Abstract
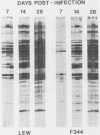
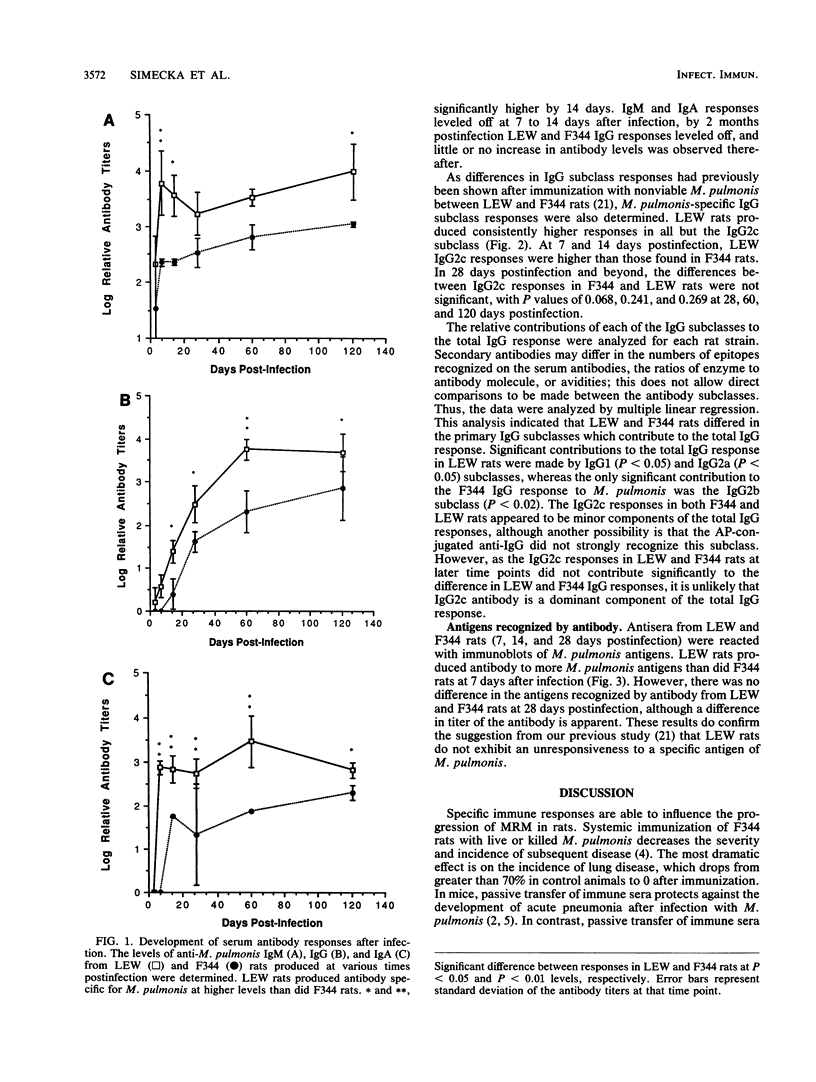
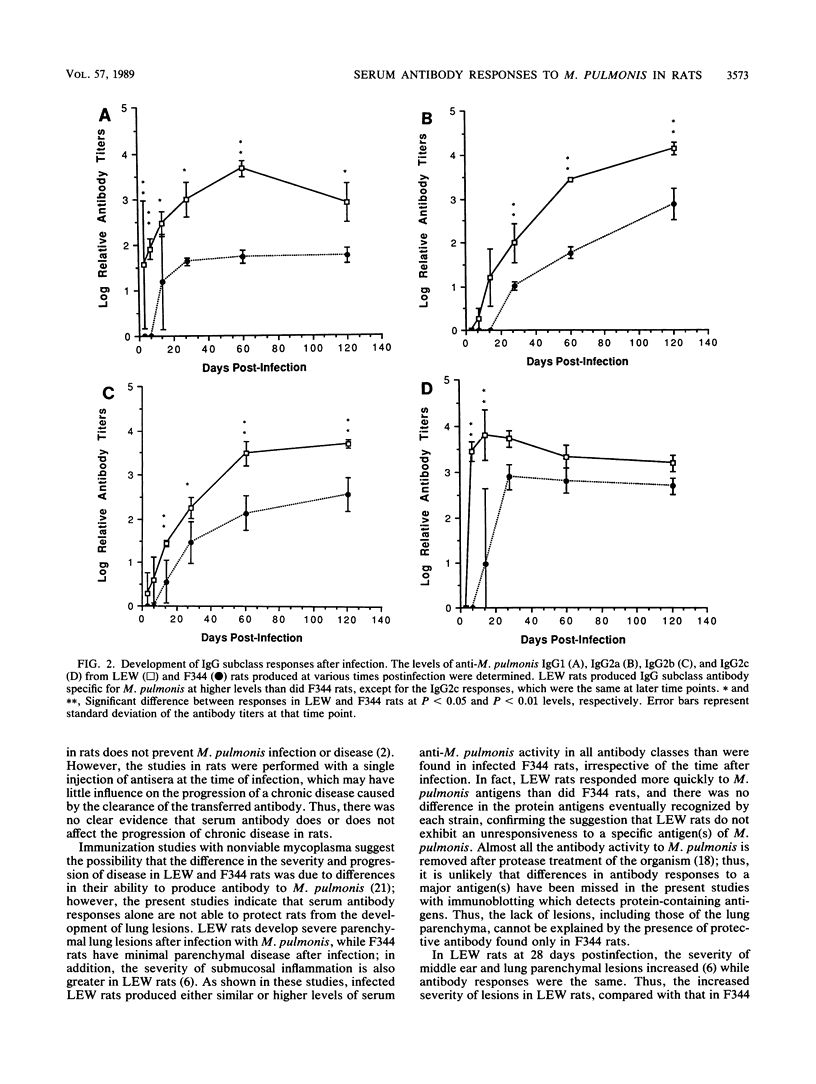
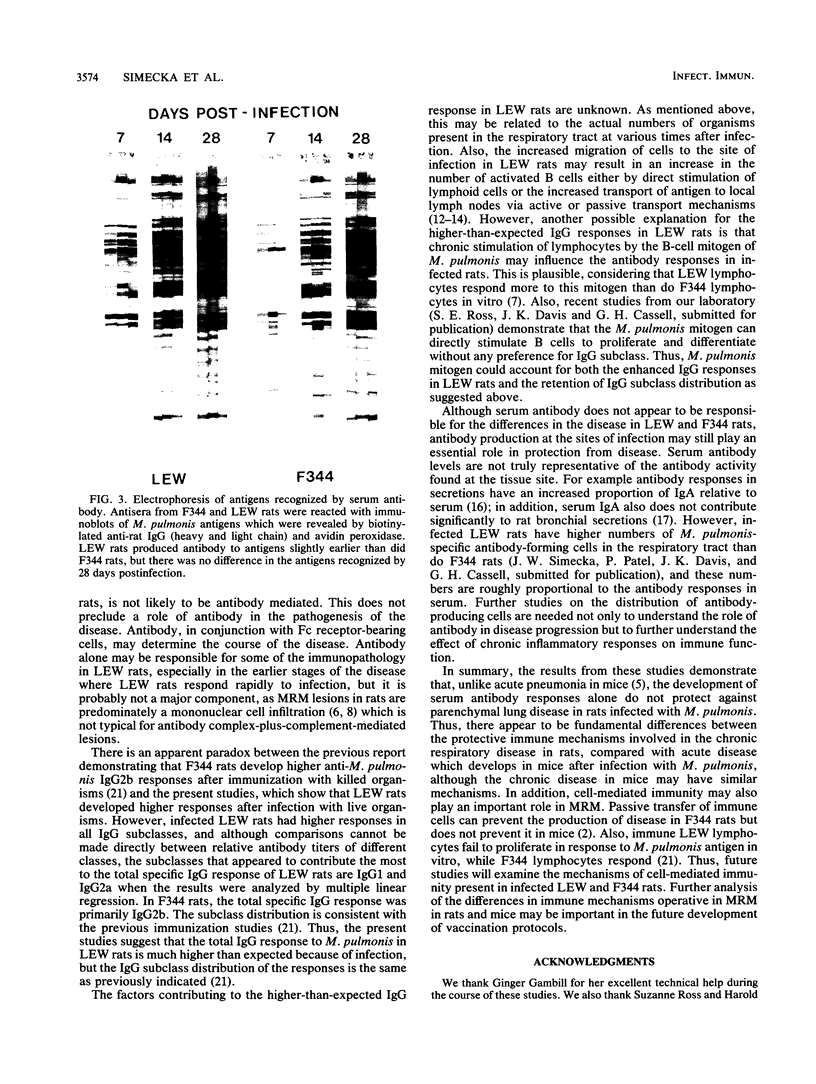
Chronic respiratory disease in rats, resulting from Mycoplasma pulmonis infection, is useful in the study of the immunological mechanisms in similar inflammatory diseases and provides a unique opportunity to study the interactions between systemic and mucosal immune systems in a naturally occurring infection. The present study examined the serum antibody responses to M. pulmonis in strains of rats which differ in disease progression and severity; LEW rats developed more severe disease than did F344 rats. Serum antibody responses were evaluated as to their levels, isotypes, and antigens recognized. Infected LEW rats produced greater or equal levels of the major classes of serum antibody to M. pulmonis than did infected F344 rats, suggesting that development of serum antibody responses alone does not resolve lesions and is not responsible for the difference in disease severity found in LEW and F344 rats. Although LEW rats produced higher responses in all subclasses of immunoglobulin G (IgG), the specific IgG response of LEW rats was composed predominately of IgG1 and IgG2a subclasses, while IgG2b was the major component of the IgG response in F344 rats. Finally, LEW rats responded more quickly to M. pulmonis antigens than did F344 rats, and there was no difference in the antigens eventually recognized by each strain, confirming previous work which suggested that LEW rats do not exhibit an unresponsiveness to a specific antigen(s) of M. pulmonis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cassell G. H., Davis J. K. Protective effect of vaccination against Mycoplasma pulmonis respiratory disease in rats. Infect Immun. 1978 Jul;21(1):69–75. doi: 10.1128/iai.21.1.69-75.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell G. H. Derrick Edward Award Lecture. The pathogenic potential of mycoplasmas: Mycoplasma pulmonis as a model. Rev Infect Dis. 1982 May-Jun;4 (Suppl):S18–S34. doi: 10.1093/clinids/4.supplement_1.s18. [DOI] [PubMed] [Google Scholar]
- Davis J. K., Cassell G. H. Murine respiratory mycoplasmosis in LEW and F344 rats: strain differences in lesion severity. Vet Pathol. 1982 May;19(3):280–293. doi: 10.1177/030098588201900306. [DOI] [PubMed] [Google Scholar]
- Davis J. K., Simecka J. W., Williamson J. S., Ross S. E., Juliana M. M., Thorp R. B., Cassell G. H. Nonspecific lymphocyte responses in F344 and LEW rats: susceptibility to murine respiratory mycoplasmosis and examination of cellular basis for strain differences. Infect Immun. 1985 Jul;49(1):152–158. doi: 10.1128/iai.49.1.152-158.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. K., Thorp R. B., Maddox P. A., Brown M. B., Cassell G. H. Murine respiratory mycoplasmosis in F344 and LEW rats: evolution of lesions and lung lymphoid cell populations. Infect Immun. 1982 May;36(2):720–729. doi: 10.1128/iai.36.2.720-729.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Guidice R. A., Barile M. F. Immunofluorescent procedures for mycoplasma identification. Dev Biol Stand. 1974;23:134–137. [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Green G. M. Alveolobronchiolar transport mechanisms. Arch Intern Med. 1973 Jan;131(1):109–114. [PubMed] [Google Scholar]
- Harmsen A. G., Mason M. J., Muggenburg B. A., Gillett N. A., Jarpe M. A., Bice D. E. Migration of neutrophils from lung to tracheobronchial lymph node. J Leukoc Biol. 1987 Feb;41(2):95–103. doi: 10.1002/jlb.41.2.95. [DOI] [PubMed] [Google Scholar]
- Harmsen A. G., Muggenburg B. A., Snipes M. B., Bice D. E. The role of macrophages in particle translocation from lungs to lymph nodes. Science. 1985 Dec 13;230(4731):1277–1280. doi: 10.1126/science.4071052. [DOI] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- Kaltreider H. B., Chan M. K. The class-specific immunoglobulin composition of fluids obtained from various levels of the canine respiratory tract. J Immunol. 1976 Feb;116(2):423–429. [PubMed] [Google Scholar]
- Lemaître-Coelho I., Yamakido M., Montgomery P. C., Langendries A. E., Vaerman J. P. Selective excretion of IgA in rat bronchial secretions: lack of significant contribution from plasma IgA. Immunol Commun. 1982;11(6):441–453. doi: 10.3109/08820138209050741. [DOI] [PubMed] [Google Scholar]
- Minion F. C., Brown M. B., Cassell G. H. Identification of cross-reactive antigens between Mycoplasma pulmonis and Mycoplasma arthritidis. Infect Immun. 1984 Jan;43(1):115–121. doi: 10.1128/iai.43.1.115-121.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R. F., Davis J. K., Blalock D. K., Thorp R. B., Simecka J. W., Cassell G. H. Pulmonary clearance of Mycoplasma pulmonis in C57BL/6N and C3H/HeN mice. Infect Immun. 1987 Nov;55(11):2631–2635. doi: 10.1128/iai.55.11.2631-2635.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeb T. R., Davidson M. K., Lindsey J. R. Intracage ammonia promotes growth of Mycoplasma pulmonis in the respiratory tract of rats. Infect Immun. 1982 Oct;38(1):212–217. doi: 10.1128/iai.38.1.212-217.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simecka J. W., Cassell G. H. Serum antibody and cellular responses in LEW and F344 rats after immunization with Mycoplasma pulmonis antigens. Infect Immun. 1987 Mar;55(3):731–735. doi: 10.1128/iai.55.3.731-735.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]