Abstract
Objectives
Establishing a connection between the emerging urogenital tract pathogen Mycoplasma genitalium and upper genital tract infection in women would be of major importance. The aim of this study was to evaluate the association between M genitalium antibodies and pelvic inflammatory disease (PID) and ectopic pregnancy (EP) using a lipid‐associated membrane protein‐enzyme immunoassay (LAMP‐EIA) method.
Methods
The LAMP‐EIA was used to analyse sera obtained from patients with clinical PID and EP collected in Sweden between February 1984 and April 1986. Sera from healthy pregnant women (Ctrl) collected during approximately the same period were used as controls. Evidence of chlamydial infection was investigated using a commercial anti‐Chlamydia trachomatis EIA assay.
Results
The LAMP‐EIA was specific as determined by a lack of cross‐reactivity with other Mycoplasma species. The LAMP‐EIA showed that 17% (33/193) of the PID patients were M genitalium positive as compared to 18% (15/82) of the EP patients and 15% (36/246) of the Ctrl women. No significant association could be demonstrated between M genitalium antibodies and PID or EP in crude or adjusted logistic regression. Antibodies against C trachomatis were demonstrated in 54% of the PID and 57% of the EP patients, and also in 37% of the Ctrl women, showing a statistically significant association.
Conclusion
No statistically significant association between PID or EP and M genitalium antibodies could be found using the LAMP‐EIA, although a slight tendency toward association was found when focusing on younger individuals.
Keywords: mycoplasma genitalium, pelvic inflammatory disease, ectopic pregnancy, serology
Pelvic inflammatory disease (PID) almost exclusively affects sexually active fertile women, who then face an increased risk of infertility, ectopic pregnancy (EP) and chronic pelvic pain. The best known causative agents of PID are Neisseria gonorrhoeae and Chlamydia trachomatis. Some studies have indicated that non‐gonococcal rather than gonococcal infections of the upper genital tract are more likely to result in infertility and EP.1,2,3 Although many cases are caused by C trachomatis, some studies have also shown an association between Mycoplasma genitalium infection and PID and infertility.4,5,6,7
M genitalium was discovered in 1981 when it was isolated from the urethra of two men with non‐gonococcal urethritis.8 The infection is sexually transmitted, but the complete pattern of diseases caused by M genitalium has not yet been completely elucidated. The infections tend to run a chronic course and are often asymptomatic.9,10 Experiments with female monkeys where M genitalium was inoculated into the oviducts, resulted in salpingitis followed by a specific antibody response.11 The cross‐reactions between M pneumoniae and M genitalium12,13 have made it difficult to use serology for diagnosis and epidemiological studies, but Wang et al developed and evaluated a Triton X‐114 extracted lipid‐associated membrane protein (LAMP) assay and found no cross‐reactions.9,14 In the present study we have used sera obtained in the 1980s from women hospitalised with a clinical diagnosis of PID or EP when the incidence of PID in Sweden was high.2
The purpose of the present study was to evaluate the association between M genitalium antibodies and PID and EP using a LAMP‐enzyme immunoassay (EIA) method.
Materials and methods
Patients and controls
A total of 303 sera from 194 women with a clinical diagnosis of PID, who were inpatients at the Department of Obstetrics and Gynecology, Örebro University Hospital, Örebro, Sweden, were obtained during a 25‐month period from February 1984 to April 1986. The PID diagnosis was based on clinical criteria, that is, pain in the lower abdomen for less than 3 weeks with a palpable adnexal mass and/or motion tenderness, fever >38.0°C and objective signs of lower genital tract infection; 60–65% of these patients underwent laparoscopy for direct visual diagnosis of acute salpingitis.15 Unfortunately, the laparoscopy data and serum specimens could not be linked, as the sera were made anonymous after the end of the initial study.
In addition, 104 sera were obtained from 83 women with a clinical diagnosis of EP who were inpatients at the same department at the same time. Some of the EP patients had a previous history of PID or EP, and when clinical evidence was uncertain the diagnosis was confirmed by laparoscopy.3 Sixty eight of the PID and 16 of the EP patients provided sera from both the acute and convalescence phases. Sixteen of the PID patients were seen twice at the same department during the study, but only data from the first visit were used in the evaluation. The median age was 23 (range 15–50) years for the PID patients and 29 (range 18–42) years for the EP patients. One serum sample from each group of patients was missing so these patients were excluded.
Sera from healthy pregnant women (Ctrl) were obtained in 1988 for rubella screening. From these stored serum samples, three samples for each EP case were matched for age and used as control material (n = 246). A second control group consisting of 150 serum samples obtained in 2002 from female blood donors (age 18–50 years) was used as a negative control group (Cb). All sera were stored at −20°C.
The ethics committee in Uppsala, Sweden, approved the study.
Enzyme immunoassays (EIA)
A LAMP‐EIA method was adapted using two different strains of M genitalium as antigen, in order to represent different antigenic variants of M genitalium. The M genitalium cells were Triton X‐114 extracted as previously described,9,14,16 and the purified fraction containing the lipid‐associated membrane protein (LAMP) of M genitalium was used as antigen in the EIA assay.
The LAMP‐EIA was performed in 96‐well microtitre plates (Maxisorb; Nunc, Roskilde, Denmark), coated with a mixture (100 μl) of the two antigen preparations (1 μg/ml) and incubated overnight at room temperature (RT). After the wells were blocked with 5% non‐fat milk (Bio‐rad, Hercules, CA, USA) in PBS+0.05% Tween 20 (PBST) for 2 h at RT, 100 μl of sera diluted 1/50 in blocking buffer was added to each well in duplicate and incubated for 2 h at RT. The plates were washed four times in PBST after each step. Peroxidase‐conjugated goat‐anti‐human IgG (Fc‐fragment, A0170; Sigma, St Louis, MO, USA) was added (100 μl) and incubated for another 2 h at RT, washed six times and followed by 30‐min development with 100 μl SIGMA FAST OPD (o‐phenylenediamine dihydrochloride; Sigma). The reaction was stopped with 50 μl 1.5 M HCl and the optical density was measured at 492 nm. Serum from a patient with a Mycoplasma genitalium PCR positive result in the urogenital tract was used as a positive control, and pooled sera from blood donors were used as a negative control in each run. Rabbit antisera against different Mycoplasma species, and sera from patients with M hominis‐ or Ureaplasma urealyticum positive cultures from urogenital tract specimens17 and 10 human sera from adults with high antibody titres against M pneumoniae in a complement fixation test, were used for validation of the assay.
Serological evidence of chlamydial infection was detected using a well‐documented18,19,20 commercial anti‐Chlamydia trachomatis EIA assay (Ani Labsystems, Oy, Finland), in order to control for confounding from C trachomatis infection in PID and EP.
Statistical methods
Logistic regression was used with outcomes PID or EP versus Ctrl. As explanatory variables, LAMP (cut‐off 0.3), Chlamydia trachomatis (CT; yes/no) and age on a continuous scale were used. Crude statistical analyses were carried out, with age also included, and adjusted with all three variables. Data were stratified according to age (15–30 years and 31–50 years). The effect parameter was expressed as an odds ratio (OR) and its 95% confidence interval (CI). All statistical analyses were done in STATA (release 9.2; Stata, College Station, TX, USA).
Results
The M genitalium LAMP‐EIA was specific as determined by a lack of cross‐reactivity with other Mycoplasma species. The negative cut‐off level was set at 0.3 OD492 and was determined as 3 standard deviations (SD) above the negative control mean.
PID patients
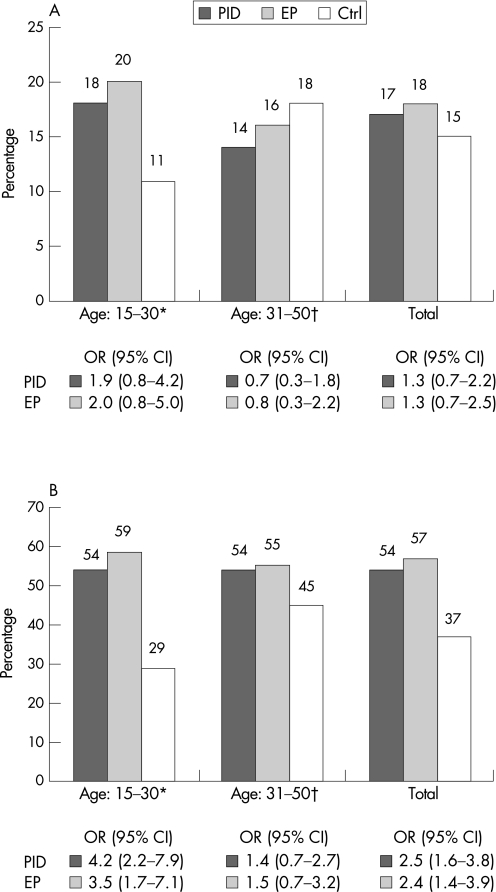
Among the PID patients, 17% (33/193) were M genitalium LAMP‐EIA seropositive. Sixty eight patients provided sera from both acute and convalescence phases. A seroconversion was seen in one patient (no. 184; table 1), but in most cases sera from both the acute and convalescence phases were negative (not shown). In two cases seroconversion occurred between the onset of the first PID and the onset of the second PID (patient nos. 87 and 188). When analysing the different age groups, 18% (26/143) of patients in the 15–30‐year‐old age group and 14% (7/50) of those in the 31–50‐year‐old age group were LAMP‐EIA positive (table 2, fig 1A).
Table 1 Results for seropositive pelvic inflammatory disease patients.
| PID patient no. | First PID onset | Second PID onset | ||||||
|---|---|---|---|---|---|---|---|---|
| LAMP* acute phase (date) | CT† | LAMP conv phase (date) | CT | LAMP acute phase (date) | CT | LAMP conv phase (date) | CT | |
| 60 | 0.48 (14/09/84) | Pos | ND‡ | ND | 0.22 (28/02/86) | Pos | ND | ND |
| 80 | 0.32 (06/03/86) | Pos | 0.32 (18/03/86) | Pos | ND | ND | ND | ND |
| 87 | 0.02 (05/11/84) | Pos | ND | ND | 1.29 (06/03/86) | Pos | ND | ND |
| 107 | 0.43 (14/01/85) | Pos | 0.46 (27/01/85) | Pos | ND | ND | ND | ND |
| 127 | 1.08 (08/08/84) | Neg | ND | ND | 1.13 (16/04/85) | Neg | 1.20 (26/04/85) | Neg |
| 140 | 0.87 (17/02/86) | Pos | 0.86 (25/02/86) | Pos | ND | ND | ND | ND |
| 146 | 0.34 (13/08/84) | Pos | 0.63 (23/08/84) | Pos | ND | ND | ND | ND |
| 154 | 2.10 (28/09/84) | Pos | 2.61 (05/10/84) | Pos | ND | ND | ND | ND |
| 184 | 0.24 (19/08/85) | Pos | 0.92 (06/09/85) | Pos | ND | ND | ND | ND |
| 188 | 0.24 (26/09/85) | Neg | ND | ND | 0.88 (20/03/86) | Neg | ND | ND |
Results from the M genitalium lipid‐associated membrane protein (LAMP)‐EIA and the Chlamydia trachomatis IgG EIA (CT) are shown. Some seropositive pelvic inflammatory disease (PID) patients provided sera from both the acute and convalescence (conv) phases, and some were seen twice at the same department with verified PID during the study period (first and/or second PID onset). Results from seronegative PID patients are not shown.
*Optical density measured at 492 nm in the LAMP‐EIA, cut off 0.3; †CT, Chlamydia trachomatis EIA results; ‡ND, not done (because no sera were obtained).
Table 2 Results for pelvic inflammatory disease (n = 194) and ectopic pregnancy patients (n = 83) compared to the control group.
| Age group | PID patients (%) | EP patients (%) | Pregnant (Ctrl) (%) | PID vs Ctrl | EP vs Ctrl | ||
|---|---|---|---|---|---|---|---|
| Crude OR (95% CI) | Adjusted OR (95% CI) | Crude OR (95% CI) | Adjusted OR (95% CI) | ||||
| 15–30 years* | |||||||
| LAMP | 18 (26/143) | 20 (9/44) | 11 (15/132) | 1.9 (0.8 to 4.2) | 1.3 (0.6 to 3.0) | 2.0 (0.8 to 5.0) | 1.6 (0.6 to 4.0) |
| CT | 54 (77/143) | 59 (26/44) | 29 (39/132) | 4.2 (2.2 to 7.8) | 4.0 (2.1 to 7.6) | 3.5 (1.7 to 7.1) | 3.3 (1.6 to 6.8) |
| 31–50 years† | |||||||
| LAMP | 14 (7/50) | 16 (6/38) | 18 (21/114) | 0.7 (0.3 to 1.8) | 0.6 (0.2 to 1.6) | 0.8 (0.3 to 2.2) | 0.7 (0.2 to 1.9) |
| CT | 54 (27/50) | 55 (21/38) | 45 (51/114) | 1.4 (0.7 to 2.7) | 1.6 (0.8 to 3.2) | 1.5 (0.7 to 3.2) | 1.6 (0.8 to 3.6) |
| Total‡ | |||||||
| LAMP | 17 (33/193) | 18 (15/82) | 15 (36/246) | 1.3 (0.7 to 2.2) | 1.0 (0.6 to 1.7) | 1.3 (0.7 to 2.5) | 1.0 (0.5 to 2.0) |
| CT | 54§ (104/193) | 57§ (47/82) | 37§ (90/246) | 2.5 (1.6 to 3.7) | 2.5 (1.6 to 3.8) | 2.4 (1.4 to 3.9) | 2.3 (1.4 to 4.0) |
Results for pelvic inflammatory disease (PID) patients (n = 194) and ectopic pregnancy (EP) patients (n = 83) seropositive in the M genitalium LAMP‐EIA and Chlamydia trachomatis IgG EIA (CT) as compared to results for the control group (Ctrl) of healthy pregnant women (three for each EP case). Statistical analyses were carried out by logistic regression. The effect parameter is expressed as the odds ratio (OR) and its 95% confidence interval (CI). Crude OR with variable age and adjusted OR with the variables age, LAMP and CT were included in the model. EP, ectopic pregnancy; PID, pelvic inflammatory disease.
*The age group for the EP patients and the control group is 18–30 years.
†The age group for the EP patients and the control group is 31–42 years.
‡One serum sample from one patients in each patient material was not found.
§Results were equivocal in the CT EIA for 7 PID patients, 5 EP patients, and 5 in the control group of pregnant women.
Figure 1 (A) M genitalium LAMP‐EIA results in percentages (%). Seropositive pelvic inflammatory disease (PID) patients (n = 194) and ectopic pregnancy (EP) patients (n = 83) are compared to the control group (Ctrl) of healthy pregnant women (n = 246). Statistical analyses were carried out by logistic regression and crude results are shown as the odds ratios (OR) and their 95% confidence intervals (CI). (B) Chlamydia trachomatis IgG EIA results in percentages (%). Seropositive pelvic inflammatory disease (PID) patients (n = 194) and ectopic pregnancy (EP) patients (n = 83) are compared to the control group (Ctrl) of healthy pregnant women (n = 246). Statistical analyses were carried out by logistic regression and crude results are shown as the odds ratios (OR) and their 95% confidence intervals (CI).*The age is 18–30 years for the EP patients and Ctrl.†The age is 31–42 for the EP patients and Ctrl.
Antibodies against C trachomatis were found in the sera of 54% (104/193) of the PID patients; sera from seven patients were equivocal in the CT‐EIA. No differences were found between the two age groups (table 2, fig 1B).
EP patients
Of the EP patients, 18% (15/82) were M genitalium LAMP‐EIA seropositive. Sixteen patients provided sera from both the acute and convalescence phases, and in no case was there any difference in the antibody level. One patient provided five sera specimens from December 1984 to 31 March 1986, and the OD value was constant (about 0.45). Twenty per cent (9/44) of the EP patients in the 18–30‐year‐old age group and 16% (6/38) of the 31–42‐year‐old age group were LAMP‐EIA positive (table 2, fig 1A).
Antibodies against C trachomatis were found in the sera of 57% (47/82) of the EP patients, while five were equivocal in the CT‐EIA. In the 18–30‐year‐old age group, 59% (26/44) were seropositive, while 55% (21/38) were CT‐EIA positive in the 31–42‐year‐old age group (table 2, fig 1B).
Control material
Fifteen per cent (36/246) of the control sera (n = 246) from healthy pregnant women (Ctrl) were M genitalium LAMP‐EIA seropositive: 11% (15/132) in the 18–30‐year‐old age group and 18% (21/114) in the 31–42‐year‐old age group.
Antibodies against C trachomatis were found in 37% (90/246) of the Ctrl sera: 29% (39/132) in the 18–30‐year‐old age group and 45% (51/114) in the 31–42‐year‐old age group were CT‐EIA positive.
In the control group of 150 female blood donors (Cb), five (3%) were M genitalium LAMP‐EIA seropositive: 2% (1/50) were seropositive in the 18–30‐year‐old age group and 4% (4/99) in the 31–50‐year‐old age group.
In the CT‐EIA, 9% (13/150) of the Cb group were positive: 2% (1/51) in the 18–30‐year‐old age group and 12% (12/99) in the 31–50‐year‐old age group. However, since these sera were collected at a later date (2002), no statistical comparisons were made.
Statistical analysis
No statistically significant association between PID and M genitalium antibodies in the LAMP‐EIA was seen in the crude logistic regression (OR 1.3, p = 0.387) or in the adjusted regression analysis (table 2, fig 1A). However, C trachomatis antibodies were significantly associated with PID in both crude and adjusted analyses. The associations were more pronounced in the younger individuals aged 15–30 years, both for M genitalium LAMP antibodies (OR 1.9, p = 0.123) and antibodies against C trachomatis (OR 4.2, p<0.001) (table 2, fig 1B). When only the CT‐EIA negative sera from the PID patients were considered, the OR was 1.3 (95% CI 0.5 to 3.5, p = 0.562) for M genitalium infection.
Also for the EP patients, no association with M genitalium LAMP antibodies could be found in the crude analysis (OR 1.3, p = 0.429), and even less so in the adjusted analysis. C trachomatis antibodies were statistically significantly associated with EP in both the crude and adjusted analyses. There was a slight trend towards an association between EP and M genitalium LAMP antibodies in the younger 18–30‐year‐old age group in both crude (OR 2.0, p = 0.133) and adjusted analyses, although this was not statistically significant. When only the CT–EIA negative sera were considered, there was a slight but insignificant trend towards an association (OR 2.3, 95% CI 0.7 to 7.0, p = 0.161) among the EP patients.
Combined antibody response
In the group of PID patients, 23 (12%) were seropositive both in the M genitalium LAMP‐EIA and in the CT‐EIA, while 10 (5%) were M genitalium LAMP antigen seropositive only. In the group of EP patients, 10 (12%) had antibodies against both pathogens, while five (6%) were M genitalium LAMP antigen seropositive only, compared to 4% of the Ctrl women.
Discussion
Findings from serological and genetic studies on the association between M genitalium and PID have been controversial. A serological study by Lind et al21 and a PCR study by Cohen et al22 were unable to show evidence for an association between M genitalium and PID, whereas a serological study by Moller et al4 and a PCR study on patients with endometritis by Cohen et al7 did show an association.
In this study we successfully adapted a Triton X‐114 extracted LAMP‐EIA to detect antibodies against M genitalium with no cross‐reactivity with other Mycoplasma species.
The LAMP antigen preparation described by Wang et al9,14 and used in this study seems to cover the antigenic variation of the different genotypes of M genitalium.
Using the LAMP‐EIA, we analysed sera from the 1980s from women with a clinical diagnosis of PID or EP. There was a trend toward an association between PID and M genitalium antibodies in the 15–30‐year‐old age group, where 18% of the patients were LAMP‐EIA positive as compared to 11% of the Ctrl women (fig 1A). There was also a trend towards an association for the EP patients in the 18–30‐year‐old age group, where 20% of the EP patients were LAMP‐EIA seropositive compared to the Ctrl women. Surprisingly, we found that the older pregnant women (31–42‐year‐old age group) had M genitalium antibodies more often than the younger age group. This was also the case for antibodies against C trachomatis, where a trend towards a higher seroprevalence was seen among the older women in the Ctrl group (fig 1B). These results may be due to the fact that the older women had a longer exposure time for the two pathogens. However, attention should also focus on behavioural differences between the groups. In the present study it was not possible to control for differences in the number of lifetime sexual partners, previous STIs and other factors since this information was not available. Future studies should address these issues. The seroprevalence for both M genitalium (3%) and C trachomatis (9%) was low in the blood donor population collected in 2002, and these findings strongly emphasises the need for careful selection of control material collected at the same time as the study material.
Key messages
Establishing a connection between Mycoplasma genitalium and upper genital tract infection in women would be of major importance. In this study we adapted a lipid‐associated membrane protein‐enzyme immunoassay (LAMP‐EIA) method that was specific as determined by a lack of cross‐reactivity with other Mycoplasma species.
The LAMP‐EIA was used to analyse sera obtained from inpatients with clinical pelvic inflammatory disease (PID) and ectopic pregnancy (EP) collected in Sweden during the 1980s.
No statistically significant association between PID or EP and M genitalium antibodies in the LAMP‐EIA could be found compared to a control group of healthy pregnant women collected at approximately the same date.
A slight tendency toward association was found in younger individuals.
In Sweden, it has been mandatory to report gonorrhoea and syphilis since 1919 under the Communicable Diseases Act, and genital infection with C trachomatis since April 1988. The sera in this study were thus obtained before the start of mandatory contact tracing, screening and treatment of asymptomatic men and women, which may explain the high percentage of women with antibodies against C trachomatis among the patients and the Ctrl women in our study. These figures are in good agreement with those reported by other Scandinavian groups from the period.23,24,25
Dual infection with both M genitalium and C trachomatis as diagnosed by PCR, has been reported in several studies,10,22,26,27,28,29 but in most investigations the two bacteria have more often been found alone than together, indicating that they may act as separate causes of disease. In the present study, some PID and EP patients had antibodies against both M genitalium and C trachomatis simultaneously, which may reflect successive infections in a population with a high burden of STIs.
In summary, we successfully adapted a LAMP‐ EIA to detect antibodies against M genitalium. Our findings did not indicate a connection between PID or EP and the presence of M genitalium antibodies. However, there was a slight trend towards an association between PID and EP and M genitalium LAMP antibodies in the younger group, although this was not statistically significant.
Further, preferably prospective, studies where serology and PCR are performed in parallel are required.
Acknowledgements
We would like to thank Emeritus Professor Dan Danielsson for providing information on the clinical findings and serum material from the PID and EP patients and Lena Jansson for skilful assistance with the EIA assays.
Authors' contribution to the manuscript
Margaretha Jurstrand initiated the study, adapted the LAMP‐EIA, collected all data and wrote the first draft of the manuscript. Jørgen Skov Jensen provided antigen and control sera for the LAMP‐EIA and contributed to the design of the study. Anders Magnusson carried out the statistical analyses, and Francis Kamwendo examined and sampled most of the patients. Hans Fredlund contributed to the design of the study and provided the serum samples from the PID and EP patients and from the control groups.
Abbreviations
CI - confidence interval
EP - ectopic pregnancy
LAMP‐EIA - lipid‐associated membrane protein‐enzyme immunoassay
OR - odds ratio
PBST - PBS+0.05% Tween 20
PID - pelvic inflammatory disease
RT - room temperature
SD - standard deviation
Footnotes
The study was supported by grants from the Örebro Medical Research Foundation, Örebro University Hospital, Örebro, Sweden.
Competing interests: None.
The ethics committee in Uppsala, Sweden, approved the study.
References
- 1.Weström L, Mårdh P. Acute pelvic inflammatory disease. In: Holmes K, Mårdh PA, Sparling PF, et al eds. Sexually transmitted diseases. New York: McGraw‐Hill, 1990593–613.
- 2.Kamwendo F, Forslin L, Bodin L.et al Programmes to reduce pelvic inflammatory disease‐‐the Swedish experience. Lancet 1998351(Suppl 3)25–28. [DOI] [PubMed] [Google Scholar]
- 3.Kamwendo F, Forslin L, Bodin L.et al Epidemiology of ectopic pregnancy during a 28 year period and the role of pelvic inflammatory disease. Sex Transm Infect 20007628–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moller B R, Taylor‐Robinson D, Furr P M. Serological evidence implicating Mycoplasma genitalium in pelvic inflammatory disease. Lancet 198411102–1103. [DOI] [PubMed] [Google Scholar]
- 5.Clausen H F, Fedder J, Drasbek M.et al Serological investigation of Mycoplasma genitalium in infertile women. Hum Reprod 2001161866–1874. [DOI] [PubMed] [Google Scholar]
- 6.Simms I, Eastick K, Mallinson H.et al Associations between Mycoplasma genitalium, Chlamydia trachomatis, and pelvic inflammatory disease. Sex Transm Infect 200379154–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen C R, Manhart L E, Bukusi E A.et al Association between Mycoplasma genitalium and acute endometritis. Lancet 2002359765–766. [DOI] [PubMed] [Google Scholar]
- 8.Tully J G, Taylor‐Robinson D, Cole R M.et al A newly discovered mycoplasma in the human urogenital tract. Lancet 198111288–1291. [DOI] [PubMed] [Google Scholar]
- 9.Wang R Y, Grandinetti T, Shih J W.et al Mycoplasma genitalium infection and host antibody immune response in patients infected by HIV, patients attending STD clinics and in healthy blood donors. FEMS Immunol Med Microbiol 199719237–245. [DOI] [PubMed] [Google Scholar]
- 10.Falk L, Fredlund H, Jensen J S. Signs and symptoms of urethritis and cervicitis among women with or without Mycoplasma genitalium or Chlamydia trachomatis infection. Sex Transm Infect 20058173–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moller B R, Taylor‐Robinson D, Furr P M.et al Acute upper genital‐tract disease in female monkeys provoked experimentally by Mycoplasma genitalium. Br J Exp Pathol 198566417–426. [PMC free article] [PubMed] [Google Scholar]
- 12.Lind K. Serological cross‐reactions between “Mycoplasma genitalium” and M. pneumoniae. Lancet 198221158–1159. [DOI] [PubMed] [Google Scholar]
- 13.Taylor‐Robinson D, Furr P M, Tully J G. Serological cross‐reactions between Mycoplasma genitalium and M. pneumoniae. Lancet 19831527. [DOI] [PubMed] [Google Scholar]
- 14.Wang R Y, Lo S ‐ C. ELISA in human urogenital infections and AIDS. In: Razin S, Tully JG, eds. Molecular and diagnostic procedures in mycoplasmology. New York: Academic Press, 1996237–245.
- 15.Kamwendo F, Forslin L, Bodin L.et al Decreasing incidences of gonorrhea‐ and chlamydia‐associated acute pelvic inflammatory disease. A 25‐year study from an urban area of central Sweden. Sex Transm Dis 199623384–391. [DOI] [PubMed] [Google Scholar]
- 16.Baseman J B, Cagle M, Korte J E.et al Diagnostic assessment of Mycoplasma genitalium in culture‐positive women. J Clin Microbiol 200442203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen J S, Borre M B, Dohn B. Detection of Mycoplasma genitalium by PCR amplification of the 16S rRNA gene. J Clin Microbiol 200341261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morre S A, Munk C, Persson K.et al Comparison of three commercially available peptide‐based immunoglobulin G (IgG) and IgA assays to microimmunofluorescence assay for detection of Chlamydia trachomatis antibodies. J Clin Microbiol 200240584–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Land J A, Gijsen A P, Kessels A G.et al Performance of five serological chlamydia antibody tests in subfertile women. Hum Reprod 2003182621–2627. [DOI] [PubMed] [Google Scholar]
- 20.Närvänen A, Puolakkainen M, Hau W.et al Detection of antibodies to Chlamydia trachomatis with peptide‐based species specific enzyme immunoassay. Infect Dis Obstet Gynecol 19975349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lind K, Kristensen G B. Significance of antibodies to Mycoplasma genitalium in salpingitis. Eur J Clin Microbiol 19876205–207. [DOI] [PubMed] [Google Scholar]
- 22.Cohen C R, Mugo N R, Astete S G.et al Detection of Mycoplasma genitalium in women with laparoscopically diagnosed acute salpingitis. Sex Transm Infect 200581463–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mardh P A, Lind I, Svensson L.et al Antibodies to Chlamydia trachomatis, Mycoplasma hominis, and Neisseria gonorrhoeae in sera from patients with acute salpingitis. Br J Vener Dis 198157125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ripa K T, Svensson L, Treharne J D.et al Chlamydia trachomatis infection in patients with laparoscopically verified acute salpingitis. Results of isolation and antibody determinations. Am J Obstet Gynecol 1980138960–964. [DOI] [PubMed] [Google Scholar]
- 25.Paavonen J. Chlamydia trachomatis in acute salpingitis. Am J Obstet Gynecol 1980138957–959. [DOI] [PubMed] [Google Scholar]
- 26.Casin I, Vexiau‐Robert D, De La Salmoniere P.et al High prevalence of Mycoplasma genitalium in the lower genitourinary tract of women attending a sexually transmitted disease clinic in Paris, France. Sex Transm Dis 200229353–359. [DOI] [PubMed] [Google Scholar]
- 27.Jensen J S, Orsum R, Dohn B.et al Mycoplasma genitalium: a cause of male urethritis? Genitourin Med 199369265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maeda S I, Tamaki M, Kojima K.et al Association of Mycoplasma genitalium persistence in the urethra with recurrence of nongonococcal urethritis. Sex Transm Dis 200128472–476. [DOI] [PubMed] [Google Scholar]
- 29.Totten P A, Schwartz M A, Sjostrom K E.et al Association of Mycoplasma genitalium with nongonococcal urethritis in heterosexual men. J Infect Dis 2001183269–276. [DOI] [PubMed] [Google Scholar]