Abstract
OBJECTIVES
To evaluate the day-night variation of acute myocardial infarction (MI) in patients with obstructive sleep apnea (OSA).
BACKGROUND
OSA has a high prevalence and is characterized by acute nocturnal hemodynamic and neurohormonal abnormalities that may increase the risk of MI during the night.
METHODS AND RESULTS
We prospectively studied 92 patients with MI, for which the time of onset of chest pain was clearly identified. The presence of OSA was determined by overnight polysomnography. For patients with and without OSA we compared the frequency of MI during different intervals of the day based on the onset time of chest pain. The groups had similar prevalence of comorbidities. MI occurred between midnight and 6am in 32% of OSA patients and 7% of non-OSA patients (P=0.01). The odds of having OSA in those patients whose MI occurred between midnight and 6am was six-fold higher than in the remaining 18 hours of the day (95% C.I: 1.3 −27.3, P=0.01). Of all patients having an MI between midnight and 6am, 91% had OSA.
CONCLUSIONS
The diurnal variation in the onset of MI in OSA patients is strikingly different from the diurnal variation in non-OSA patients. Patients with nocturnal onset of MI have a high likelihood of having OSA. These findings suggest that OSA may be a trigger for MI. Patients having nocturnal onset of MI should be evaluated for OSA, and future research should address the effects of OSA therapy for prevention of nocturnal cardiac events.
CONDENSEND ABSTRACT
We studied 92 patients with MI, for whom the time of onset of chest pain was clearly identified. MI occurred between midnight and 6am in 32% of OSA patients and 7% of non-OSA patients (P=0.01). The odds of having OSA in those patients whose MI occurred between midnight and 6am was 6 fold higher than in the remaining 18 hours of the day (95% C.I: 1.3 −27.3, P=0.01). The diurnal variation in the onset of MI in patients with OSA is strikingly different; patients with nocturnal onset of MI have a high likelihood of having OSA.
Keywords: obstructive sleep apnea, myocardial infarction, circadian variation
INTRODUCTION
Obstructive sleep apnea (OSA) is an increasingly prevalent condition that remains underdiagnosed and undertreated (1). OSA may increase the risk of cardiovascular diseases, including hypertension, ischemic heart disease, stroke, heart failure, pulmonary hypertension and cardiac arrhythmias(2,3). The prevalence of OSA is two to three-fold higher in patients with a history of MI(4).
In the general population MI and SCD occur with a diurnal periodicity that peaks between 6am and noon(5). In a previous study, we found that SCD was more frequent during the night in OSA patients(6). Mechanisms of SCD could have included MI, stroke, arrhythmias, pulmonary embolus, aortic dissection, or other cardiovascular causes.
Acute nocturnal pathophysiological responses to OSA, including hypoxemia, sympathetic activation and surges in blood pressure, may lead to plaque rupture, coronary thrombosis, and MI. Should OSA be a trigger of MI, we would expect a peak of onset of symptoms of MI during the night. Whether OSA patients are more likely to have nocturnal MI has not been previously studied.
METHODS
The study was approved by the Institutional Review Board of the Mayo Clinic, and all subjects provided informed consent. We prospectively studied 92 patients admitted with incident MI to our hospital. While consecutive patients were eligible, recruitment was based on exclusion criteria listed below, on availability of research personnel, and on patients consenting to participate. The exclusion criteria were: patients without typical chest pain; uncertain time of onset of MI; and previous CPAP treatment.
The diagnosis of MI was made by the patients’ attending physician and confirmed by the following: increase in creatine-phosphokinase concentration ≥2 times the upper limit of normal, and elevation of troponin T activity (>0.03 ng/ml).
The time of onset of MI was determined by each patient’s report of the chest pain that prompted hospital admission. This strategy to assess the time of MI has been previously validated(7).
Every subject underwent comprehensive polysomnography at 17 ± 2.4 days after MI, performed with an attended complete overnight polysomnographic monitoring system. Obstructive apneas and hypopneas were classified according to standard criteria(8). An apnea-hypopnea index (AHI) ≥ 5 established the diagnosis of OSA. All polysomnographic measurements and diagnoses were made blinded to the timing of symptoms of MI.
Statistical analysis
Patients’ characteristics are presented as means (± SD) or percentages. Quantitative variables were compared with a two-tailed t-test. Qualitative data and the frequency distributions of MI for the four 6-hour intervals of the day between subjects with and without OSA were compared with the χ2 test or Fisher’s exact test (when expected frequencies below 5 occurred). Intra-group comparisons were conducted to determine the odds ratio of having OSA in patients who had an MI during each 6-hour interval compared to the remaining 18 hours of the day.
RESULTS
We studied 92 patients (71 men), mean age 61 ± 13 years and BMI 30 ± 5 kg/m2. Using a threshold of AHI ≥ 5 events/hour, OSA was present in 70% of patients. Using a more conservative threshold of AHI ≥ 10 events/hour, about half (52%) of our patient population was diagnosed with OSA. Patients’ characteristics are shown in Table 1. The two groups had similar prevalence of comorbidities. There was no difference between groups regarding medication use (Table 2 and Table 3).
Table 1.
Characteristics of the study population at the time of myocardial infarction, according to the presence or absence of obstructive sleep apnea.
| Characteristics | OSA Status | ||
|---|---|---|---|
| OSA (N=64) | No OSA (N=28) | p-value | |
| Age, year | 64 ± 12 | 57 ± 12 | 0.02 |
| Male sex (%) | 78 | 75 | 0.7 |
| Body mass index, kg/m2 | 31 ± 6 | 28 ± 4 | 0.01 |
| AHI, events/hour | 22 ± 2.1 | 1.6 ± 0.3 | <0.0001 |
| LVEF (%) | 51 ± 2 | 55 ± 2 | 0.11 |
| Peak CK, U/L | 1392 ± 296 | 1417 ± 264 | 0.9 |
| Peak CK-MB, ng/mL | 143 ± 23 | 134 ± 20 | 0.8 |
| Hypertension (%) | 57 | 57 | 1 |
| Hypercholesterolemia (%) | 67 | 61 | 0.6 |
| Diabetes mellitus (%) | 25 | 11 | 0.1 |
| Prior MI (%) | 13 | 18 | 0.5 |
| Congestive heart failure (%) | 5 | 0 | 0.5 |
| Current smoker (%) | 27 | 39 | 0.2 |
| Systolic BP, mmHg* | 120 ± 2 | 116 ± 3 | 0.2 |
| Diastolic BP, mmHg* | 69 ± 1 | 67 ± 2 | 0.4 |
| Cholesterol, mg/dL | 178 ± 5 | 161 ± 6 | 0.06 |
| Triglycerides, mg/dL | 153 ± 14 | 101 ± 11 | 0.02 |
| HDL cholesterol, mg/dL | 43 ± 2 | 41 ± 2 | 0.4 |
| LDL cholesterol, mg/dL | 108 ± 4.9 | 101 ± 6.2 | 0.4 |
| Fasting Glucose, mg/dL | 118 ± 4 | 112 ± 5 | 0.4 |
AHI= Apnea hypopnea index, BP = Blood pressure (*values at the time of PSG), CK = creatine kinase, CK-MB = creatine kinase MB isoenzyme, HDL = High-density lipoproteins, LDL = low-density lipoproteins, LVEF= left ventricular ejection fraction, measured within one week after MI, MI = myocardial infarction, OSA = obstructive sleep apnea.
Table 2.
Medications taken at the time of myocardial infarction, according to the presence or absence of obstructive sleep apnea.
| OSA Status | |||
|---|---|---|---|
| OSA (N=64) | No OSA (N=28) | p-value | |
| Aspirin (%) | 35 | 25 | 0.4 |
| Beta-blockers (%) | 25 | 29 | 0.7 |
| ACE inhibitors (%) | 16 | 21 | 0.5 |
| Statins (%) | 35 | 39 | 0.7 |
| Calcium antagonists (%) | 9 | 7 | 1 |
ACE = angiotensin converting enzyme, OSA = obstructive sleep apnea.
Table 3.
Medications at the time of the sleep study, according to the presence or absence of obstructive sleep apnea
| OSA Status | |||
|---|---|---|---|
| OSA (N=64) | No OSA (N=28) | p-value | |
| Aspirin (%) | 98 | 89 | 0.08 |
| Beta-blockers (%) | 100 | 96 | 0.3 |
| ACE inhibitors (%) | 73 | 78 | 0.6 |
| Statins (%) | 98 | 93 | 0.2 |
| Calcium antagonists (%) | 3 | 0 | 1 |
ACE = angiotensin converting enzyme, OSA = obstructive sleep apnea.
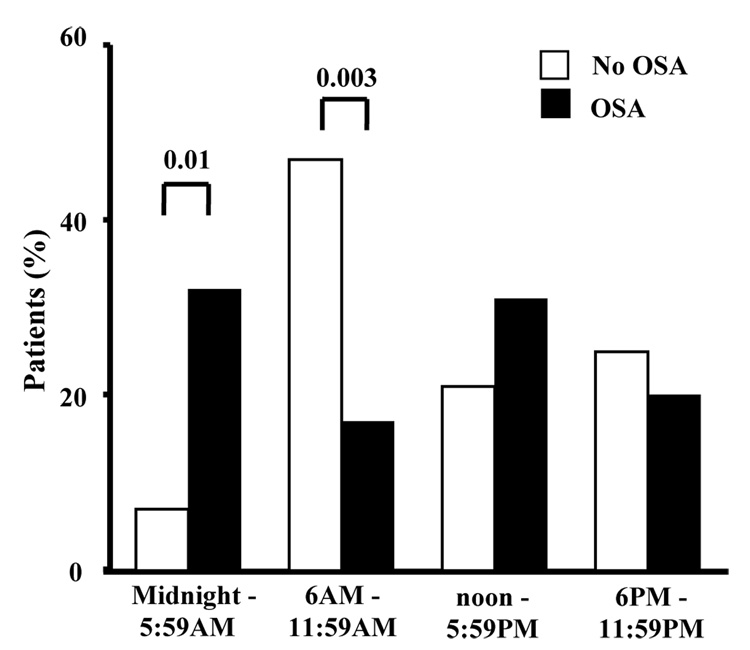
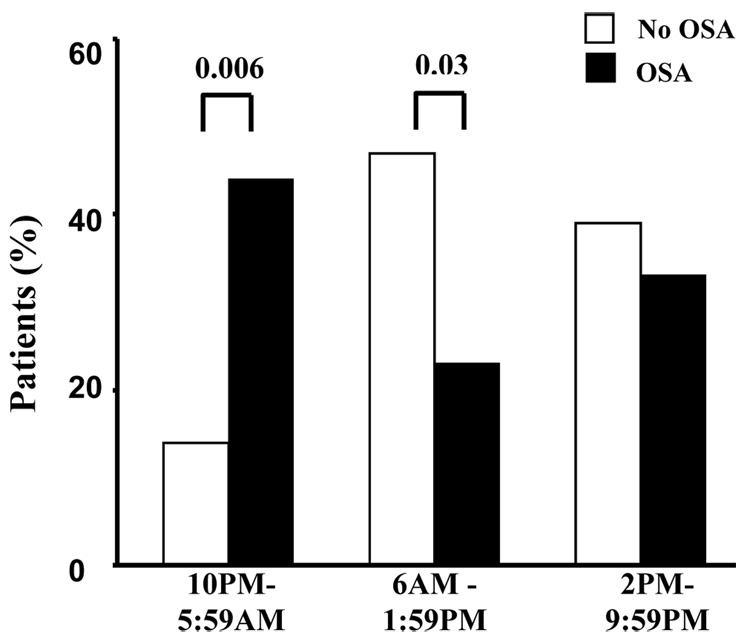
The diurnal variation in the onset of MI in OSA patients was different from that observed in non-OSA patients (Figure 1). From midnight to 6 am, the frequency of MI was significantly higher in OSA patients compared to non-OSA patients (32% vs. 7%; p=0.01). Using a threshold of AHI ≥ 10 events/hour we observed similar results (33% vs. 14%; p=0.03). From 6am to noon, the frequency of MI was higher in non-OSA patients compared to OSA patients (47% vs. 17%; p=0.003). Similar results were evident for the analyses based on three 8-hour time intervals (Figure 2). We found no difference in the use of beta-blocker therapy and the frequency of MI during the various intervals of the day.
Figure 1.
Day–night pattern of MI based on four six-hour time intervals in OSA (64) and no-OSA (28) patients.
Figure 2.
Day–night pattern of MI based on three eight-hour time intervals in OSA (64) and no-OSA (28) patients.
Patients whose MI occurred between midnight to 6am had an odds ratio of 6 for having OSA (95% C.I: 1.3 − 27.3, p=0.01). Of 22 patients who had an MI between midnight and 6am, 20 (91%) had OSA. If we used a more conservative threshold for OSA the likelihood of nocturnal MI occurring in OSA patients remains significantly higher (73%).
DISCUSSION
The novel finding of this study is that OSA patients have an increased risk of MI between midnight and 6 am, compared to non-OSA patients. Our data suggest that OSA may be a trigger for MI, with a striking reversal in the expected diurnal timing of MI onset. Conversely, non-OSA patients had a nadir of MI onset at night and a peak in the morning, similar to the diurnal distribution of MI seen in the general population. Previous studies suggest that beta-blockers(7) and diabetes(9) may attenuate the morning peak of MI. Our findings identify OSA as the first disease condition recognized to actually reverse the usual day-night variation in the incidence of MI.
OSA has been implicated in increased risk of MI, stroke and SCD(10,11). While OSA patients have a higher frequency of nocturnal ST-segment depression than those without OSA(12,13), it remained unknown whether OSA may directly cause nocturnal MI. Our findings suggest that the pathophysiology of OSA leads to an increased risk of MI during the night.
Several acute pathophysiological mechanisms during sleep in OSA patients may be responsible for their altered diurnal variation of MI. Cessation of airflow results in hypoxemia and hypercapnia, with consequent activation of the chemoreflex(14) and increased sympathetic nerve activity and blood pressure (BP) (15). Obstructed breathing with negative intra-thoracic pressures increases cardiac wall stress(16).
Peripheral vasoconstriction and increased cardiac output (due to changes in cardiac transmural pressures upon termination of apneas) lead to dramatic surges in arterial BP. These hemodynamic stresses in the setting of simultaneous hypoxemia and increased myocardial oxygen demand may promote acute nocturnal cardiac ischemia(13,17). OSA is also associated with factors that may increase the risk of nocturnal coronary thrombosis, including platelet activation during sleep(18), elevated fibrinogen levels(19), increased whole blood viscosity, and decreased fibrinolytic activity(20). These processes may be responsible for the shift in the timing of MI from the morning hours to the night in OSA patients.
Strengths of the current study include first, its prospective design. Second is the use of complete polysomnography, interpreted while blinded as to time of onset of MI. Third, the influence of OSA on timing of MI onset could not be explained by comorbidities or medications, which were similar in both groups. Potential limitations include first, the inherent uncertainty in identifying the exact timing of onset of an MI. The pathophysiology of coronary plaque rupture and arterial thrombosis is dynamic and occurs over varying time periods before symptoms or signs may manifest. These limitation parallel those of the entire body of evidence that has demonstrated the timing of MI in the general population and other subgroups(9). Previous studies have shown a strong correlation between the timing of MI, based on cardiac biomarker levels, and the onset of pain(7). Second, based on criteria noted earlier, we did not study every patient admitted with MI. Therefore, our data cannot be used to estimate the overall prevalence of OSA in patients with recent MI. Identifying the prevalence of OSA in the post-MI patient population was not a goal of this study. Nevertheless, the characteristics of our study sample are similar to those of the general MI patient population in Olmsted County(21) and although the prevalence of OSA in our population is relatively high, our findings are comparable to those noted in a prior study of OSA prevalence in the post-MI patient population(22). A third concern relates to whether OSA developed as an acute consequence of MI. Of patients found to have OSA on polysomnography, 76% had a high risk for OSA as assessed by the Berlin Questionnaire, suggesting that the OSA was indeed present prior to the MI. Furthermore, polysomnography was conducted when patients were stable. Most important, this limitation cannot account for our findings of a higher nocturnal occurrence of MI in OSA patients. Last, these studies represent survivors of MI, and do not necessarily represent all patients with acute MI.
In summary, we have demonstrated that patients with OSA have an altered diurnal variation of MI, which is consistent with the unique nocturnal pathophysiology of OSA. These findings highlight a potential causative role of OSA in the development of acute coronary syndromes, and suggest that nocturnal MI may contribute to the increased likelihood of nocturnal SCD observed in OSA patients(6). Our data further suggest that those patients who experience the onset of MI during the usual sleep hours should be evaluated for the presence of OSA. Further research is necessary to understand the effects of OSA therapy on modifying the timing of MI in these patients and in altering their overall risk of acute coronary syndromes and SCD.
Acknowledgments
FUNDING SOURCES: These studies were supported in part by funding from the Respironics Foundation for Sleep and Breathing and NIH Grants HL65176 and M01-RR00585. FHS is supported by American Heart Association grant 06-15709Z, a Perkins Memorial Award from the American Physiological Society and Espirito Santo Science and Technology Foundation. FLJ is supported by the American Heart Association. TK is supported by an unrestricted educational grant from General Electric (Europe).
ABBREVIATIONS LIST
- AHI
apnea-hypopnea index
- BMI
body mass index
- BP
blood pressure
- MI
myocardial infarction
- OSA
obstructive sleep apnea
- SCD
sudden cardiac death
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST: VKS serves as a consultant for ResMed and Respironics, and has spoken at meetings sponsored by Respironics, ResMed and Medtronic. He has also served as consultant for GlaxoSmithKline, Sepracor, and Cardiac Concepts. He has received research grants from the ResMed Foundation, the Respironics Sleep and Breathing Foundation, Sorin, Inc., and Select Research, Inc. He also works with Mayo Health Solutions and iLife on intellectual property related to sleep and to obesity.
REFERENCES
- 1.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–245. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 3.Parish JM, Somers VK. Obstructive sleep apnea and cardiovascular disease. Mayo Clin Proc. 2004;79:1036–1046. doi: 10.4065/79.8.1036. [DOI] [PubMed] [Google Scholar]
- 4.Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in men with coronary artery disease. Chest. 1996;109:659–663. doi: 10.1378/chest.109.3.659. [DOI] [PubMed] [Google Scholar]
- 5.Cohen MC, Rohtla KM, Lavery CE, Muller JE, Mittleman MA. Meta-analysis of the morning excess of acute myocardial infarction and sudden cardiac death. Am J Cardiol. 1997;79:1512–1516. doi: 10.1016/s0002-9149(97)00181-1. [DOI] [PubMed] [Google Scholar]
- 6.Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352:1206–1214. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 7.Muller JE, Stone PH, Turi ZG, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313:1315–1322. doi: 10.1056/NEJM198511213132103. [DOI] [PubMed] [Google Scholar]
- 8.Iber C, Ancoli-Israel S, Chesson A, Quan S. Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM Manual for the Scoring of Sleep and Associated Events; pp. 45–47. [Google Scholar]
- 9.Rana JS, Mukamal KJ, Morgan JP, Muller JE, Mittleman MA. Circadian variation in the onset of myocardial infarction: effect of duration of diabetes. Diabetes. 2003;52:1464–1468. doi: 10.2337/diabetes.52.6.1464. [DOI] [PubMed] [Google Scholar]
- 10.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 11.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 12.Schafer H, Koehler U, Ploch T, Peter JH. Sleep-related myocardial ischemia and sleep structure in patients with obstructive sleep apnea and coronary heart disease. Chest. 1997;111:387–393. doi: 10.1378/chest.111.2.387. [DOI] [PubMed] [Google Scholar]
- 13.Alonso-Fernandez A, Garcia-Rio F, Racionero MA, et al. Cardiac rhythm disturbances and ST-segment depression episodes in patients with obstructive sleep apnea-hypopnea syndrome and its mechanisms. Chest. 2005;127:15–22. doi: 10.1378/chest.127.1.15. [DOI] [PubMed] [Google Scholar]
- 14.Narkiewicz K, van de Borne PJ, Pesek CA, Dyken ME, Montano N, Somers VK. Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation. 1999;99:1183–1189. doi: 10.1161/01.cir.99.9.1183. [DOI] [PubMed] [Google Scholar]
- 15.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Floras JS, Bradley TD. Treating obstructive sleep apnea: is there more to the story than 2 millimeters of mercury? Hypertension. 2007;50:289–291. doi: 10.1161/HYPERTENSIONAHA.107.092106. [DOI] [PubMed] [Google Scholar]
- 17.Mooe T, Franklin KA, Wiklund U, Rabben T, Holmstrom K. Sleep-disordered breathing and myocardial ischemia in patients with coronary artery disease. Chest. 2000;117:1597–1602. doi: 10.1378/chest.117.6.1597. [DOI] [PubMed] [Google Scholar]
- 18.Sanner BM, Konermann M, Tepel M, Groetz J, Mummenhoff C, Zidek W. Platelet function in patients with obstructive sleep apnoea syndrome. Eur Respir J. 2000;16:648–652. doi: 10.1034/j.1399-3003.2000.16d14.x. [DOI] [PubMed] [Google Scholar]
- 19.Chin K, Ohi M, Kita H, et al. Effects of NCPAP therapy on fibrinogen levels in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 1996;153:1972–1976. doi: 10.1164/ajrccm.153.6.8665063. [DOI] [PubMed] [Google Scholar]
- 20.Rangemark C, Hedner JA, Carlson JT, Gleerup G, Winther K. Platelet function and fibrinolytic activity in hypertensive and normotensive sleep apnea patients. Sleep. 1995;18:188–194. doi: 10.1093/sleep/18.3.188. [DOI] [PubMed] [Google Scholar]
- 21.Gerber Y, Jacobsen SJ, Killian JM, Weston SA, Roger VL. Participation bias assessment in a community-based study of myocardial infarction, 2002–2005. Mayo Clin Proc. 2007;82:933–938. doi: 10.4065/82.8.933. [DOI] [PubMed] [Google Scholar]
- 22.Mehra R, Principe-Rodriguez K, Kirchner HL, Strohl KP. Sleep apnea in acute coronary syndrome: high prevalence but low impact on 6-month outcome. Sleep Med. 2006;7:521–528. doi: 10.1016/j.sleep.2006.03.012. [DOI] [PubMed] [Google Scholar]