Abstract
Objective
To explore behaviour change, baseline risk behaviour, perception of risk, HIV disclosure and life events in health centre‐based voluntary counselling and testing (VCT) clients.
Design and setting
Single‐arm prospective cohort with before–after design at three (one urban and two rural) government health centres in Kenya; study duration 2 years, 1999–2001.
Subjects
Consecutive eligible adult clients.
Main outcome measures
Numbers of sexual partners, partner type, condom use, reported symptoms of sexually transmitted infection, HIV disclosure and life events.
Results
High rates of enrolment and follow‐up provided a demographically representative sample of 401 clients with mean time to follow‐up of 7.5 months. Baseline indicators showed that clients were at higher risk than the general population, but reported a poor perception of risk. Clients with multiple partners showed a significant reduction of sexual partners at follow‐up (16% to 6%; p<0.001), and numbers reporting symptoms of sexually transmitted infection decreased significantly also (from 40% to 15%; p<0.001). Condom use improved from a low baseline. Low rates of disclosure (55%) were reported by HIV‐positive clients. Overall, no changes in rates of life events were seen.
Conclusion
This study suggests that significant prevention gains can be recorded in clients receiving health centre‐based VCT services in Africa. Prevention issues should be considered when refining counselling and testing policies for expanding treatment programmes.
The promotion of diagnostic counselling and testing (DCT) is welcome,1 although efforts to strengthen voluntary counselling and testing (VCT) must continue, as DCT, which is vital for treatment uptake, is less concerned with primary prevention. It is critically important to invest in primary prevention models especially with incident HIV cases rising faster than treatment programme enrolment. With the evolution of new testing approaches, it is important to continue to explore potential primary prevention opportunities in HIV testing programmes.
The effectiveness of VCT in preventing new HIV infections is an evolving field of research, and subject to some debate. An early meta‐analysis of largely cohort data (27 studies performed before 1994, of which only six were African studies) suggested that VCT was mainly a secondary HIV prevention strategy, as existing published data suggested that effectiveness focused on discordant couples and HIV‐positive men.2 Subsequently, two large randomised controlled trials,3,4 one based in developing countries including Kenya, showed that quality controlled VCT approaches led to significant reductions in risk behaviours, new sexually transmitted infections (STIs), or both. Three recent African studies have conflicting outcomes. In the Côte d'Ivoire, condom use increased only in male VCT clients with tuberculosis.5 In Mozambique, condom use increased more significantly in VCT clients, but casual sex also increased.6 A study of population‐based VCT found no difference in sexual behaviour,7 although these were not clients self‐referring for VCT. Rapid testing has been shown to be as effective as counselling around standard HIV testing.8
Different counselling and testing service models (such as DCT, stand‐alone sites, or VCT health centre‐based sites) might vary in behaviour change outcomes. This study was conducted in government primary healthcare centre VCT services, nested in a feasibility and costing study. Primary health centres have been shown to be a cost effective9 and feasible10 base for VCT services in resource‐poor settings, and countries are using this approach.11,12,13 It is important to consider primary healthcare settings for VCT services in addition to both provider‐initiated DCT at primary healthcare centres and VCT stand‐alone sites to enhance VCT coverage (primary healthcare centres reach very rural populations), and integration of VCT with other activities—for example, STI services, family planning. Limiting primary healthcare services to DCT has opportunity costs if VCT can aid prevention.
It has been unclear whether VCT is associated with increased negative life events,14,15,16,17,18 hampered as we are by a lack of information on background rates for comparison. Low disclosure rates may affect VCT impacts on behaviour change and stigma.
Here we present the findings of a before–after study of behaviour change and life events for health centre‐based VCT services. We report on risk behaviour, disclosure rates and life‐event outcomes of VCT. This provides insights into the potential prevention benefits and negative outcomes of VCT provision in health centres. There is considerable interest in enhancing primary care HIV and STI services in developed countries, and this study can inform new approaches in the North.
Methodology
This study was nested in a pilot study of integrating VCT into government primary healthcare centres in Kenya. It aimed to measure sexual behaviour before and at the 6‐month follow‐up after VCT in consecutive clients. Main outcome variables were numbers of sexual partners, unprotected intercourse and STI symptoms over a 6‐month period, measured by self‐report. Client's risk perception, plans and actions to prevent HIV, life events, and disclosure were also recorded.
Three health centres were selected systematically after a review of all health centres in Thika and Nairobi districts. Clinics were selected on catchment area (serving large, poor populations) and facility type (standard, with average number and types of staff) to represent government facilities serving distinct poor populations. This study took place before antiretroviral therapy and DCT activities were in place in Kenya, and when VCT existed predominantly in a few urban private clinics, non‐governmental organisations and district hospitals.19 As rural VCT activities were under‐developed, the study focused on two rural sites in Thika District (clinics A and B) and one urban Nairobi site (clinic C). Formal permission and consent were sought locally. The new free counselling and simple rapid on‐site testing service used existing infrastructure to mirror future government services. Each had one project‐salaried counsellor, with testing performed by nurses and counsellors. Condom demonstrations and free condoms were provided. Further study details are provided elsewhere.10 Counselling sessions (guideline20,21,22 based) used a client‐centred23 approach involving personalised, interactive counselling, and included risk assessment and coping strategies.
Consecutive VCT clients were approached for recruitment. Transport costs and small gifts were given at follow‐up. Enrolment criteria were: age at least 18 years or married, never tested for HIV, regular partner never tested. Fully informed verbal consent was ascertained. Using a McNemar test for the difference between correlated proportions, with estimates from the multi‐centre VCT efficacy trial data,4 a sample size was estimated at 586 patients (assuming 30% attrition and power of 80).
Basic demographic data were collected (including socioeconomic score using household amenities at recruitment), before pretest counselling, by field interviewers (not counsellors), trained in all aspects of data collection. The structured face‐to‐face behaviour survey, adapted from a fully validated Kenyan questionnaire,4 was completed by the field interviewer after pretest counselling while clients awaited results. At a 6‐month follow‐up visit, a second two‐part behaviour survey was completed by the field interviewer and counsellor, and repeat HIV testing was offered by counsellors.
Self‐reported recall of STI symptoms and treatment over the preceding 6 months was recorded at baseline and for the follow‐up period (∼6 months). Numbers of sexual partners were recorded at baseline (over the past 2, 6 and 12 months) and at follow‐up (for the preceding 2 months and follow‐up period). Partner‐specific behaviour (up to five partners for 2 months) for each time point was recorded. Clients were asked to assess their chance of testing HIV positive. Specific life events were recorded at both time points, and personal HIV‐prevention plans and actions were ascertained. Field interviewers were blinded to HIV status.
With prior consent, up to three tracing attempts were made for follow‐up. Disclosure and life‐event data that might reveal HIV status were not collected in field interviews.
Data were protected and double‐entered using FoxPro and validated. All analyses were performed using SPSS for Windows V9 (SPSS, Chicago, Illinois, USA) and Stata V6.0 (Stata Corporation, College Station, Texas, USA). Bivariate comparisons of categorical variables used χ2, and means were compared using the two‐sample Wilcoxon rank‐sum (Mann‐Whitney) test, Kruskal‐Wallis tests and paired t test. A two‐tailed α level of 0.05 was used as criterion for significance throughout. The generalised estimating equations technique investigates the effect of baseline explanatory factors (clinic site, gender, age, socioeconomic score, education, HIV status and clinic site) on outcome variables, measured before and after VCT. Odds ratios refer to after VCT, and are adjusted for explanatory variables (clinic, age, sex, marital status and HIV status; these had no effect on any of the outcomes).
Ethical approval was obtained from the ethics committees of Kenya Medical Research Institute and Liverpool School of Tropical Medicine.
Results
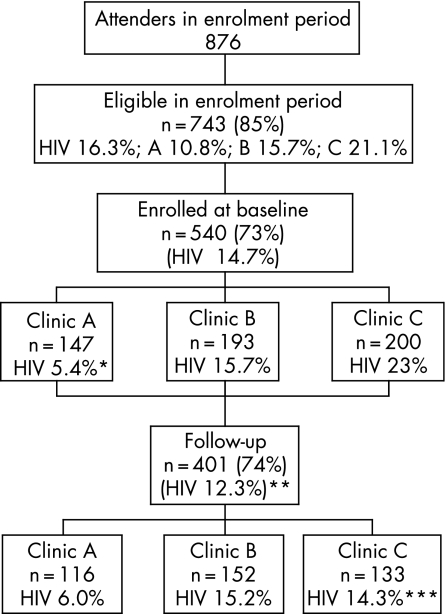
Recruitment ran from January 1999 for 11 months. Of consecutive VCT attenders, 743/876 (85%) were eligible and 540 (73% of those eligible) were enrolled equally across clinics (fig 1). Figure 1 shows how, owing to under‐recruitment at clinic A and increased loss to follow‐up in clinic C, the follow‐up group under‐represents HIV‐positive clients. This loss of follow‐up of HIV‐positive clients is particularly related to loss of female HIV‐positive clients. Otherwise there were no significant differences in follow‐up by gender or age of client. Those reporting multiple sex partners also tended to be lost to follow‐up (data not shown). Overall 401/540 (74%) were successfully followed up (“follow‐up clients”). The mean time to follow‐up was 7.5 (2.4) months, median 6.9 months, with no site variation. Duration of follow‐up had no significant effect on any reported outcomes. The feasibility of this VCT pilot in terms of uptake, client characteristics, acceptability, quality of counselling, and cost effectiveness are reported elsewhere.9 Of note, during the feasibility study at these centres, of 1823 pretest clients, 93% (1688/1823) opted for testing and 91% received their results.
Figure 1 Summary of attrition rates and HIV. *Clinic A HIV prevalence in enrolled versus non‐enrolled (5.4% vs 22.7%, p<0.001). **HIV prevalence in follow‐up group versus non‐follow‐up group (12.3% vs 21.9%, p<0.01). ***Clinic C HIV prevalence in follow‐up versus non‐follow‐up group (14.3% vs 33.3%, p = 0.002).
Baseline data
There were no significant differences for age, sex or marital status by enrolment or follow‐up status at any site. Table 1 shows baseline demographics for follow‐up clients. Men received significantly more secondary education than women (52% (100/206) vs 31% (60/191); p<0.001), and significantly more women than men were married (51% vs 36%, p = 0.01). Urban clients (clinic C) received more education and had higher socioeconomic status, but otherwise clinic clienteles had similar demographics. HIV seroprevalence was 12% (female, 16% (30/192); male, 9% (19/208)).
Table 1 Baseline demographics of follow‐up clients showing interclinic variation and comparison with those lost to follow‐up.
| FU clients | Intraclinic comparison (p value) | Clients lost to FU (n = 139) | Comparison of FU versus lost to FU (p value) | ||||
|---|---|---|---|---|---|---|---|
| Overall (n = 401)* | By clinic | ||||||
| A (n = 116) | B (n = 152) | C (n = 133) | |||||
| Age | |||||||
| Male | 29 (10.1) (n = 208) | 31 (12.9) (n = 64) | 28 (9.8) (n = 82) | 28 (6.5) (n = 62) | 0.468 | 30 (9.2) (n = 66) | 0.927 |
| Female | 28 (9.1) (n = 192) | 29 (10.5) (n = 52) | 28 (10.2) (n = 69) | 27 (6.6) (n = 71) | 0.837 | 28 (8.1) (n = 73) | 0.559 |
| % male | 52% (209/401) | 4% (64/116) | 55% (83/152) | 47% (62/133) | 0.298 | 47.5% (66/139) | 0.384 |
| % married/cohabiting | 47% (187/397) | 49% (57/116) | 46% (69/149) | 46% (61/132) | 0.445 | 49.6% (68/137) | 0.44 |
| Education level | |||||||
| No formal education | 3% (12/397) | 2% (2/116) | 4% (6/149) | 3% (4/132) | <0.01 | 2% (3/138) | 0.407 |
| Primary education | 55% (219/397) | 69% (80/116) | 58% (86/149) | 40% (53/132) | 62% (85/138) | ||
| Secondary education | 42% (166/397) | 29% (34/116) | 38% (57/149) | 57% (75/132) | 36% (50/138) | ||
| SES† | 2.1 (1.9) | 1.1 (1.3) | 1.9 (1.7) | 3.2 (2.1) | <0.01 | 2.2 (2.1) | 0.997 |
| HIV prevalence (%) | |||||||
| Overall | 12 | 6 | 15 | 14 | 0.053 | 22 | 0.008 |
| Male | 9 | – | – | – | – | 9 | 1.00 |
| Female | 16 | – | – | – | – | 33 | 0.003 |
Values are mean (SD) unless otherwise indicated.
*Age was missing for one male.
†Socioeconomic score (SES) was calculated from responses to questions about household amenities such as electricity and piped water; the higher the score, the better the standard of living.
FU, follow‐up.
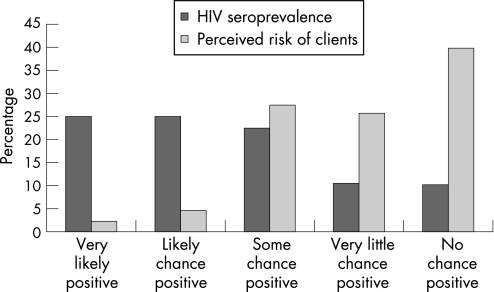
The majority (65.5%) perceived very little or no chance of testing HIV positive (fig 2), with a loose correlation between expectation and the HIV result itself. Three‐quarters (27/36) of those who thought that they were likely or very likely to test positive ended up testing HIV negative.
Figure 2 Perceived risk of testing positive for HIV.
Of those followed‐up, men reported more partners at baseline than women: 24% of men had two or more partners over 6 months compared with 8% of women (odds ratio (OR) 3.66 (95% CI 1.97 to 6.79)), and similarly for the 2‐month and 12‐month time periods at all three sites (table 2, accessible online). Only 1% of married women reported having more than one sexual partner in the preceding 2 months compared with 9% of married men (p = 0.011). The majority from all clinics (98% of women and 90% of men) reported either one primary partner or no sexual activity over the preceding 2 months (table 2). A minority of 9% (35/401) (19 (9%) male clients; 16 (8%) female clients)) reported having casual partners. There was some clinic variation in women reporting casual partners: 17%, 4% and 6% for clinic A, B and C respectively (p = 0.022). Married women were much less likely than single women to have casual partners (2.4% vs 19%; OR 0.19 (95% CI 0.05 to 0.67); p = 0.010) whereas for men, marital status made no difference. Commercial sex was uncommon and much more likely to be reported by men (nine (4%) male clients; one (0.5%) female client; p = 0.01).
Of follow‐up clients, 35% (134/385) had ever used a condom. At baseline, only 6% of clients reported using condoms consistently with their primary partner, and none used condoms consistently with non‐primary partners (table 2). On average, 95% of casual sex acts and 77% of commercial sex acts were unprotected at baseline. STI symptoms were common over the preceding 6 months (40% (159/401)). Reporting an STI symptom was associated in logistic regression with female gender, HIV positivity and attending a rural clinic: male versus female, 36% vs 48% (OR 0.65, 95% CI 0.43 to 0.97, p = 0.037); HIV positive versus HIV negative, 61% vs 38% (OR 1.92, 95% CI 1.02 to 3.63, p = 0.043); urban versus rural, 32% vs 48% (OR 0.48, 95% CI 0.28 to 0.80, p = 0.005). Approximately two‐thirds of those reporting symptoms had sought treatment, and these rates were similar at both time points (data not shown).
Follow‐up data
There was a marked reduction in clients having two or more partners in the follow‐up (∼6 months) time period (table 3). Overall, 16% (66/401) reported two or more sexual partners in 6 months at baseline compared with 6% (23/401) at follow‐up (OR 0.30, 95% CI 0.20 to 0.47, p<0.001). Although there were no significant differences by gender, women showed a stronger trend than men (falling from 8% before to 2% after compared with 24% before and 10% after for men). There was a trend for reduction in non‐primary partners (11% (44/401) at baseline vs 8% (30/401) at follow‐up), although this was not a significant change (p = 0.08). Significantly fewer STI symptoms were reported for the follow‐up period after VCT compared with the 6‐month period before VCT across all clinics: 40% (159/401) vs 15% (61/401), OR 0.27, 95% CI 0.20 to 0.36, p<0.001. Sexual intercourse was more often protected at follow‐up, although condom use remained low for all partner types: clients reporting unprotected sex with any partner type went down from 230/242 (95%) to 217/244 (89%) (OR 0.45, 95% CI 0.23 to 0.89, p = 0.021) (table 3). Looking at reported sexual episodes, there was little change in the mean number of episodes of sexual intercourse for either primary or non‐primary partners before and after VCT. However, there was a trend for decreasing unprotected episodes of sex with non‐primary partners (93% to 49% sex acts with non‐primary partners were unprotected at baseline versus follow‐up).
Table 3 Behaviour change at follow‐up.
| Before | After | p Value | |
|---|---|---|---|
| Number of sexual partners | N (%) | N (%) | |
| ⩾2 in past 6 months | 66/401 (16.4) | 23/401 (5.8) | <0.001 |
| ⩾2 in past 2 months | 23/401 (5.7) | 15/401 (3.7) | <0.02 |
| Unprotected sexual intercourse | N (%) | N (%) | |
| Any partner type | 230/242 (95) | 217/244 (89) | 0.021 |
| With primary partners | 195/207 (94) | 192/214 (90) | 0.152 |
| With non‐primary partners | 44/44 (100) | 25/30 (83) | NA |
| Episodes of sexual intercourse by partner type | |||
| Primary partners | (n = 207) | (n = 217) | |
| All episodes | |||
| Mean (SD) | 9.9 (10.1) | 12.2 (16.9) | 0.825 |
| SE | 0.7 | 1.2 | |
| Median | 6.0 | 8.0 | |
| Unprotected episodes | 0.001 | ||
| Mean (SD) | 10.3 (12.7) | 11.2 (14.3) | |
| SE | 0.9 | 1.0 | |
| Median | 6.0 | 8.0 | |
| Average rate of unprotected episodes (%) | 100 | 92 | |
| Non‐primary partners | (n = 44) | (n = 30) | |
| All episodes | |||
| Mean (SD) | 6.59 (12.64) | 6.9 (9.47) | 0.001 |
| SE | 1.9 | 1.7 | |
| Median | 2.0 | 2.5 | |
| Unprotected episodes | 0.002 | ||
| Mean | 6.14 (11.90) | 3.4 (4.6) | |
| SE | 1.79 | 0.84 | |
| Median | 2.0 | 1.5 | |
| Average rate of unprotected episodes (%) | 93 | 49 | |
| Proportion reporting any STI symptoms | 159/401 (39.7%) | 61/401 (15.3%) | <0.02 |
NA, not available as numbers insufficient for robust analysis.
Negative attitudes towards condom use improved but remained commonplace at follow‐up: embarrassment in using condoms fell from 42% to 33% (p<0.001); “condoms symbolise unfaithfulness” fell from 54% to 44% (p<0.001). Eighty five percent (316/373) thought that condoms were easily accessible, and only 6% (18/296) could not afford condoms (data not in table 3).
Before receiving results, 93% planned to disclose if they were HIV positive (table 4). At follow‐up, 55% of HIV‐positive and 82% of HIV‐negative clients (both men and women) had disclosed. One‐third of HIV‐positive clients had disclosed to a primary partner, and one‐fifth to a doctor. Of 49 HIV‐positive clients, few (nine (18%)) reported having sought help for HIV in the 2 months before follow‐up (there was no local antiretroviral provision at that time). There were no reported differences in disclosure by gender.
Table 4 Personal plans and actions for HIV prevention, disclosure and life events.
| Plans and actions (n = 401) | Action in 2 months before testing | Plans after testing | Action 2 months before follow‐up visit |
|---|---|---|---|
| Faithful to one partner | 88 (22) | 189 (47) | 176 (44) |
| Abstain from sex | 112 (28) | 116 (29) | 115 (29) |
| Use of condoms/discussed condoms | 19 (5) | 94 (23) | 47 (12) |
| None | 184 (46) | 35 (9) | 81 (20) |
| Disclosure | Plans to disclose if HIV positive (all) | Actual disclosure (90 missing responses) | |
|---|---|---|---|
| HIV negative | HIV positive | ||
| Disclosure to anyone | 373/401 (93) | 227/278 (82) | 18/33 (55) |
| Disclosure to primary partner (includes spouse) | 183/234 (78) | 149/223 (66) | 9/28 (32) |
| Disclosure to non‐primary partner | 27/42 (64) | 12/31 (39) | 2/4 (50) |
| Disclosure to doctor | 351/393 (89) | 28/240 (12) | 6/32 (19) |
| Life events | All clients | HIV positive clients | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| Positive life events | ||||
| Emotional support from family/friends | 153/383 (40) | 123/371 (33) | 15/47 (32) | 11/43 (26) |
| Strong relationship with sexual partner | 112/324 (35) | 148/324 (46) | 8/42 (19) | 11/37 (30) |
| Emotional support by peers | 111/381 (29) | 119/368 (32) | 19/47 (40) | 15/44 (34) |
| Emotional support from employers | 8/129 (6) | 13/120 (10) | 1/18 (6) | 1/16 (6) |
| Negative life events | ||||
| Breakdown of sexual relations | 77/333 (23) | 111/347 (32) | 11/43 (26) | 16/40 (40) |
| Physical abuse by sexual partner | 26/314 (8) | 14/311 (5) | 3/40 (8) | 1/38 (3) |
| Break up of marriage | 12/199 (6) | 8/177 (5) | 1/30 (3) | 5/28 (18) |
| Neglect by family | 12/384(3) | 9/369 (2) | 3/47 (6) | 4/45 (9) |
| Shunned by peers | 8/382 (2) | 11/368 (3) | 0 | 4/44 (9) |
Values in parentheses are percentages. Of those followed‐up, there was a 78% response rate for the disclosure section.
Self‐reported HIV prevention measures concurred with sexual behaviour reports (table 4). Condoms were the least popular prevention measure reported before VCT. Immediately after VCT, the most popular plan was to be faithful to one partner (47% (189/401)), and 23% (94/401) planned to use condoms. At follow‐up, actions mainly involved being faithful or abstaining, and only half of those planning condom use had acted on this.
There were both more strengthened relationships (35% to 46%; OR 1.59 (95% CI 1.15 to 2.21); p = 0.004) and more relationship breakdowns (23% before vs 32% after; OR 1.56 (95% CI 1.1 to 2.23); p = 0.01) after VCT. Rates of physical abuse or neglect by family were unaltered by a positive result, with a baseline rate of 8%. No suicides were reported to the counselling programme during the duration of the first 2 years of the programme.
Discussion
This prospective study evaluating health centre‐based VCT services found that clients attending services reported a significant reduction in high‐risk sexual behaviours from baseline (before services) to the 6‐month (average 7.5 month) follow‐up. VCT clients reported a significant decrease in multiple sexual partners and STI symptoms. Condom use, although increased, was still relatively low at follow‐up, with 95% of sexually active clients reporting any unprotected sex at baseline compared with 89% at follow‐up. Average episodes of unprotected sex with a non‐primary partner fell from 93% to 49% in those reporting sex with a non‐primary partner. There were very high rates of disclosure by HIV‐negative clients, but low rates for HIV‐positive clients. VCT did not increase negative life events reported regardless of the outcome of the test.
As part of a larger operational research study evaluating new strategies for VCT provision, the design has limitations, as it could not include a control group or biological measures of STI. Social desirability bias may have led to under‐reporting of risky behaviours. In addition, risky behaviours and events may prelude a visit for VCT and may not be typical of a person's behaviour and therefore may “regress” with time regardless of any intervention effect. This is another limitation of an uncontrolled intervention study where people self‐select for the intervention. However, in this study, 58% of clients stated no specific trigger for attending for VCT (just wanting to know status), and only 17% said that they had attended because of risky behaviour. Only 19% were internal referrals from within the clinic (eg, STI attenders). High rates of recruitment and follow‐up meant that the study population appeared to broadly represent those eligible, although some under‐recruitment and increased loss to follow‐up meant that the study population does under‐represent HIV‐positive clients. In addition, the participants may not have been representative of VCT attenders in Kenya generally. These data were collected in the pre‐antiretroviral era; any recommendations must be made cautiously, as behaviour may have changed with antiretroviral availability. However, this new era has also moved the focus towards diagnostic testing, and it remains important to consider missed opportunities for prevention.
Many VCT clients failed to perceive their higher HIV risk compared with the general public (reported elsewhere24). There was also poor correlation between individual risk perception and HIV result, as noted elsewhere,25 making counselling and testing a vital tool in this population.
In addition to a reduction in the number of sexual partners, decrease in STI symptoms, and a small increase in condom use, there was also a trend for change in partner type, with a reduction in sexual intercourse with non‐primary partners after VCT. These changes did not vary by gender, age, marital status or HIV test outcome.
Key messages
Clients attending primary healthcare‐based voluntary counselling and testing (VCT) services showed significant reductions in the number of sexual partners, fewer sexually transmitted infection symptoms, and increased condom use at the 6‐month follow‐up after VCT.
High background rates of violence were recorded, with no increase in life events noted after VCT.
VCT in healthcare centres should be considered alongside diagnostic counselling and testing to aid primary prevention opportunities, which are vital as incident HIV infections continue to rise, exceeding treatment enrolment.
The concurrence of a reduction in numbers of sexual partners with reduction in reported STI symptoms suggests a real change, but without a control group it is not possible to conclude that this is due to the intervention. However, the magnitude of the change and the clients' own perceptions of their HIV prevention behaviour before and after the intervention support this interpretation. Behaviour change was mainly via partner reduction not condom use, which may go against social desirability bias.
Behaviour change has been described for other VCT services.4,25,26,27,28 It is encouraging that health centre VCT may mirror these successes. It is disappointing that this study, like others26 but unlike the multicentre efficacy trial,4 did not find more impact on condom use despite good knowledge and access to condoms. This may be related to low baseline rates of condom use and prevalent negative attitudes towards condoms.
Disclosure rates for HIV‐positive clients were low, although similar to other studies.17,29 This highlights the importance of seeking improved strategies for disclosure for HIV‐positive clients—for example, using couple counselling.18,27 High rates of HIV‐negative disclosure suggest low stigma for HIV testing itself.
In comparison with earlier reports,17 this study concurs with the multicentre trial in recording no significant harm caused by VCT.18 Our findings need to be interpreted with care in view of the small numbers of HIV‐positive clients involved and low disclosure rates. Importantly, we found high background rates of violence; this has also been noted in studies in other countries.30
At this time of emphasis on HIV treatment, it is critically important to focus on primary HIV prevention strategies. This study found that clients planned risk reduction after pretest counselling and showed significant changes in sexual behaviour at follow‐up. These findings are in line with randomised controlled trials suggesting that primary health centre services can help primary prevention efforts. Ongoing monitoring for negative impacts is recommended. The challenge to policy makers is to now weigh the prevention benefits of VCT against the possibility that it may not offer greatest efficiency in increasing treatment uptake. This study suggests that future health centre‐based VCT services emphasising prevention outcomes should be considered in counselling and testing packages, alongside DCT and antiretroviral treatment programmes, to ensure that substantial prevention opportunities are not missed.
Table 2 can be accessed at http://sti.bmj.com/supplemental.
Acknowledgements
We thank all the clients, staff and communities for their huge contribution to this work. We also thank Andrew Copas and Catherine Mercer for fantastic support with statistical analysis.
Contributors
GA and CG had the original idea for this study. All the authors (GA, CG, VN, RM, SF, JO) contributed to the planning of the study and data collection. GA, VN and SF collected and analysed the data. GA wrote the first draft of the paper, and all authors contributed to revisions.
Abbreviations
DCT - diagnostic counselling and testing
VCT - voluntary counselling and testing
STI - sexually transmitted infection
Footnotes
Sponsorship: The study was funded by the UK Department for International Development, through The Futures Group International.
Competing interests: None declared.
Note: The UK Department for International Development and the Futures Group International can accept no responsibility for any information presented or views expressed.
Table 2 can be accessed at http://sti.bmj.com/supplemental.
References
- 1.Branson B M, Handsfield H H, Lampe M A.et al Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health‐care settings. Morb Mortal Wkly Rep 200655(RR‐14)1–17. [PubMed] [Google Scholar]
- 2.Weinhardt L S, Carey M P, Johnson B T.et al Effects of HIV counselling and testing on sexual risk behaviour: a meta‐analytic review of published research, 1985–1997. Am J Public Health 1999891397–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamb M L, Fishbein M, Douglas J M J.et al Efficacy of risk‐reduction counselling to prevent human immunodeficiency virus and sexually transmitted diseases: a randomised controlled trial. JAMA 19982801161–1167. [DOI] [PubMed] [Google Scholar]
- 4.The Voluntary HIV‐1 Counselling and Testing Efficacy Study Group Efficacy of voluntary HIV‐1 counselling and testing in individuals and couples in Kenya, Tanzania, and Trinidad: a randomised trial. Lancet 2001356 [PubMed] [Google Scholar]
- 5.Wiktor S Z, Abouya L, Angoran H.et al Effect of an HIV counseling and testing program on AIDS‐related knowledge and practices in tuberculosis clinics in Abidjan, Cote d'Ivoire. Int J Tuberc Lung Dis 20048445. [PubMed] [Google Scholar]
- 6.Mola O D, Mercer M A, Asghar R J.et al Condom use after voluntary counselling and testing in central Mozambique. Trop Med Int Health 200611176–181. [DOI] [PubMed] [Google Scholar]
- 7.Matovu J K, Gray R H, Makumbi F.et al Voluntary HIV counseling and testing acceptance, sexual risk behavior and HIV incidence in Rakai, Uganda. AIDS 200519503–511. [DOI] [PubMed] [Google Scholar]
- 8.Metcalf C A, Douglas J M, Malotte C K.et al Relative efficacy of prevention counseling with rapid and standard HIV testing: a randomized, controlled trial (RESPECT‐2). Sex Transm Dis 200532130–138. [DOI] [PubMed] [Google Scholar]
- 9.Forsythe S, Arthur G, Ngatia G.et al Assessing the cost and willingness to pay for voluntary HIV counselling and testing in Kenya. Health Policy Plan 200217187–195. [DOI] [PubMed] [Google Scholar]
- 10.Arthur G, Ngatia G, Rachier C.et al The role of government health centres in provision for government health centres. I. Provision of same‐day voluntary HIV counselling and testing in Kenya. J Acquir Immune Defic Syndr 200540329–335. [DOI] [PubMed] [Google Scholar]
- 11.Pronyk P M, Kim J C, Makhubele M B.et al Introduction of voluntary counselling and rapid testing for HIV in rural South Africa: from theory to practice. AIDS Care 200414859–865. [DOI] [PubMed] [Google Scholar]
- 12.USAID Success stories in HIV/AIDS: new voluntary counselling and testing sites reach growing numbers. USAID. 1‐5‐2003. Washington DC: USAID.
- 13.Marum E, Taegtmeyer M, Chebet K. Scale‐up of voluntary HIV counseling and testing in Kenya. JAMA 2006296859–862. [DOI] [PubMed] [Google Scholar]
- 14.Keogh P, Allen S, Almedal C.et al The social impact of HIV infection on women in Kigali, Rwanda: a prospective study. Soc Sci Med 1994381047–1053. [DOI] [PubMed] [Google Scholar]
- 15.Kamenga M, Ryder R W, Jingu M.et al Evidence of marked sexual behaviour change associated with low HIV‐1 seroconversion in 149 married couples with discordant HIV‐1 serostatus: experience at an HIV counselling centre in Zaire. AIDS 1991561–67. [DOI] [PubMed] [Google Scholar]
- 16.Van der Straten A, King R, Grinstead O A.et al Couple communication, sexual coercion and HIV risk reduction in Kigali, Rwanda. AIDS 19959935–944. [DOI] [PubMed] [Google Scholar]
- 17.Temmerman M, Ndinya‐Achola J, Ambani J.et al The right not to know HIV‐test results. Lancet 1995345969–970. [DOI] [PubMed] [Google Scholar]
- 18.Grinstead O A, Gregorich S E, Choi K‐H, Coates T J, The Voluntary HIV‐1 Counselling and Testing Efficacy Study Group Positive and negative life events after counselling and testing: the voluntary HIV‐1 Counselling and Testing Efficacy Study. AIDS 2001151045–1052. [DOI] [PubMed] [Google Scholar]
- 19.Population Council and Family Health International HIV/AIDS counselling, testing, care and support services in Nairobi, Kenya. Population Council and Family Health International 1999
- 20.Kenya Association of Professional Counsellors HIV/AIDS counselling training manual. Nairobi: KAPC, 1990
- 21.Centers for Disease Control and Prevention HIV prevention counselling: a training program. 1993. Atlanta, GA: United States Department of Health and Human Services 1993
- 22.Ministry of Health, Government of Kenya National AIDS and STDs Control Programme, Kenya. Counselling policy guidelines. Nairobi: Ministry of Health, Government of Kenya 1989
- 23.Rogers C R.Client‐centred therapy. London: Constable, 1951
- 24.Nduba V, Arthur G, Ngatia G.et al Health Centre based voluntary counselling and testing (VCT) services in resource poor settings: are users a high risk group? Republic of South Africa, International AIDS Society. 13th World AIDS Conference 2000
- 25.Lindan C, Allen S, Carael M. Knowledge, attitudes, and perceived risk of AIDS among urban Rwandan women: relationship to HIV infection and behaviour change. AIDS 19911069–75. [DOI] [PubMed] [Google Scholar]
- 26.Jackson D, Rakwar J, Richardson B.et al Decreased incidence of sexually transmitted diseases among trucking company workers in Kenya: results of a behavioural risk‐reduction programme. AIDS 199711903–909. [DOI] [PubMed] [Google Scholar]
- 27.Allen S, Serufilira A, Bogaerts J.et al Confidential HIV testing and condom promotion in Africa. Impact on HIV and gonorrhea rates. JAMA 19922683338–3343. [PubMed] [Google Scholar]
- 28.Allen S. Effects of serotesting with counselling on condom use and seroconversion among HIV discordant couples in Africa. BMJ 20013041605–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baingana G, Choi K ‐ H, Barrett D C.et al Female partners of AIDS patients in Uganda: reported knowledge, perceptions and plans. AIDS 19959S15–S19. [PubMed] [Google Scholar]
- 30.Koenig L J, Moore J. Women, violence, and HIV: a critical evaluation with implications for HIV services. Maternal Child Health 20004103–109. [DOI] [PubMed] [Google Scholar]