Abstract
Objectives
Sentinel surveys in Bissau, the capital of Guinea‐Bissau, have shown low prevalence of HIV‐1 but high HIV‐2 prevalence before 1998. Guinea‐Bissau experienced a civil war in 1998–1999. To examine specifically the trends of HIV prevalence from antenatal surveys in Bissau, Guinea‐Bissau in 1987–2004, and whether the civil war in 1998–1999 could have an effect on HIV prevalence levels after the conflict.
Methods
Since 1987, anonymous HIV testing in delivering women has been performed at the maternity clinic, Simão Mendes National Hospital, Bissau, as part of the national sentinel surveillance programme. Consecutive sampling was performed for approximately 3 months between September and December each year. Serological analyses were performed at the National Public Health Laboratory in Guinea‐Bissau.
Results
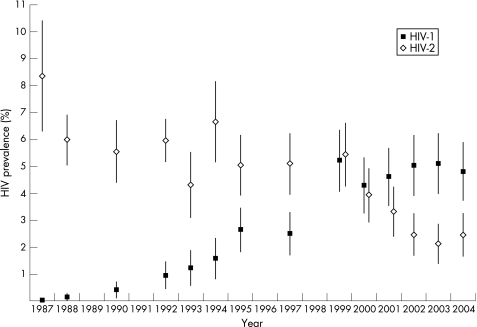
A total of 20 422 women were tested for HIV between 1987 and 2004. The total HIV‐1 prevalence increased from 0.0% in 1987 to 4.8% in 2004 and the total HIV‐2 prevalence decreased from 8.3% in 1987 to 2.5% in 2004. The HIV‐1 prevalence increased from 2.5% in 1997 to 5.2% in 1999, but stabilized in subsequent years.
Conclusions
There was a significant increase in HIV‐1 prevalence in the years 1987–2004 and a significant decline in HIV‐2 prevalence over the same period. The civil war in 1998–1999 may have sparked HIV‐1 transmission, as HIV‐1 prevalence more than doubled between 1997 and 1999, but there is no evidence of a long‐term effect on the trends of HIV‐1 or HIV‐2 prevalence.
Keywords: HIV‐1, HIV‐2, surveillance, Guinea‐Bissau, war
Key messages
HIV‐2 prevalence is declining among pregnant women in Bissau
HIV‐1 prevalence increased until 1999 and is now the dominant HIV type among pregnant women in Bissau
There was an increase in HIV‐1 prevalence directly after the civil war in 1998–1999 but HIV‐1 prevalence stabilized in the subsequent years and there is no evidence of a long‐term war effect
At the beginning of the HIV epidemic when the infection spread rapidly in East and Central Africa, very few cases were found in West Africa.1 In 1986, a similar virus with lower pathogenicity, which was subsequently named HIV‐2, was discovered in two patients from Guinea‐Bissau and the neighbouring Cabo Verde.2 Seroepidemiological surveys showed that HIV‐2 was mainly confined to West Africa, with the highest prevalence in Guinea‐Bissau, 8.9% in the adult urban population of Bissau in the first survey of seroprevalence in 1987.3 Declining prevalence rates of HIV‐2 along with increasing prevalence rates of HIV‐1 have, however, since then been reported from Guinea‐Bissau.4,5 In the neighbouring countries Senegal6 and The Gambia7, the prevalence of HIV‐1 and HIV‐2 has continued at a comparatively low level among pregnant women, at approximately 1% or below, whereas HIV‐2 prevalence declined from 7.0% in 1988–1991 to 4.0% in 2001–2003 in a recent study of clinical patients in The Gambia.8
The war in Guinea‐Bissau in 1998–1999 started on 7 June 1998, with a military uprising against the regime. Fierce fighting followed, which made approximately 250 000 individuals leave the capital city of Bissau for interior parts of the country. A ceasefire was agreed upon on 24 July 1998, after which some of the people returned to Bissau but had to flee again during renewed outbreaks of fighting in October 1998 and February 1999.9 The vast majority of the population resided inside the country and did not cross borders during the conflict. During the conflict there was a general decline in public services such as health institutions.10,11 In this study we specifically wanted to examine the trends of HIV prevalence from antenatal surveys in Bissau, Guinea‐Bissau in 1987–2004, and examine whether the civil war in 1998–1999 could have a possible effect on HIV prevalence levels, as monitored in the antenatal surveys before and after the conflict.
Methods
Study population
Guinea‐Bissau is a country of approximately 1.3 million inhabitants. In the United Nations Development Programme Human Development Report of 2005 it was ranked as the sixth poorest out of 177 countries.12 The capital Bissau has 250 000–300 000 inhabitants. The majority of women in the city normally give birth at the Simão Mendes National Hospital.13 Within the national sentinel surveillance system, and according to the recommendations of the World Health Organisation regarding sentinel surveillance of selected population groups, anonymous HIV testing of women giving birth at the Simão Mendes National Hospital has been performed since 1987. The testing has taken place every one or two years between August and December. During the first years the sample size varied between 700 and 2500, but since 1992 the number of women tested per year has been approximately 1500. The data from 1989–1991 have been pooled in the presentation because of lower sampling numbers during these years. Questionnaires have differed slightly over the years, but the age of the women has been recorded throughout. Age data are completely missing from the years 1987–1991 and for the first 460 women from 1995; they were probably lost in a fire during the conflict in 1998–1999 when the national laboratory was almost totally burnt down after being hit by a missile. No sampling was planned for 1998, but even if it had been planned it would probably not have been possible to perform during the civil war. For 1992–2004 when age records are available, age data are missing on average in 6.4% of cases. Results for the years 1987–1997 have been reported previously.5
Laboratory methods
HIV testing was performed at the National Public Health Laboratory, Bissau. In 1987–1994 sera were screened for HIV‐1 and HIV‐2 antibodies by ELISA using the Behring anti‐HIV‐1/HIV‐2 (Behring, Marburg, Germany) and/or Wellcozyme recombinant anti‐HIV‐1 (Wellcome, Dartford, UK) and an in‐house HIV‐2 (SBL6669) ELISA assay.14 From 1995 and onwards, screening has been performed using the Behring Enzygnost HIV‐1/HIV‐2 Plus ELISA (Behring). Confirmation of positive results was performed with Western blot analysis (Diagnostic Biotechnology anti‐HIV‐1 blot 2.2; Science Park, Singapore, or in‐house anti‐HIV‐2),15 and dually HIV‐1/HIV‐2 positive samples were confirmed by Pepti‐lav (Sanofi Diagnostics Pasteur, Marnes‐la‐Coquette, France) in the years 1987–1997. Since 1999, an alternative confirmation strategy has been used with Capillus HIV‐1/HIV‐2 (Cambridge Biotech Limited, Galway, Ireland) and Immunocomb II HIV‐1 and 2 BiSpot RST (Orgenics, Yavne, Israel).16 The three screening assays used have all been evaluated in parallel with each other in order to avoid changes to assays with too different performance characteristics. The change from Behring anti‐HIV‐1/HIV‐2 to Enzygnost HIV‐1/HIV‐2 Plus ELISA was caused by an upgrade of the assays by the manufacturer mainly to include HIV‐1 subtype O (no cases of that subtype have been found in Guinea‐Bissau). The alternative confirmatory strategy introduced in 1999 was found to have very similar sensitivity and specificity to the ELISA/Western blot‐based strategy used until then. The new strategy allowed screening with rapid/simple tests and confirmation without Western blot 16. The gold standard for the diagnostics has over the years been our in‐house Western blot for HIV‐2 14 and the Diagnostic Biotechnology anti‐HIV‐1 blot for HIV‐1. The Pepti‐lav and Immunocomb assays are virtually equal in their capacity to differentiate between HIV‐1 and HIV‐2. 16,17
Statistical analysis
For the years 1987 to 2004 HIV‐1 and HIV‐2 prevalence and trends were calculated from the whole statistic material of tested women. Ninety‐five per cent confidence intervals (CI) were derived from approximation based on normal distribution. For the years when data on age were present (not 1987–1991) we analysed the material stratified by age groups 15–19, 20–24, 25–29 and 30–45 years. The reason for grouping the women between 30 and 45 years in only one group was that there were very few individuals above the age 35 years, and grouping in the age groups 35–39 and 40–45 years would have given very small groups. The data were also analysed for the age groups 15–24 years and 25–45 years, as the age group 15–24 years is a core indicator recommended by UNAIDS guidelines.18 The mean age was compared for the different years and an age‐controlled analysis was performed to exclude age‐dependent reasons for a change in HIV prevalence. When calculating the total HIV‐1 and HIV‐2 prevalence, dually HIV‐1/HIV‐2 reactive samples were added both to the HIV‐1 and HIV‐2 group. SPSS 12 software was used for statistical analysis (SPSS Inc., Chicago, Illinois, USA).
Ethics
The study received ethical approval from the Ministry of Health of Guinea‐Bissau.
Results
A total number of 20 422 women have been HIV tested over a period of 18 years. Between 1987 and 2004 the total HIV‐1 prevalence (including dually HIV‐1/HIV‐2 infected) increased from 0% to 4.8% (p<0.0001, χ2 test for trend). From a total HIV‐1 prevalence of 2.5% (95% CI 1.7–3.3) in 1997, the prevalence more than doubled to 5.2% (95% CI 4.1–6.3) in 1999 (odds ratio (OR) 2.15, p = 0.00012, 95% CI 1.44–3.20). In comparison, a linear regression weighted by the number of observations for the years 1987–1997, gave a predicted HIV prevalence value for 1999 of 3.2% (95% CI 2.6–3.7). In subsequent years the total HIV‐1 prevalence has been fairly stable at approximately 5%. Between 1987 and 2004, the total HIV‐2 prevalence (including dually HIV‐1/HIV‐2 infected) decreased from 8.3% to 2.5% (χ2 test for trend, p<0.0001). The HIV‐2 prevalence in 1999 was slightly higher compared with 1997 (5.4% versus 5.1%), but after 1999 the prevalence continued to decrease (figure 1, table 1).
Figure 1 HIV‐1 and HIV‐2 total prevalence (with 95% confidence intervals) of all women for the years 1987–2004. HIV‐1 and HIV‐2 double infections are added into each group.
Table 1 HIV prevalence among pregnant women giving birth at the maternity ward of Simão Mendes National Hospital 1987–2004.
| Year | Number | HIV‐1 % (n) | HIV‐2 % (n) | HIV‐1/2 % (n) | HIV‐1 total %a | HIV‐2 total %a |
|---|---|---|---|---|---|---|
| 1987 | 707 | 0.0 (0) | 8.3 (59) | 0.0 (0) | 0.0 | 8.3 |
| 1988 | 2539 | 0.1 (3) | 6.0 (152) | 0.0 (0) | 0.1 | 6.0 |
| 1989–1991 | 1514 | 0.4 (6) | 5.5 (84) | 0.0 (0) | 0.4 | 5.5 |
| 1992 | 1494 | 0.9 (14) | 6.0 (89) | 0.0 (0) | 0.9 | 6.0 |
| 1993 | 1087 | 0.9 (10) | 4.1 (44) | 0.3 (3) | 1.2 | 4.3 |
| 1994 | 1095 | 1.4 (15) | 6.5 (71) | 0.2 (2) | 1.6 | 6.7 |
| 1995 | 1487 | 1.9 (28) | 4.3 (64) | 0.7 (11) | 2.0 | 5.0 |
| 1996 | No survey performed | |||||
| 1997 | 1491 | 2.0 (30) | 4.6 (69) | 0.5 (7) | 2.5 | 5.1 |
| 1998 | No survey performed | |||||
| 1999 | 1505 | 3.9 (59) | 4.2 (63) | 1.3 (19) | 5.2 | 5.4 |
| 2000 | 1498 | 3.6 (54) | 3.2 (49) | 0.7 (10) | 4.3 | 3.9 |
| 2001 | 1502 | 4.2 (63) | 2.9 (44) | 0.4 (6) | 4.6 | 3.3 |
| 2002 | 1498 | 4.7 (70) | 2.2 (32) | 0.3 (5) | 5.0 | 2.5 |
| 2003 | 1499 | 4.7 (71) | 1.8 (27) | 0.3 (5) | 5.1 | 2.1 |
| 2004 | 1506 | 4.2 (63) | 1.9 (28) | 0.6 (9) | 4.8 | 2.5 |
aHIV‐1/HIV‐2 double‐infected included.
For the surveys from 1992 to 2004, the mean age calculated for all women (HIV‐negative and HIV‐positive) showed a slight increase over time that was slightly significant (Spearman's rank correlation coefficient 0.047). The maximum difference in mean age between the years was 0.87 years (23.08 in 1995 and 23.95 in 2003).
To exclude the possibility that the observed changes in HIV prevalence were confounded by age, an age‐correlated logistic regression analysis was performed, showing that trends of HIV‐1 prevalence (p<0.0001) and HIV‐2 prevalence (p<0.0001) were not dependent on the overall mean age of the women.
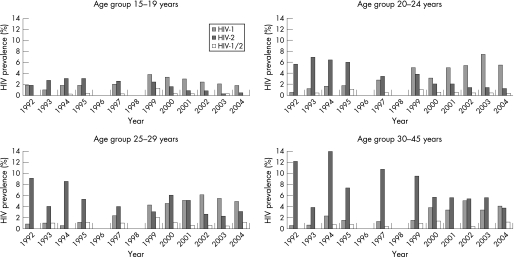
We then stratified the women by the age groups 15–19, 20–24, 25–29 and 30–45 years for the years 1992–2004, in which 14 225 women had recorded age (table 2). A total of 64 subjects were under 15 years or over 45 years and were excluded from the analysis. The HIV‐1 prevalence increased in all age groups in the 1999 sample, after that we observed a steady decline in the age group 15–19 years, from 5.1% in 1999 to 1.8% in 2004. In contrast to this, in the age groups 20–24 and 25–29 years, the HIV‐1 prevalence has continued at a level of approximately 6%, and in the oldest age group (30–45 years) the HIV‐1 prevalence has thereafter varied on a level of approximately 4%. HIV‐2 prevalence was higher in the older age groups throughout the observation period, and a gradual decline was seen in all age groups (figure 2).
Table 2 Number of women for each age group for the years 1992–2004, when age data were available (see HIV prevalence in fig 2).
| Age group | 1992 | 1993 | 1994 | 1995 | 1997 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 15–19 | 432 | 293 | 323 | 272 | 386 | 373 | 368 | 368 | 371 | 383 | 379 |
| 20–24 | 366 | 276 | 311 | 273 | 435 | 505 | 544 | 496 | 517 | 489 | 511 |
| 25–29 | 276 | 200 | 211 | 188 | 303 | 352 | 266 | 312 | 346 | 363 | 357 |
| 30–45 | 214 | 156 | 137 | 137 | 251 | 220 | 212 | 251 | 242 | 251 | 246 |
| Total | 1288 | 925 | 982 | 870 | 1375 | 1450 | 1390 | 1427 | 1475 | 1486 | 1493 |
Figure 2 HIV‐1, HIV‐2 and HIV‐1/2 prevalence stratified by age groups for the years 1992–2004, when age data were available.
From 1992 to 2004, when stratified by the age groups 15–24 and 25–45 years only, the HIV‐1 prevalence among women aged 15–24 years increased from 1.2% to 5.7%, whereas in the same time period the overall HIV‐2 prevalence in the same age group decreased from 3.6% to 1.7%. In the age group 25–45 years the HIV‐1 prevalence increased from 1.4% to 6.9%, whereas the HIV‐2 prevalence decreased from 10.4% to 5.8%.
Discussion
We observed a significant increase in HIV‐1 prevalence and a significant decrease in HIV‐2 prevalence among 20 422 women between 1987 and 2004. It is noteworthy that in the initial survey in 1987, no HIV‐1‐positive subject was found.
There are no exact data on the percentage of women giving birth at the Simão Mendes National Hospital. There is, however, no indication of changing numbers of deliveries over the study period, so this would hardly bias the results. Age is self‐reported in the surveillance system as in most studies of antenatal prevalence in African countries, thus the individual age might be disputable in some cases. As described in the Methods section, HIV tests have changed over time, with a possible variation in sensitivity and specificity. A further weakness of the study is the lack of systematically repeated behavioural data, creating difficulties in the interpretation of the trends and limiting the use of the data in responding to the epidemic.
Compared with the majority of other West African countries there was a sharper increase of HIV‐1 prevalence until 1999. Since 2000 the HIV‐1 prevalence has been fairly stable in the region, including Guinea‐Bissau. In Bissau, however, the prevalence stays on a higher level compared with figures from sentinel studies from Senegal and The Gambia. In the whole West African sub‐region only Ivory Coast, Burkina Faso and Togo have reported higher total HIV prevalence in antenatal surveys since 2000.19 In a recent report from Ivory Coast, a decrease in overall HIV prevalence (no distinction was made between HIV‐1 and HIV‐2) in pregnant women was reported, from 14–15% in 1995–1996 to 11% in 2002, with a drop from 15% to 8% in 18–22‐year‐old women. The changes were presumed to be the result of different phenomena such as the ageing of the epidemic and behavioural changes.20 Differences in resources for prevention programmes, general health services and education, levels of political stability and population migration, as well as cultural, religious and behavioural differences makes comparisons between countries difficult. The HIV‐1 epidemic in West Africa is generally dominated by an A/G recombinant strain,21,22 which makes a biological explanation for the differences less likely. In many countries no distinction is made between HIV‐1 and HIV‐2, in spite of substantial differences in transmission rates, disease progression and mortality between the two infections.23 In this study, without distinction, there would only have been a recorded difference in overall HIV prevalence from 8.3% to 6.6% over these 18 years.
The differences in HIV‐1 prevalence between the different age groups were mostly marked between the older groups and the age group 15–20 years, in which HIV‐1 prevalence increased in the first survey after the war (1999) but in the subsequent years declined to a level equivalent to the pre‐war situation. An explanation for declining HIV‐1 prevalence in this group could be behavioural change, with better disease and prevention awareness, as reported from Ivory Coast,20 but the recorded HIV‐1 prevalence in 1999 might also be the recorded result of a group of younger women having been more sexually active during the war. The increase in HIV‐1 prevalence between 1997 and 1999 in all age groups was striking, 5.2% in 1999 compared with a predicted HIV‐1 prevalence of 3.2% based on the years 1987–1997. The stable HIV‐1 prevalence level in the subsequent years demonstrates the importance of long‐term investigations to avoid precipitative conclusions. One possible scenario is that the HIV‐1 epidemic has now entered a steady state and will continue at this level. Another possible scenario is that the HIV‐1 epidemic curve reached a peak after the civil war in 1998–1999, stabilized in subsequent years and is now returning to the increasing trend curve seen before the war, with maybe further increase in the coming years.
We can only speculate on the number of newly infected women during the war. The sampling of pregnant women giving birth in 1999 took place from August until December 1999, thus indicating conceptions from November 1998 to March 1999, i.e. in a time that was characterized by repetitive major population displacements. The observed increase in HIV‐1 prevalence in pregnant women might be a result of war‐related effects such as changed sexual behaviour and increased female vulnerability, as seen in other places,24 but there is no evidence that it has affected the long‐term trend of HIV‐1 prevalence. Conflicts have in some cases been shown to increase the transmission of HIV in societies by mechanisms such as population movements, increase in commercial sexual activities, sexual abuse, transitory sexual relations as well as the collapse of health structures leading to unsafe injections and blood transfusion routines.25,26 Armed troops have been shown in some places to have significantly higher HIV prevalence than the general population,27 and this has also been the case for peacekeeping forces.28 There are also several examples of settings in which armed conflict has retarded the transmission of HIV by means such as the reduced mobility and accessibility of populations and increased death rates among high‐risk groups.29,30
We observed a decline in the HIV‐2 prevalence among pregnant women in Bissau from 8.3% to 2.5% between 1987 and 2004. Similar results of declining HIV‐2 prevalence have also been observed in surveys of the general population4 and of a professional cohort5 in Guinea‐Bissau, as well as of patients attending clinics in The Gambia.8 The reasons for the previously high HIV‐2 prevalence in Guinea‐Bissau are not clear, but could partly be a result of the prolonged war of independence in 1963–1974. It has been suggested that transmission then might have been driven by multiple mechanisms, such as inoculation campaigns, non‐sterile surgical procedures or injections, blood transfusions and sexual transmission.31 None of the neighbouring countries had a similar period of prolonged armed conflict during the 1960s and 1970s and surveys in those countries have shown lower levels of HIV‐2 prevalence in the 1980s.6,7 The declining prevalence of HIV‐2 observed in this study could thus be the return of HIV‐2 prevalence to a steady state level similar to levels in The Gambia and Senegal. Few data regarding changes in sexual behaviour are available to support any hypothesis of declining HIV‐2 prevalence caused by sexual behaviour changes, and increasing HIV‐1 prevalence during the same period contradicts this hypothesis. An alternative explanation to the declining prevalence of HIV‐2 is offered in a mathematical model by Anderson et al.32, which implies that in countries where both HIV‐1 and HIV‐2 are being transmitted, HIV‐1 will outcompete and in a longer perspective displace HIV‐2 because of higher reproductive rates. We could not find any evidence of the civil war in 1998–1999 halting the trend in the long‐term decline in HIV‐2 prevalence. Only in the 1999 measurement was a slight increase recorded, but after that came a clear post‐war decline in all age groups. Further research is needed to gain knowledge on behavioural patterns in Bissau, and future surveillance will tell whether treatment and prevention efforts can help to stabilize or halt the HIV‐1 epidemic, or if HIV‐1 prevalence will again increase as seen before the civil war.
In conclusion, there has been a significant shift in prevalence rates of HIV‐1 and HIV‐2 in women giving birth at the maternity clinic in Bissau, away from the less virulent HIV‐2 infection towards a dominance by the more aggressive HIV‐1 infection. The observed increase in HIV‐1 prevalence in pregnant women might have been sparked by war effects such as population movements, but there is no evidence that the war affected the long‐term trends or the prevalence of HIV‐1 or HIV‐2.
Acknowledgements
The authors would like to thank all the staff at the maternity ward at Simão Mendes National Hospital, Bissau, and Amadu Baldé, Ansu Biai, Cidia Camara, Braima Dabo(deceased), Carla Pereira, Julieta Pinto Delgado, Leonvengilda Fernandes Mendes and Ana Monteiro at the National Public Health Laboratory in Bissau for their help with the collection and analysis of the samples. The authors would also like to thank Håkan Lövkvist at RSKC, Lund University Hospital, Sweden and Per‐Erik Isberg, Department of Statistics, Faculty of Social Sciences, Lund University, Sweden, for help with the statistics.
Contributors
A.A. was medically responsible for the clinical site, Z.J.dS. and F.D. were responsible for the HIV testing at the laboratory, S.A. developed the HIV testing algorithm, G.B. and E.M.F. were responsible for project coordination. F.M. and H.N. coordinated the laboratory and clinical work and were lead authors of the manuscript, all other authors scrutinized the manuscript and suggested corrections.
Footnotes
Funding: The sentinel surveys were funded by the Department for Research Cooperation (SAREC) at the Swedish International Development Agency (Sida).
Competing interests: None.
References
- 1.World Health Organisation WHO global AIDS survey. Wkly Epidem Rec 198762237 [Google Scholar]
- 2.Clavel F, Guetard D, Brun‐Vezinet F.et al Isolation of a new human retrovirus from West African patients with AIDS. Science 1986233343–346. [DOI] [PubMed] [Google Scholar]
- 3.Poulsen A G, Aaby P, Gottschau A.et al HIV‐2 infection in Bissau, West Africa, 1987–1989: incidence, prevalences, and routes of transmission. J Acquir Immune Defic Syndr 19936941–948. [PubMed] [Google Scholar]
- 4.Larsen O, da Silva Z, Sandstrom A.et al Declining HIV‐2 prevalence and incidence among men in a community study from Guinea‐Bissau. AIDS 1998121707–1714. [DOI] [PubMed] [Google Scholar]
- 5.Norrgren H, Andersson S, Biague A J.et al Trends and interaction of HIV‐1 and HIV‐2 in Guinea‐Bissau, west Africa: no protection of HIV‐2 against HIV‐1 infection. AIDS 199913701–707. [DOI] [PubMed] [Google Scholar]
- 6.Meda N, Ndoye I, M'Boup S.et al Low and stable HIV infection rates in Senegal: natural course of the epidemic or evidence for success of prevention? AIDS 1999131397–1405. [DOI] [PubMed] [Google Scholar]
- 7.Schim van der Loeff M F, Sarge‐Njie R, Ceesay S.et al Regional differences in HIV trends in The Gambia: results from sentinel surveillance among pregnant women. AIDS 2003171841–1846. [DOI] [PubMed] [Google Scholar]
- 8.Schim van der Loeff M F, Awasana A A, Sarge‐Njie R.et al Sixteen years of HIV surveillance in a West African research clinic reveals divergent epidemic trends of HIV‐1 and HIV‐2. Int J Epidemiol 2006351322–1328. [DOI] [PubMed] [Google Scholar]
- 9.Massey S. Multi‐faceted mediation in the Guinea‐Bissau civil war. Scientia Militaria 20043276–95. [Google Scholar]
- 10.Rudebeck L.On democracy's sustainability: transition in Guinea‐Bissau. Stockholm: Sida, 2001
- 11.Gustafson P, Gomes V F, Vieira C S.et al Tuberculosis mortality during a civil war in Guinea‐Bissau. JAMA 2001286599–603. [DOI] [PubMed] [Google Scholar]
- 12.United Nations Development Programme Human Development Report 2005: International cooperation at a crossroads: aid, trade and security in an unequal world. New York: Human Development Reports, 2005
- 13.Labbe A C, Mendonca A P, Alves A C.et al The impact of syphilis, HIV‐1, and HIV‐2 on pregnancy outcome in Bissau, Guinea‐Bissau. Sex Transm Dis 200229157–167. [DOI] [PubMed] [Google Scholar]
- 14.Biberfeld G, Thorstensson R, Bergstrom M.et al Enzyme immunoassays for the demonstration of antibodies to HIV‐2SBL‐6669 and HTLV‐IV (SIVmac). AIDS 19882195–199. [PubMed] [Google Scholar]
- 15.Albert J, Bredberg U, Chiodi F.et al A new human retrovirus isolate of West African origin (SBL‐6669) and its relationship to HTLV‐IV, LAV‐II, and HTLV‐IIIB. AIDS Res Hum Retroviruses 198733–10. [DOI] [PubMed] [Google Scholar]
- 16.Andersson S, da Silva Z, Norrgren H.et al Field evaluation of alternative testing strategies for diagnosis and differentiation of HIV‐1 and HIV‐2 infections in an HIV‐1 and HIV‐2‐prevalent area. AIDS 1997111815–1822. [DOI] [PubMed] [Google Scholar]
- 17.Walther‐Jallow L, Andersson S, da Silva Z.et al High concordance between polymerase chain reaction and antibody testing of specimens from individuals dually infected with HIV types 1 and 2 in Guinea‐Bissau, West Africa. AIDS Res Hum Retroviruses 199915957–962. [DOI] [PubMed] [Google Scholar]
- 18.UNAIDS Monitoring the Declaration of Commitment on HIV/AIDS – Guidelines on Construction of Core Indicators. Geneva: UNAIDS, 2005
- 19.Asamoah‐Odei E, Garcia Calleja J M, Boerma J T. HIV prevalence and trends in sub‐Saharan Africa: no decline and large subregional differences. Lancet 200436435–40. [DOI] [PubMed] [Google Scholar]
- 20.Msellati P, Sakarovitch C, Bequet L.et al Decrease of human immunodeficiency virus prevalence in antenatal clinics in Abidjan, Cote d'Ivoire, 1995–2002. Int J STD AIDS 20061757–60. [DOI] [PubMed] [Google Scholar]
- 21.Andersson S, Norrgren H, Dias F.et al Molecular characterization of human immunodeficiency virus (HIV)‐1 and ‐2 in individuals from Guinea‐Bissau with single or dual infections: predominance of a distinct HIV‐1 subtype A/G recombinant in West Africa. Virology 1999262312–320. [DOI] [PubMed] [Google Scholar]
- 22.Montavon C, Toure‐Kane C, Liegeois F.et al Most env and gag subtype A HIV‐1 viruses circulating in West and West Central Africa are similar to the prototype AG recombinant virus IBNG. J Acquir Immune Defic Syndr 200023363–374. [DOI] [PubMed] [Google Scholar]
- 23.Schim van der Loeff M F, Aaby P. Towards a better understanding of the epidemiology of HIV‐2. AIDS. 1999;13(Suppl. A)S69–S84. [PubMed]
- 24.Mills E J, Singh S, Nelson B D.et al The impact of conflict on HIV/AIDS in sub‐Saharan Africa. Int J STD AIDS 200617713–717. [DOI] [PubMed] [Google Scholar]
- 25.Hankins C A, Friedman S R, Zafar T.et al Transmission and prevention of HIV and sexually transmitted infections in war settings: implications for current and future armed conflicts. AIDS 2002162245–2252. [DOI] [PubMed] [Google Scholar]
- 26.Khaw A J, Salama P, Burkholder B.et al HIV risk and prevention in emergency‐affected populations: a review. Disasters 200024181–197. [DOI] [PubMed] [Google Scholar]
- 27.UNAIDS 2004 Report on the global AIDS epidemic: 4th global report. Geneva: UNAIDS, 2004175–182.
- 28.Tripodi P, Patel P. HIV/AIDS, peacekeeping and conflict crises in Africa. Med Confl Surviv 200420195–208. [DOI] [PubMed] [Google Scholar]
- 29.Spiegel P B. HIV/AIDS among conflict‐affected and displaced populations: dispelling myths and taking action. Disasters 200428322–339. [DOI] [PubMed] [Google Scholar]
- 30.Mock N B, Duale S, Brown L F.et al Conflict and HIV: a framework for risk assessment to prevent HIV in conflict‐affected settings in Africa. Emerg Themes Epidemiol 200416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poulsen A G, Aaby P, Jensen H.et al Risk factors for HIV‐2 seropositivity among older people in Guinea‐Bissau. A search for the early history of HIV‐2 infection. Scand J Infect Dis 200032169–175. [DOI] [PubMed] [Google Scholar]
- 32.Anderson R M, May R M. The population biology of the interaction between HIV‐1 and HIV‐2: coexistence or competitive exclusion? AIDS 1996101663–1673. [DOI] [PubMed] [Google Scholar]