Abstract
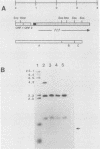
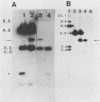
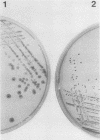
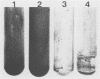
The Streptococcus mutans extracellular fructosyltransferase (FTF) enzyme may play a role in the formation of dental caries by synthesizing a fructan polymer that serves as an extracellular storage polysaccharide. We sought to determine if an FTF-deficient strain of S. mutans was less virulent than wild-type cells in a rat animal model system. Cloned ftf gene sequences from S. mutans GS5 were used to generate a defective copy of the ftf gene by inserting into the ftf coding region a DNA fragment which encoded erythromycin resistance. The plasmid which carried the defective ftf construct was introduced into S. mutans V403 by using genetic transformation. This defective construct replaced, by allelic exchange, the wild-type copy of the ftf gene carried on the V403 chromosome. FTF activity assays indicated that the recombinant strain, V1741, was deficient in fructan synthesis. However, extracellular protein preparations from this strain displayed an increased ability to generate glucose polymers (glucans) compared with V403 preparations. Levels of adherence to glass and rat tooth surfaces by strain V1741 were similar to those of the V403 strain. Both strains caused moderate decay on rat tooth surfaces; however, the FTF-deficient strain was less pathogenic compared with the wild-type strain. These results suggest that FTF activity contributes to the pathogenicity of S. mutans V403, possibly by generating extracellular fructans which serve as storage compounds.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barletta R. G., Michalek S. M., Curtiss R., 3rd Analysis of the virulence of Streptococcus mutans serotype c gtfA mutants in the rat model system. Infect Immun. 1988 Feb;56(2):322–330. doi: 10.1128/iai.56.2.322-330.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. F., Barrett T. A., Curtiss R., 3rd Purification and partial characterization of the multicomponent dextranase complex of Streptococcus sobrinus and cloning of the dextranase gene. Infect Immun. 1987 Mar;55(3):792–802. doi: 10.1128/iai.55.3.792-802.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkhed D., Rosell K. G., Granath K. Structure of extracellular water-soluble polysaccharides synthesized from sucrose by oral strains of Streptococcus mutans, Streptococcus salivarius, Streptococcus sanguis and Actinomyces viscosus. Arch Oral Biol. 1979;24(1):53–61. doi: 10.1016/0003-9969(79)90175-4. [DOI] [PubMed] [Google Scholar]
- Carlsson J. A levansucrase from Streptococcus mutans. Caries Res. 1970;4(2):97–113. doi: 10.1159/000259632. [DOI] [PubMed] [Google Scholar]
- Chassy B. M., Beall J. R., Bielawski R. M., Porter E. V., Donkersloot J. A. Occurrence and distribution of sucrose-metabolizing enzymes in oral streptococci. Infect Immun. 1976 Aug;14(2):408–415. doi: 10.1128/iai.14.2.408-415.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- DaCosta T., Gibbons R. J. Hydrolysis of levan by human plaque streptococci. Arch Oral Biol. 1968 Jun;13(6):609–617. doi: 10.1016/0003-9969(68)90139-8. [DOI] [PubMed] [Google Scholar]
- Ehrlich J., Stivala S. S., Bahary W. S., Garg S. K., Long L. W., Newbrun E. Levans: I. Fractionation, solution viscosity, and chemical analysis of levan produced by Streptococcus salivarius. J Dent Res. 1975 Mar-Apr;54(2):290–297. [PubMed] [Google Scholar]
- Fitzgerald R. J., Adams B. O., Sandham H. J., Abhyankar S. Cariogenicity of a lactate dehydrogenase-deficient mutant of Streptococcus mutans serotype c in gnotobiotic rats. Infect Immun. 1989 Mar;57(3):823–826. doi: 10.1128/iai.57.3.823-826.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUSTAFSSON B. E., QUENSEL C. E., LANKE L. S., LUNDQVIST C., GRAHNEN H., BONOW B. E., KRASSE B. The Vipeholm dental caries study; the effect of different levels of carbohydrate intake on caries activity in 436 individuals observed for five years. Acta Odontol Scand. 1954 Sep;11(3-4):232–264. doi: 10.3109/00016355308993925. [DOI] [PubMed] [Google Scholar]
- Germaine G. R., Schachtele C. F., Chludzinski A. M. Rapid filter paper assay for the dextransucrase activity from Streptococcus mutans. J Dent Res. 1974 Nov-Dec;53(6):1355–1360. doi: 10.1177/00220345740530061101. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. Dental caries. Annu Rev Med. 1975;26:121–136. doi: 10.1146/annurev.me.26.020175.001005. [DOI] [PubMed] [Google Scholar]
- Gold W., Preston F. B., Lache M. C., Blechman H. Production of levan and dextran in plaque in vivo. J Dent Res. 1974 Mar-Apr;53(2):442–446. doi: 10.1177/00220345740530024401. [DOI] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman J. D., Yaphe B. I., Johnson K. P. Colonization of the human oral cavity by a strain of Streptococcus mutans. J Dent Res. 1985 Nov;64(11):1272–1274. doi: 10.1177/00220345850640110301. [DOI] [PubMed] [Google Scholar]
- Jacobs A. A., Roosendaal B., van Breemen J. F., de Graaf F. K. Role of phenylalanine 150 in the receptor-binding domain of the K88 fibrillar subunit. J Bacteriol. 1987 Nov;169(11):4907–4911. doi: 10.1128/jb.169.11.4907-4911.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYES P. H. Dental caries in the molar teeth of rats. I. Distribution of lesions induced by high-carbohydrate low-fat diets. J Dent Res. 1958 Nov-Dec;37(6):1077–1087. doi: 10.1177/00220345580370060801. [DOI] [PubMed] [Google Scholar]
- KEYES P. H. Dental caries in the molar teeth of rats. II. A method for diagnosing and scoring several types of lesions simultaneously. J Dent Res. 1958 Nov-Dec;37(6):1088–1099. doi: 10.1177/00220345580370060901. [DOI] [PubMed] [Google Scholar]
- Kametaka S., Hayashi S., Miyake Y., Suginaka H. Electrophoretic studies of extracellular glucosyltransferases and fructosyltransferases from seventeen strains of Streptococcus mutans. Arch Microbiol. 1987 Apr;147(3):207–212. doi: 10.1007/BF00463476. [DOI] [PubMed] [Google Scholar]
- Keyes P. H. Research in dental caries. J Am Dent Assoc. 1968 Jun;76(6):1357–1373. doi: 10.14219/jada.archive.1968.0186. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Macrina F. L., Jones K. R., Welch R. A. Transformation of Streptococcus sanguis with monomeric pVA736 plasmid deoxyribonucleic acid. J Bacteriol. 1981 May;146(2):826–830. doi: 10.1128/jb.146.2.826-830.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Reider J. L., Virgili S. S., Kopecko D. J. Survey of the extrachromosomal gene pool of Streptococcus mutans. Infect Immun. 1977 Jul;17(1):215–226. doi: 10.1128/iai.17.1.215-226.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Tobian J. A., Jones K. R., Evans R. P., Clewell D. B. A cloning vector able to replicate in Escherichia coli and Streptococcus sanguis. Gene. 1982 Oct;19(3):345–353. doi: 10.1016/0378-1119(82)90025-7. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Virgili S. S., Scott C. L. Extrachromosomal gene systems in Streptococcus mutans. Adv Exp Med Biol. 1978;107:859–868. doi: 10.1007/978-1-4684-3369-2_96. [DOI] [PubMed] [Google Scholar]
- Manly R. S., Richardson D. T. Metabolism of levan by oral samples. J Dent Res. 1968 Nov-Dec;47(6):1080–1086. doi: 10.1177/00220345680470061301. [DOI] [PubMed] [Google Scholar]
- Marshall K., Weigel H. Evidence of multiple branching in the levan elaborated by Streptococcus salivarius strain 51. Carbohydr Res. 1980 Aug 15;83(2):321–326. doi: 10.1016/s0008-6215(00)84544-9. [DOI] [PubMed] [Google Scholar]
- Michalek S. M., McGhee J. R., Navia J. M. Virulence of Streptococcus mutans: a sensitive method for evaluating cariogenicity in young gnotobiotic rats. Infect Immun. 1975 Jul;12(1):69–75. doi: 10.1128/iai.12.1.69-75.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D., Kuramitsu H. K. Genetic transformation of Streptococcus mutans. Infect Immun. 1981 Jun;32(3):1295–1297. doi: 10.1128/iai.32.3.1295-1297.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D., Wondrack L. M., Kuramitsu H. K. Genetic transformation of putative cariogenic properties in Streptococcus mutans. Infect Immun. 1983 Aug;41(2):722–727. doi: 10.1128/iai.41.2.722-727.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rölla G., Iversen O. J., Bonesvoll P. Lipoteichoic acid - the key to the adhesiveness of sucrose grown Streptococcus mutans. Adv Exp Med Biol. 1978;107:607–617. doi: 10.1007/978-1-4684-3369-2_69. [DOI] [PubMed] [Google Scholar]
- Sato S., Kuramitsu H. K. Isolation and characterization of a fructosyltransferase gene from Streptococcus mutans GS-5. Infect Immun. 1986 Apr;52(1):166–170. doi: 10.1128/iai.52.1.166-170.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroza T., Kuramitsu H. K. Sequence analysis of the Streptococcus mutans fructosyltransferase gene and flanking regions. J Bacteriol. 1988 Feb;170(2):810–816. doi: 10.1128/jb.170.2.810-816.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Mizuno F., Takamori K. Purification and preliminary characterization of exo-beta-D-fructosidase in Streptococcus salivarius KTA-19. Infect Immun. 1985 Jan;47(1):271–276. doi: 10.1128/iai.47.1.271-276.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M. Essential dependence of smooth surface caries on, and augmentation of fissure caries by, sucrose and Streptococcus mutans infection. Infect Immun. 1979 Aug;25(2):526–531. doi: 10.1128/iai.25.2.526-531.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Freedman M. L., Fitzgerald R. J., Larson R. H. Diminished virulence of glucan synthesis-defective mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):197–203. doi: 10.1128/iai.10.1.197-203.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. J., Hare M. D., Morrey-Jones J. G. Activity of fructanase in batch cultures of oral streptococci. Carbohydr Res. 1983 Feb 16;113(1):101–112. doi: 10.1016/0008-6215(83)88222-6. [DOI] [PubMed] [Google Scholar]
- Wood J. M. The amount, distribution and metabolism of soluble polysaccharides in human dental plaque. Arch Oral Biol. 1967 Jul;12(7):849–858. doi: 10.1016/0003-9969(67)90107-0. [DOI] [PubMed] [Google Scholar]
- van Houte J., Jansen H. M. Levan degradation by streptococci isolated from human dental plaque. Arch Oral Biol. 1968 Jul;13(7):827–830. doi: 10.1016/0003-9969(68)90102-7. [DOI] [PubMed] [Google Scholar]