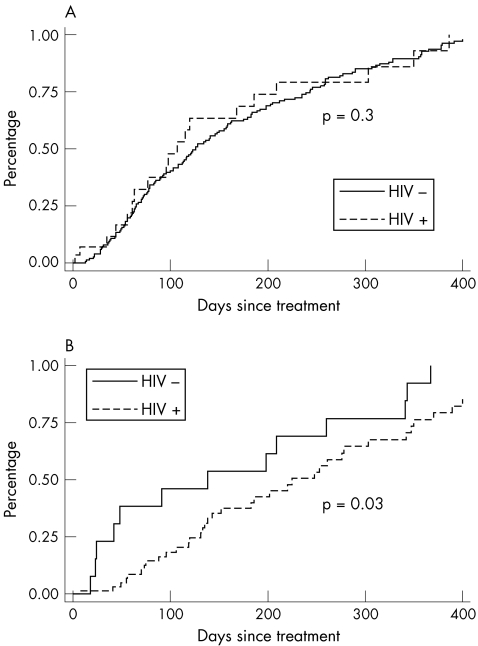
Overall, among patients with documented serological response to treatment (fig 2), the median time to response between the HIV‐positive and HIV‐negative patients was 278 (95% CI 209 to 350) and 126 days (95% CI 108 to 157), respectively (p<0.001). When stratified by syphilis stage, we found no difference in time to serological responses between the two groups with early syphilis (log rank p = 0.3; median time to response 168 (95% CI 77 to 350) v 126 days (95% CI 108 to 155), respectively). However, HIV‐positive patients with late‐latent infection had a slower response time to treatment than HIV‐negative patients (log rank p = 0.03; median time to response 342 (95% CI 248 to 370) v 138 days (95% CI 24 to 341), respectively).
Figure 2 Kaplan–Meier curves comparing time to serological response between HIV‐positive and HIV‐negative patients with (A) early (primary, secondary or early‐latent) syphilis and (B) late‐latent syphilis or syphilis of unknown duration.
Discussion
Our evaluation of outcomes in a clinical cohort of patients with syphilis highlights two important findings: (1) HIV‐positive patients with early‐stage syphilis are more likely to experience serological failure after syphilis treatment than HIV‐negative patients; and (2) routine follow‐up of serological response after treatment is equally poor in both groups.
Our findings on patients with early syphilis are consistent with those reported by others.10,15,16,17 In the most rigorously designed study,10 HIV‐coinfected patients with primary syphilis were more likely to experience serological failure than their HIV‐negative counterparts (22% v 8%, respectively) 6 months after treatment, as well as a trend towards increased odds of failure among coinfected patients with secondary syphilis (23% v 10%).
We observed a trend for increased failure in patients with late‐latent syphilis or syphilis of unknown duration among the coinfected patients compared with the HIV‐negative patients (18.6% v 7.1%, respectively). The relatively few HIV‐negative patients with late‐stage disease may have limited the power of our study to detect such differences. In the multivariate Cox's proportional hazards model, stage of syphilis was not an independent predictor of failure. The distinction between serological failure and clinical failure is important. Of the patients who had evidence of serological failure and who were followed at the STD clinics (10/27), none had documented evidence of clinical failure.
Although some studies reported a slower serological response in HIV‐positive patients compared with HIV‐negative patients,10,16 other studies did not.18 Thus, higher failure rates in HIV‐positive patients may be the result of slower declines in non‐treponemal serologies rather than a lack of response to treatment. Among the patients who failed in our study, equal proportions in the HIV‐positive and HIV‐negative groups failed because of a lack of a fourfold drop in serological titres, suggesting that the higher failure rates among HIV‐positive patients in our study were probably not due to a slower response time, at least not at 12 months after treatment.
The second important finding in our study is the lack of follow‐up data on nearly 65% of HIV‐positive and 77% of HIV‐negative patients treated for syphilis. A similar lack of compliance with post‐treatment serological follow‐up has been reported in the UK.19 It is possible that some patients did not have follow‐up titres documented in the statewide registry because they died, moved out of Maryland or they seroreverted (and thus mandatory reporting by laboratories and doctors of positive serologies does not apply). In HIV‐negative patients, RPR seroreversion at 3 and 12 months has been documented to occur in about 13% and 44% of patients with a first episode of primary syphilis, 0.7% and 22% of patients with secondary syphilis and 3% and 13% of patients with early‐latent syphilis,20 respectively. These data suggest that, although seroreversion could account for some of the loss to follow‐up, a high number of patients with syphilis are not being routinely monitored. Given the high rates of serological failure in HIV‐coinfected patients and risks of clinical consequences related to such failures, improving the mechanisms to ensure more consistent follow‐up is prudent to avoid long‐term sequelae. One possible solution is to expand the role of DISs to include follow‐up of coinfected patients after treatment. This initiative in a city with a high STD burden such as Baltimore is prohibitively expensive.
Our study has several limitations. The eligible patients represented a fraction of the patients diagnosed with syphilis; thus, selection bias is of concern. We have provided data on patients who were excluded from the study. These patients were more likely to be men and to report fewer sex partners in the past month at the time of evaluation compared with both the HIV‐positive and HIV‐negative patients. Whether these differences affect the outcome is unknown. Nearly 45% of patients were excluded because they did not have an HIV test documented in our clinic database. HIV testing is routinely offered to all patients who attend STD clinics in Baltimore. Only 19% refused HIV testing at the time of their clinic visit and had reported not being tested in the previous 6 months. The remaining patients refused HIV testing but reported having been tested elsewhere in the previous 6 months.
As in many series of patients with syphilis, it is difficult to ascertain whether a patient has experienced treatment failure or reinfection. This is especially important because HIV‐positive patients may be linked to sexual networks with a higher prevalence of syphilis, creating a bias toward higher rates of failure. In our study, for example, more coinfected patients with early syphilis failed because of a fourfold rise in titres, suggesting potential reinfection. To classify failures appropriately, we reviewed the DIS charts of all patients who fit the definition of treatment failure, and excluded all those who were not interviewed or who were thought by a trained DIS to have been reinfected (5 HIV‐positive and 2 HIV‐negative patients were excluded), on the basis of the interviews of the index case and follow‐up of their sex partners.
Key messages
Follow‐up serologies among HIV‐positive patients treated for syphilis in our sexually transmitted disease clinics were as inconsistent as follow‐up in HIV‐negative patients despite recommendations for more aggressive screenings at shorter intervals
Coinfected patients with early syphilis who had follow‐up serologies were more likely to experience serological failures than HIV‐negative patients
Controlled trials suggest that more aggressive initial treatment for coinfected patients does not alter the risk of serological relapse, underscoring the need for consistent serological follow‐up in this high‐risk population to detect possible failure early
HIV‐positive patients had more follow‐up serological titres than HIV‐negative patients. This may have led to two potential biases. First, HIV‐positive patients had more opportunity to be diagnosed as treatment failures because they had more follow‐up serologies. To minimise that, we adjusted for the number of follow‐up titres in the multivariate model. Second, HIV‐positive patients had more follow‐up titres, which may have underestimated time to serological response in the HIV‐negative group (fewer serologies leading to an apparent slower response time). This, however, would bias our results towards the null and strengthen our findings. Finally, as this study was performed at an STD clinic, CD4 cell counts and HIV load determinations were not available. Thus, whether the degree of immunosuppression enhances failure cannot be determined. Previous studies, however, suggest that CD4 count does not correlate with serological failure.6,10
In summary, our data suggest that HIV‐positive patients with syphilis are more likely to experience serological failure, consistent with findings from several studies including a randomised trial. Importantly, most of these coinfected patients do not return for timely follow‐up serologies to document therapeutic response. Efforts are warranted to ensure more consistent serological follow‐up among patients treated for syphilis, especially those who are HIV‐positive.
Acknowledgements
We acknowledge the help of the Baltimore City Health Department staff in coordinating access for abstraction of the data.
Abbreviations
DIS - disease intervention specialist
RPR - rapid plasma reagin
STD - sexually transmitted disease
Footnotes
Funding: This study was supported by grant NIH 5R01AI045724 (to AMR).
Competing interests: None.
This paper was presented in part as an oral presentation at the International Society for Sexually Transmitted Diseases Research (ISSTDR), July 2005, Amsterdam, The Netherlands.
Ethical approval: This study was approved by the institutional review boards of the Johns Hopkins Medical Institutions and the Baltimore City Health Department, Baltimore, Maryland, USA.
Contributors: KGG: study design, data collection, data analysis, drafting of manuscript; EJE: study design, data collection, drafting of manuscript; ZSW: data collection; AMR: study design, data analysis, drafting of manuscript.
References
- 1.Quinn T C, Glasser D, Cannon R O.et al Human immunodeficiency virus infection among patients attending clinics for sexually transmitted diseases. N Engl J Med 1988318197–203. [DOI] [PubMed] [Google Scholar]
- 2.Ansell D A, Hu T C, Straus M.et al HIV and syphilis seroprevalence among clients with sexually transmitted diseases attending a walk‐in clinic at Cook County Hospital. Sex Transm Dis 19942193–96. [DOI] [PubMed] [Google Scholar]
- 3.Blocker M E, Levine W C, St Louis M E. HIV prevalence in patients with syphilis, United States. Sex Transm Dis 20002753–59. [DOI] [PubMed] [Google Scholar]
- 4.Gadkari D A, Quinn T C, Gangakhedkar R R.et al HIV‐1 DNA shedding in genital ulcers and its associated risk factors in Pune, India. J Acquir Immune Defic Syndr Hum Retrovirol 199818277–281. [DOI] [PubMed] [Google Scholar]
- 5.Buchacz K, Patel P, Taylor M.et al Syphilis increases HIV viral load and decreases CD4 cell counts in HIV‐infected patients with new syphilis infections. AIDS 2004182075–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kofoed K, Gerstoft J, Mathiesen L R.et al Syphilis and human immunodeficiency virus (HIV)‐1 coinfection: influence on CD4 T‐cell count, HIV‐1 viral load, and treatment response. Sex Transm Dis 200633143–148. [DOI] [PubMed] [Google Scholar]
- 7.Berry C D, Hooton T M, Collier A C.et al Neurologic relapse after benzathine penicillin therapy for secondary syphilis in a patient with HIV infection. N Engl J Med 19873161587–1589. [DOI] [PubMed] [Google Scholar]
- 8.Radolf J D, Kaplan R P. Unusual manifestations of secondary syphilis and abnormal humoral immune response to Treponema pallidum antigens in a homosexual man with asymptomatic human immunodeficiency virus infection. J Am Acad Dermatol 198818(2 Pt 2)423–428. [DOI] [PubMed] [Google Scholar]
- 9.Rompalo A M, Joesoef M R, O'Donnell J A.et al Clinical manifestations of early syphilis by HIV status and gender: results of the syphilis and HIV study. Sex Transm Dis 200128158–165. [DOI] [PubMed] [Google Scholar]
- 10.Rolfs R T, Joesoef M R, Hendershot E F.et al A randomized trial of enhanced therapy for early syphilis in patients with and without human immunodeficiency virus infection. The Syphilis and HIV Study Group. N Engl J Med 1997337307–314. [DOI] [PubMed] [Google Scholar]
- 11.Long C M, Klausner J D, Leon S.et al Syphilis treatment and HIV infection in a population‐based study of persons at high risk for sexually transmitted disease/HIV infection in Lima, Peru. Sex Transm Dis 200633151–155. [DOI] [PubMed] [Google Scholar]
- 12.Marra C M, Maxwell C L, Smith S L.et al Cerebrospinal fluid abnormalities in patients with syphilis: association with clinical and laboratory features. J Infect Dis 2004189369–376. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention Sexually transmitted diseases treatment guidelines 2002. MMWR Recomm Rep 2002511–78. [PubMed] [Google Scholar]
- 14.Wharton M, Chorba T L, Vogt R L.et al Case definitions for public health surveillance. MMWR Recomm Rep 1990391–43. [PubMed] [Google Scholar]
- 15.Telzak E E, Greenberg M S, Harrison J.et al Syphilis treatment response in HIV‐infected individuals. AIDS 19915591–595. [PubMed] [Google Scholar]
- 16.Yinnon A M, Coury‐Doniger P, Polito R.et al Serologic response to treatment of syphilis in patients with HIV infection. Arch Intern Med 1996156321–325. [PubMed] [Google Scholar]
- 17.Malone J L, Wallace M R, Hendrick B B.et al Syphilis and neurosyphilis in a human immunodeficiency virus type‐1 seropositive population: evidence for frequent serologic relapse after therapy. Am J Med 19959955–63. [DOI] [PubMed] [Google Scholar]
- 18.Gourevitch M N, Selwyn P A, Davenny K.et al Effects of HIV infection on the serologic manifestations and response to treatment of syphilis in intravenous drug users.Ann Intern Med 1993118350–355. [DOI] [PubMed] [Google Scholar]
- 19.Chauhan M, Serisha B, Sankar K N.et al Audit of the use of benzathine penicillin, post‐treatment syphilis serology and partner notification of patients with early infectious syphilis. Int J STD AIDS 200617200–202. [DOI] [PubMed] [Google Scholar]
- 20.Romanowski B, Sutherland R, Fick G H.et al Serologic response to treatment of infectious syphilis. Ann Intern Med 19911141005–1009. [DOI] [PubMed] [Google Scholar]