Abstract
In experiments that measured food consumption, Holland (1981; Learning and Motivation, 12, 1–18) found that food aversions were formed when an exteroceptive associate of food was paired with illness, but not when such an associate was paired with shock. By contrast, measuring the ability of food to reinforce instrumental responding, Ward-Robinson and Hall (1999; Quarterly Journal of Experimental Psychology, 52B, 335–350) found that pairing an associatively-activated representation of food with shock readily established an aversion to that food. Two experiments considered the origins of these apparently discrepant results. The results did not support either the possibility that instrumental reinforcement power is a more sensitive measure of aversion learning than consumption, nor the hypothesis that illness particularly devalues properties of food representations that determine consumption (such as palatability) whereas shock devalues more general properties critical to reinforcement. The results suggested instead that whereas the effects of pairings of a food associate with illness are mediated by changes in the value of the food itself, the effects of pairings with shock are mediated by the conditioning of fear or other competing responses to the site of food delivery, and not by modification of the value of food itself.
Many learning theorists assert that, as a result of associative learning, a conditioned stimulus (CS) comes to activate an internal representation of the unconditioned stimulus (US) with which it is paired. Research over the past 30 years shows that such associatively-activated US representations (Hall, 1996) share many properties with the USs themselves, and may substitute for them in a variety of behavioral functions (for a recent review, see Holland & Wheeler, 2008). For example, Holland (1981, Exp. 1) found that a food-illness association could be learned when an associatively-activated food representation was paired with illness. In that experiment, rats received pairings of a tone with a flavored food, designed to endow the tone with the ability to activate a representation of that food. Next, the tone was paired with an illness-inducing injection of lithium chloride (LiCl), in the absence of the food. Finally, food consumption was assessed in the rats’ home cages, in the absence of the tone. As would be anticipated if the tone-activated representation of the food entered into an association with illness, consumption of the flavored food itself was reduced, relative to consumption of various control groups of rats that did not have the opportunity to associate a food representation with illness.
Holland (1981) suggested that the success of this procedure in establishing such a mediated food aversion was due in part to the privileged relation between flavor and illness in aversion learning. A great deal of data show that flavors are far more readily associated with illness than are auditory, visual, tactile, or olfactory cues (e.g. Garcia & Koelling, 1966; Garcia, Kovner & Green, 1970). In Holland’s (1981) experiment, this privileged relation between flavor and illness may have permitted establishment of flavor-illness associations, despite the absence of the flavor itself. Although the associatively-activated food representation is likely to have been relatively weak compared to the directly-activated tone that evoked it, such cue-consequence selectivity might have nonetheless favored formation of flavor-illness associations, because substantial overshadowing of that learning by tone-illness learning would be unlikely. Indeed, in that experiment, tone-LiCl pairings had no observable effect on responding to the tone itself. Furthermore, Holland (1981, Exp. 3) found no evidence for a learned food aversion when in a similar experiment the tone was paired with shock rather than illness. In that experiment, however, there was substantial suppression of learned responding to the tone as a result of those tone-shock pairings. Thus, Holland (1981) concluded that associatively-activated food representations, like representations activated directly by food itself, also exhibited privileged flavor-illness learning.
In contrast to Holland’s (1981, Experiment 3) results, Ward-Robinson and Hall (1999, Exp. 1) provided evidence that suggested an associatively-activated flavor representation could readily enter into an association with shock. Rats received discrimination training in which one auditory stimulus (CS+) signaled the delivery of flavored food, and another CS signaled nothing (CS−). Half of the rats then received pairings of CS+ with shock, and the other half received pairings of CS− with shock. Finally, the rats’ food preferences were assessed by giving them access to the food, contingent on lever-pressing, in the absence of either CS. Ward-Robinson and Hall (1999) found that the rats for which CS+ had been paired with shock showed longer lever-press inter-response times, as if the food had acquired aversive properties (or lost appetitive value) when its associatively-activated representation was paired with shock. Ward-Robinson and Hall (1999) suggested that they, unlike Holland (1981), were able to detect a flavor-shock association because food’s ability to serve as a reinforcer for instrumental learning may be a more sensitive measure of food aversion learning than food consumption itself.
A potentially more interesting account for the differences in Holland’s (1981) and Ward-Robinson and Hall’s (1999) conclusions is that illness and shock selectively devalue different aspects of associatively-activated representations of food, one responsible for consumption and one responsible for reinforcement value. For example, although both flavor-illness and flavor-shock pairings can result in flavor avoidance, only flavor-illness learning also reduces the palatability of the flavor (e.g., Berridge et al., 1981), as measured by taste-reactivity responses (e.g. Berridge, 2000). Thus, measures of consumption might be especially sensitive to pairings of a food representation with illness, even when the illness did not produce large losses in the overall value of the taste, whereas comparable pairings with shock might substantially reduce food’s overall value (including its reinforcing properties), but have relatively less impact on food consumption, because the food flavor might retain attractive oral hedonic aspects. Indeed, Berridge et al. (1981) found that although rats avoided flavors that were paired with shock, they still performed positive rather than negative taste-reactivity responses when those flavors were infused directly into their oral cavities.
Holland (1981) only measured changes in food consumption after opportunities for mediated learning, and Ward-Robinson and Hall (1999) only assessed instrumental reinforcement. Thus, there is currently no basis on which to evaluate either of these possibilities. Here we did so by directly comparing consumption and instrumental reinforcement measures of aversion learning after pairing an associatively-activated food representation with shock or LiCl. In Experiment 1, we used a single reinforcer and simple between-subjects designs similar to those used by Holland (1981; Exp. 1) and Ward-Robinson and Hall (1999). In Experiment 2 we used multiple reinforcers and a within-subjects design, similar to that used by Holland (1981, Exp. 3).
Experiment 1
In Experiment 1, rats first received tone-food pairings, followed by either tone-shock or tone-LiCl pairings, in separate groups of rats. Finally, the ability of the food to reinforce instrumental responding was assessed in half of the rats in each group, and food consumption was assessed in the other half. If Ward-Robinson and Hall’s (1999) observation of mediated aversion learning with shock reinforcers was due to the greater sensitivity of their instrumental test procedure, then that procedure might reveal even larger mediated aversions if the tone CS was paired with illness. By contrast, if the reinforcement value of food depends on different food attributes than food consumption, tone-shock pairings might especially reduce the food’s ability to serve as a reinforcer, whereas tone-LiCl pairings might especially reduce food consumption.
Methods
Subjects
The subjects were 48 male naive Sprague-Dawley strain rats (Charles River Laboratories, Raleigh, NC, USA) which weighed between 300–350 g when they arrived in the laboratory vivarium. They had free access to lab chow (2018 Rodent Diet, Harlan Teklad Laboratory, Madison, WI, USA) for a week before their food was restricted to maintain them at 85% of their ad-libitum weights. The rats were caged individually in a colony room illuminated from 6:00am to 6:00pm. The research was approved by the Duke University (Experiment 1) and Johns Hopkins University (Experiment 2) Animal Care and Use committees.
Apparatus
The experiments were conducted in 8 training chambers (22.9 × 20.3 × 20.3 cm) with aluminum front and back walls and clear acrylic side walls and top. An infrared activity monitor (Coulbourn Instruments, Allentown, PA, USA) was placed on the top of each chamber. An illuminated (neon glow-tube) food tray was recessed behind a 5.0 × 5.0 cm square hole in the center of the front wall. A photocell beam in the food cup recess was used to detect head entries and time spent in the tray. A steel chain could be suspended from a microswitch mounted above the chamber, to the right of the food tray, centered between the tray and the side wall. The chain was only available during the instrumental reinforcement tests. The floor of the chamber was composed of 0.48-cm stainless steel bars, spaced 1.9 cm apart; they could be electrified with a constant current shock generator and relay scrambler system. A speaker which was used to present auditory cues was placed on the back wall of a double-walled sound-resistant shell which enclosed each experimental chamber. Constant low-level illumination was provided by a 6-w lamp behind a red lens mounted near the speaker. A low-light TV camera in each chamber provided images during each trial, which were recorded.
Procedure
The rats first received two sessions designed to establish consistent food tray entry responses to the delivery of the food pellet reinforcer. In each 64-min session, 16 2-pellet USs (45 mg, Formula A, P.J. Noyes Co., Lancaster, NH) were delivered at random times. Next, all rats received 8 pairings of a 5-s, 80-db, 1,500-hz tone with food pellets in each of 4 64-min sessions, with variable intertrial intervals (ITIs) that averaged 8 min, rectangularly distributed within a range of 4 to 12 min. About 2 hr after each of these sessions, each rat was given 10 min exposure to an empty ceramic bowl in its home cage, to familiarize them with the consumption test procedure to be used at the end of the experiment. Then, two groups of rats received treatments designed to establish a mediated aversion to food by pairing the tone with either shock or LiCl (in the absence of food pellets), relative to a third group, which did not receive tone presentations. In the first 5-min session of this mediated aversion phase, the rats in Group Shock received 4 5-s presentations of the tone, each followed immediately by administration of a 1-s 0.5-mA electric shock. The rats in Group LiCl received the 4 tone presentations alone and were injected with 5 ml/kg of 0.6-M LiCl as they were removed from the chambers (0.5 to 5 min after the last tone). The rats in Group Control received no tone presentations in that session; half received 4 unsignaled shocks and half were injected with LiCl as they were removed from the chamber. In all groups, the tone and/or shock presentations occurred at 75-s intervals, with the first presentation occurring 75-s after the beginning of the session. The next day, each rat was placed in the chamber for 5 min, with no CS presentations, but with delivery of whichever aversive event it did not receive in the first session (i.e., 4 shocks or LiCl injection). Shocks were distributed as described for session 1. Thus, the rats in all 3 groups had similar exposure to both aversive events.
Finally, half of the rats in each group received consumption tests and half received an instrumental reinforcement test. Assessment of food consumption included two tests. In the first test, each rat was given 10 min access to a ceramic bowl containing 60 food pellets, which was placed in its home cage. In the second test, conducted the next day, the rats received 10 min access to 60 pellets placed in the food tray in the experimental chambers. The rats that received instrumental reinforcement tests were placed in the chambers for 60 min, with a chain available in each chamber. A small amount of a paste of sucrose and water was placed on each chain prior to placing the rats in the chambers to encourage initial contact. Each chain pull was reinforced with the delivery of a food pellet.
Results
Conditioning to the tone proceeded rapidly. On the final session the percentages of time the rats spent with their heads in the food tray were 62.2 ± 2.6% and 11.1 ± 1.5% during the 5-s tone and pre-CS periods, respectively. In the devaluation session, casual observation of video tapes showed that rats that received shocks quickly came to freeze, which reduced the level of food tray behavior on the final tone trial to 9.8 ± 2.8% in Group Shock, whereas the rats that received LiCl injections after the session showed only a minor reduction in food tray time (to 48.0 ± 3.7% in Group LiCl), which probably reflected extinction.
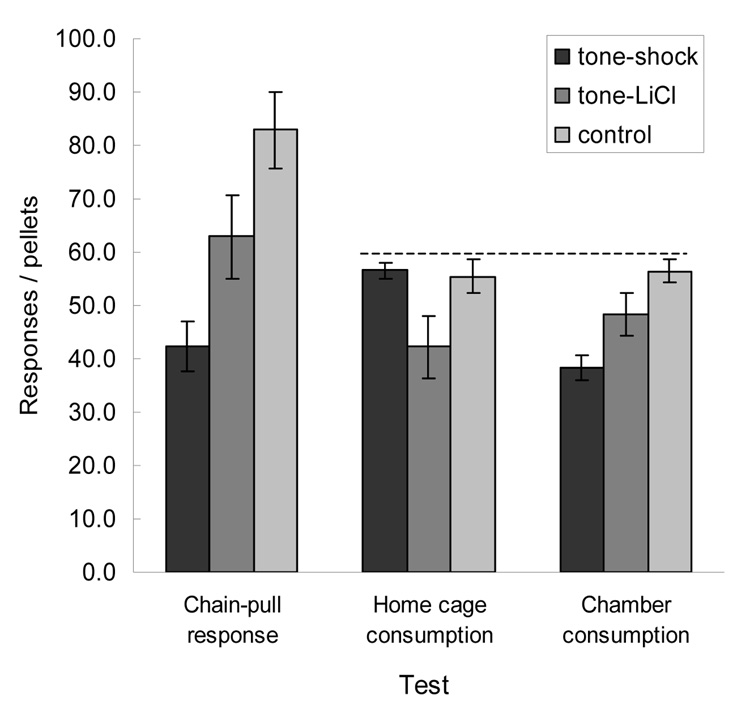
Figure 1 shows the results of the instrumental reinforcement (left pair of bars) and consumption tests (center and right bars). Both mediated aversion treatments apparently reduced the ability of the food to serve as a reinforcer for instrumental chain-pull learning relative to the control treatment. Notably, the rats in Group Shock, which had received tone-shock pairings, made fewer chain-pulls in the instrumental training session than the rats in Group LiCl, which had the tone paired with LiCl. Furthermore, the rats in each of those groups made fewer chain-pulls than the rats in the Control group. A one-way ANOVA was significant, F(2, 21) = 9.06, p = 0.001, and planned comparisons showed that each group differed significantly from each other group, ps < 0.048. By contrast, only tone-LiCl pairings reduced home-cage consumption of food pellets (center bars). An ANOVA was significant, F(2, 21) = 4.18, p = 0.030, and planned comparisons showed that Group LiCl showed significantly less consumption than either of the other groups, Ps < 0.027.
Figure 1.
Test results of Experiment 1. The left set of bars show mean ± sem instrumental chain pull responses reinforced by food after pairings of the tone with shock, lithium chloride (LiCl) or neither aversive event. The center and right sets of bars show mean ± sem food pellets consumed in the initial homecage (center) and subsequent experimental chamber (right) consumption tests after comparable tone treatments. The dotted line signifies the 60 pellets made available in each of those tests. Chain-pull and consumption tests were conducted with separate groups of rats.
Thus, at first glance these results support the notion that shock devalued food’s ability to serve as a reinforcer more than LiCl, but LiCl devalued its palatability more. However, the results of the consumption test in the experimental chamber (right bars of Figure 1), conducted after the home cage consumption test, suggest different explanations (to be considered in the discussion). In the experimental chamber, the rats in Group Shock consumed fewer pellets than the rats in Groups LiCl or Control, the opposite pattern as was observed in the home cage tests, but the same pattern as was observed for instrumental chain-pulling. An ANOVA of chamber consumption scores was significant, F(2, 21) = 9.48, p = 0.001, and planned comparisons showed that consumption of Group Shock was significantly lower than that in the other 2 groups, ps < 0.026. The difference in consumption of Groups LiCl and Control approached significance, p = 0.065.
To directly contrast home cage and chamber consumption, we performed a devaluation US (shock, LiCl, or control) × test (home cage or chamber) ANOVA, which showed a significant devaluation US × test interaction, F(2, 21) = 55.27, p < 0.001. Post-hoc comparisons using Tukey’s honest-significant-difference (HSD) procedure showed all pairwise comparisons to be significant, p < 0.025, except those between home cage consumption of Group LiCl and chamber consumption of Group Shock, p = 0.234, between Group Shock’s consumption in the home cage and Group Control’s consumption in either test, and between Group Control’s consumption in the two tests.
Discussion
We observed a double dissociation between devaluation by tone-shock or tone-LiCl pairings and assessment by a home cage consumption test (as in Holland’s, 1981, experiment) or an instrumental reinforcement test (as in Ward-Robinson & Hall’s, 1999, study). Tone-LiCl pairings reduced home cage food consumption more than tone-shock pairings, whereas tone-shock pairings reduced food’s ability to serve as a reinforcer for chain pulling more than tone-LiCl pairings. Thus, there was no evidence that the instrumental reinforcement test was simply more sensitive to changes in the food’s value than the consumption test, as suggested by Ward-Robinson and Hall (1999). However, our hypothesis that tone-shock pairings might preferentially devalue food’s reinforcing properties whereas tone-LiCl pairings might selectively devalue properties that determine consumption, was contradicted by the results of the consumption test in the experimental chamber. In that test, food consumption was lower after tone-shock pairings than after tone-LiCl pairings. Taken together with the results of the instrumental reinforcement test, that outcome could indicate that, contrary to Holland’s (1981, Experiment 3) claims, tone-shock pairings are simply more effective at devaluing food than tone-LiCl pairings, at least under these circumstances.
Two alternative accounts for this pattern of outcomes come to mind. First, it is notable that shock and pain occurred in the experimental chamber but not in the home cage, whereas LiCl-induced illness occurred in the home cage but not in the experimental chamber. Thus, to the extent that the mediated food aversions established were context-dependent, it would not be surprising that both chamber measures showed greater aversions after tone-shock pairings but the home cage measure showed greater aversion after tone-LiCl pairings. Indeed, the rats that received tone-shock pairings showed greater suppression of consumption in the chamber than in the home cage, whereas the opposite was true for the rats that received tone-LiCl pairings. On the other hand, most evidence, including that from this laboratory, suggests substantial if not complete transfer of flavor aversions established by direct flavor-LiCl pairings from home cage to chamber (e.g., Holland & Straub, 1979; Pickens et al., 2003, 2004). In this regard, it is notable that the chamber tests were conducted after the home cage tests and thus would reflect extinction of the mediated food aversion, which would be expected to occur in the initial home cage test session.
A simpler account for the pattern of data observed here is that tone-shock pairings punished food-tray approach or established conditioned fear to that site. Although both groups of rats received the same shocks in the chamber, the rats in Group Shock had approached the food tray and had their heads in that tray when shocks were delivered, whereas on the whole the rats in Group LiCl received shocks when they were not in or near the food tray. If the rats in Group Shock were less likely to enter the food tray, then it would hardly be surprising that they both consumed fewer pellets there and were less likely to acquire an instrumental response to earn pellets delivered there. Indeed, during the final devaluation trial the rats in Group Shock entered the food tray less often than the rats in Group LiCl. Thus, it is possible that the mediated aversion observed in Group Shock may not reflect an aversion to food at all (recall there was no evidence of a food aversion in the home cage in this group), but rather the establishment of a direct aversion to the chamber food tray. Ward-Robinson and Hall’s (1989) observation could be interpreted in a similar fashion. Notably, this account is consistent with Holland’s (1981) view that associatively-activated food representations, like ones directly activated by food itself, are preferentially associated with illness rather than shock/pain. Experiment 2 evaluated this account.
Experiment 2
Experiment 2 compared the effectiveness of shock and illness USs in establishing mediated food aversions under conditions in which mediation of those aversions by conditioning of an aversion to the food site, rather than to food itself, was unlikely. As in Holland’s (1981) Experiment 3, rats were trained with two different USs delivered to the same food site, and a mediated aversion was established to one of them, selectiviely. An auditory CS and a visual CS were each paired with a different US (e.g., clicker-orange sucrose, light-plain sucrose), both delivered to the same food cup. After this treatment, one of the CSs was reinforced by either LiCl (e.g., clicker-LiCl) or shock (clicker-Shock), in separate groups. Then, half of the rats in each group received two consumption tests, one with each of the USs, placed in the food cups in the experimental chambers. The other half of the rats received two instrumental training test sessions designed to assess the reinforcing power of each of the two USs. In these tests, performance of a lever press yielded the delivery of one US, and performance of a chain-pull yielded the other US, again both to the same food cup. Notably, any tendency to avoid the food cup established by CS-LiCl or, especially, CS-shock, training would contribute equally to the assessment of both USs. Thus, selectivity in test performances would indicate mediation by associatively-activated stimulus features of the two USs, rather than by conditioning that resulted in the reduced likelihood or speed of approach to the site of reinforcer delivery.
Method
Subjects
The subjects were 32 naïve rats similar to those used in Experiment 1. They were maintained as in Experiment 1.
Apparatus
The apparatus was similar to that used in Experiment 1, with four differences. First, the food tray was replaced by a brass liquid cup, with a capacity of about 15 ml, which was also recessed behind the square hole in the center of the front wall. Second, in addition to the chain, which could be mounted to the right of the liquid cup, an aluminum lever (2.0 × 2.0 cm) could be mounted to the left of the food cup. Levers and chains were centered between the cup and the side walls. These response devices were only available during the instrumental reinforcement tests. Third, a 6-w lamp, located on the wall of the sound proof shell opposite the speaker, served as a visual cue. Fourth, no background illumination was provided; only the small amount of light that shone from the illuminated food cup was available.
Procedure
Rats first received four 64-minute sessions designed to train them to approach the food cup and consume each of the 2 flavored sucrose USs. Each of these sessions included 16 0.1-ml deliveries of either 0.2M plain sucrose or 0.2M orange sucrose (1g orange Koolaid flavoring in 1 l of 0.2 M sucrose solution), which served as the 2 USs. US presentations were made at random times throughout the sessions. The first and fourth sessions included presentations of one US and the second and third sessions included presentations of the other US; the identity of the two USs was counterbalanced. Next, the rats received eight sessions in which a 10-s 5-Hz clicker was paired with delivery of sucrose and a 10-s illumination of the 6-w lamp was paired with orange. Each 64-min session included four presentations of each of the two CS-US pairs, randomly intermixed, with rectangularly-distributed ITIs ranging from 4 to 12 min, mean = 8 min. The measure of responding reported is the percentage of time spent in the food cup during the last 5-s of the 10-s clicker or light CSs, and the 5-s periods prior to CS presentations. Responding during the last 5-s of CS presentations is presented both to be consistent with the 5-s reporting periods of Experiment 1, and because many previous studies from this laboratory show that food cup responding is more frequent and less variable across the last 5-s of 10-s CSs paired with food (e.g., Holland, 1977).
Next, each rat received two 5-min CS-devaluation sessions in the experimental chamber, one followed by IP injection of 5 ml/kg 0.6-M LiCl and one that included the administration of 4 1-s 0.8 mA grid shocks spaced evenly throughout the session, as in Experiment 2. For half of the rats (Group LiCl), the session followed by LiCl injection included 4 nonreinforced 10-s presentations of one of the CSs and the shock session included no CSs, and for the other half of the rats (Group Shock), the shocked session included presentations of one of the CSs immediately before each shock, and the LiCl session had no presentations of any event. The order of the shock and LiCl sessions and the identity of the presented CS (and hence that of the represented flavor USs) were counterbalanced within each group.
The effects of the CS-devaluation sessions on the rats’ evaluations of the fluid USs were then assessed. Half of the rats in each group received consumption tests and half received instrumental reinforcement tests. The rats that received consumption tests were placed in the chambers for 5 min on two consecutive days with access to 10 ml of orange or sucrose in the usual liquid cup. The order of presentation of the two USs was fully counterbalanced with all of the earlier counterbalancing variables. The rats that received instrumental reinforcement tests were placed in the chambers for 60 min on each of two consecutive sessions. In one session, a lever was available in each chamber and in the other session, a chain was available in each chamber. A small amount of a paste of lab chow and water was placed on each chain or lever prior to placing the rats in the chambers to encourage initial contact. In each of these sessions, each lever press (or chain pull) was reinforced with the delivery of 0.2 ml of orange or sucrose. The order of the sessions (chain pull or lever) and the identity of the reinforcer produced by each response device were completely counterbalanced with respect to all of the preceding counterbalancing variables.
Next, all rats received two test sessions in the experimental chambers, which evaluated the effects of the CS devaluation sessions on responding to the CSs. Each 32-min session included 4 10-s presentations of either the light or clicker CS. The order of these sessions was counterbalanced across previous training conditions.
Finally, consumption of the two flavors in a different location in the experimental chambers was assessed in all rats. First, the ceramic cups were again placed in the rats’ home cages for 2 hr to make them familiar to the rats. Next, these cups were placed in the experimental chambers, at the end opposite from the liquid cup used in training. The rats were placed in the chambers for 5 min on two consecutive days, with access to 20 ml of orange or sucrose in the ceramic cups, and nothing in the liquid cups.
Results
Liquid cup responding was acquired quickly during the initial conditioning phase, at similar rates for both clicker-sucrose and light-orange pairs. On the final session the percentages of time in the liquid cup were 62.0 ± 4.2%, 54.3 ± 5.6%, and 8.3 ± 1.8% during the 5-s clicker, light, and pre-CS periods, respectively.
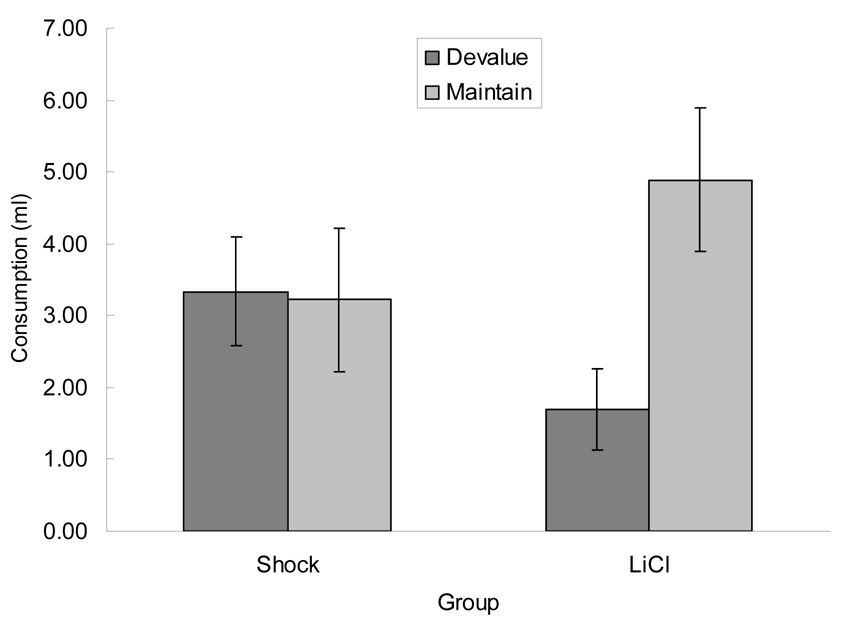
Figure 2 shows the results of the initial consumption tests, conducted in the experimental chambers in half of the rats. Rats that had received one of the CSs paired with LiCl injection displayed a selective mediated aversion. That is, they consumed less of that CS’s fluid associate than of the other fluid. By contrast, rats that had received one of the CSs paired with shock failed to show such a selective aversion, consuming only small amounts of both solutions. Initial ANOVAs that included CS/US counterbalancing showed no significant effects of that variable or any of its interactions, ps > 0.250, so that variable was dropped from subsequent analyses. A Phase 2 devaluing US (shock or LiCl) × test fluid (devalued or nondevalued CS partner) ANOVA showed a significant effect of test fluid, F(1, 14) = 5.66, p = 0.032, and a significant devaluing US × test fluid interaction, F(1, 14) = 6.52, p = 0.023. A subsequent Tukey HSD test confirmed the greater consumption of the test fluid whose CS partner had not been devalued among the rats that had received CS-LiCl pairings, p = 0.017. No other individual post-hoc comparison was significant, ps > 0.305.
Figure 2.
Mean ± sem consumption (in ml) of the foods whose conditioned stimulus partners had been paired (Devalue) or not paired (Maintain) with either shock or lithium chloride in Experiment 2. Liquid foods were placed in the experimental chamber liquid cups that were used in training.
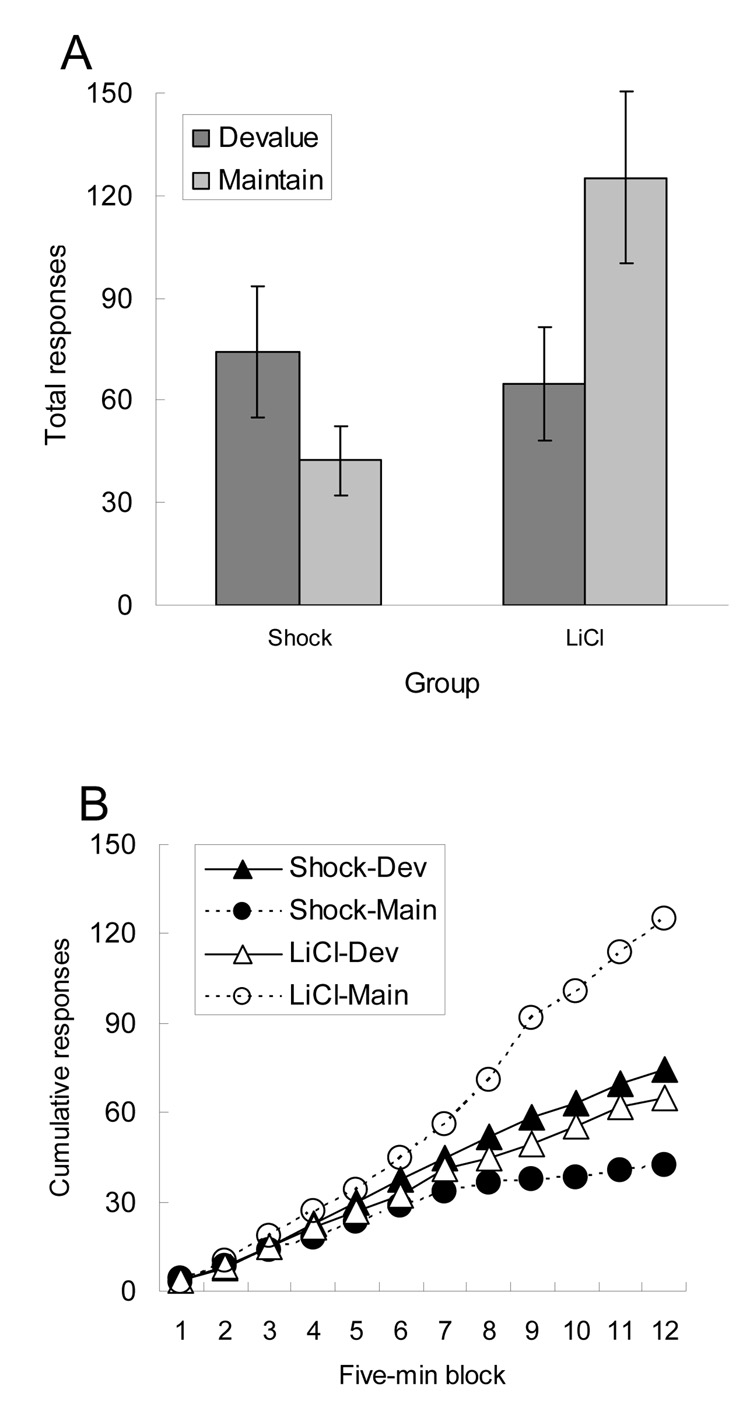
Figure 3 shows the results of the instrumental reinforcement training sessions, conducted with the other half of the rats. Figure 3A shows total responding over each of the two sessions (one with each reinforcer), and Figure 3B shows cumulative responding by 5-min intervals in those sessions. As with fluid consumption, the fluid whose CS partner had been paired with LiCl injection was less effective as a reinforcer than the other fluid, whereas no such tendency was observed among the rats that had received CS-shock pairings. Initial ANOVAs that included CS/US and response counterbalancing showed no significant effects of either of those variables nor any of their interactions, ps > 0.376, so they were dropped from subsequent analysis. A Phase 2 devaluing US × test fluid ANOVA showed a significant devaluing US × test fluid interaction, F(1, 14) = 20.87, p < 0.001. A subsequent Tukey HSD test confirmed that responding to earn the fluid whose CS partner had not been paired with LiCl was significantly greater than responding under any other condition, ps < 0.015. No other individual post-hoc comparison was significant, ps > 0.162.
Figure 3.
Instrumental responding reinforced by the food whose conditioned stimulus partners had been paired (Devalue) or not paired (Maintain) with either shock or lithium chloride (LiCl) in Experiment 2. Panel A shows total responding over the 60-min sessions, and panel B shows the acquisition of that responding over 5-min blocks.
After the consumption or instrumental reinforcer tests, the rats received a test of responding to the CSs. Liquid cup responding to the CS that had been paired with shock was substantially reduced (9.9 ± 1.9%) relative to responding to the CS that had not been paired with shock (44.8 ± 3.0%). LiCl injections had no effect on responding to the CS that had been paired with that injection (47.6 ± 3.0%) compared to responding to the other CS (46.9 ± 3.1%). A devaluing US (shock or LiCl) × test CS (devalued or unpaired) ANOVA showed significant effects of both variables and their interaction, Fs(1, 30) > 31.26, ps < 0.001, and Tukey HSD tests showed that responding to the CS that was paired with shock was significantly less than responding to the CS in each other condition, ps < 0.001. A one-way ANOVA of pre-CS responding showed no effect of devaluing US, F < 1.
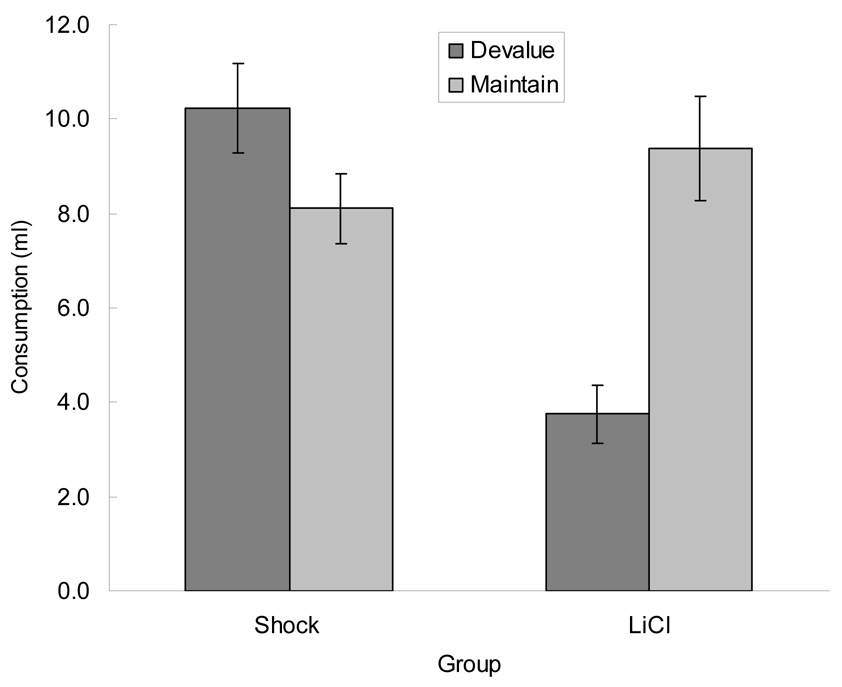
Finally, all rats received a test of consumption of each of the flavored fluids from the ceramic cups, placed in the experimental chambers, but away from the original liquid cups. If the reduced consumption of both fluids in the rats that had received CS-shock pairings was due to an aversion established to the liquid cup rather than to the fluids in general, those rats should show relatively high levels of consumption of both fluids, whereas rats that had received CS-LiCl pairings should continue to show selective aversions to the flavor whose CS partner had been paired with LiCl. Figure 4 shows the results of this test, which conformed exactly to those predictions. An ANOVA, which included type of prior test (consumption or instrumental), devaluing US, and test fluid (devalued or unpaired) as variables, showed significant main effects of devaluing US, F(1, 28) = 6.03, p = 0.021 and test fluid, F(1, 28) = 6.70, p = 0.015, and, more important, a significant interaction between those two variable, F(1, 28) = 32.89, p < 0.001. Post-hoc HSD tests showed that consumption of the fluid whose CS partner had been paired with LiCl was lower than consumption in any other condition, ps < 0.001. No other individual post-hoc comparison was significant, ps > 0.141.
Figure 4.
Mean ± sem consumption (ml) of the foods whose conditioned stimulus partners had been paired with either shock or lithium chloride (LiCl) in Experiment 2. Liquid foods were place in ceramic bowls in the experimental chambers, opposite from the liquid cups used in training. The data from these tests are from all rats, including both those that were initially tested for food consumption (Figure 2) and those that were previously tested for instrumental reinforcement (Figure 3).
Discussion
In this experiment, auditory and visual CSs themselves were more readily associated with shock than with LiCl: rats suppressed responding directed to the liquid cup considerably more if the CS had been paired with shock than if it had been paired with LiCl. Indeed, there was no evidence that CS-LiCl pairings had any effect on responding to the CS. By contrast, the results of the food aversion tests suggest that the food representations activated by those CSs were more readily associated with illness than with shock, as is typically found with food itself (e.g., Garcia & Koelling, 1966). In both tests of consumption and tests of the fluids’ abilities to serve as reinforcers for instrumental learning, the fluid aversions established when the clicker or light CS was paired with illness (Group LiCl) were highly flavor-specific, whereas there was no evidence for such specificity after CS-shock pairings (Group Shock). Thus, when potentially confounding effects of liquid cup fear/response punishment were equated, there was no evidence for selective reduction in either consumption or reinforcement power of a food whose CS partner had been paired with shock.
Comparison of the results of the liquid cup and ceramic bowl consumption tests supports the view that the nonselective reduction in fluid consumption in Group Shock was due to conditioning of fear to the liquid cup site or to punishment of liquid cup approach responses. When the fluids were placed in the liquid cup used in training, consumption of both fluids was low (that is, comparable to consumption of the fluid whose CS partner had been paired with LiCl), but when the fluids were instead placed in the ceramic cups, consumption of both fluids was high (comparable to consumption of the fluid whose partner had not been paired with LiCl). However, because the bowl test was always conducted after the liquid cup test, these results do not rule out the possibility that CS-shock pairings established nonselective aversions to both fluids. That is, the low consumption of both fluids in the initial liquid cup test may have revealed a nonselective aversion, and the greater consumption of both fluids in the subsequent bowl test may have reflected extinction of those aversions. Likewise, in the instrumental test, all reinforcers were delivered to the liquid cup, making it difficult to determine if the general reduction in instrumental learning rate observed in Group Shock was the result of learned avoidance of the liquid cup or of the fluids themselves. Thus, it remains possible that CS-shock pairings established nonselective aversions to both fluids, by devaluing a property of the associatively-activated fluid representation other than its unique flavor (e.g., Garcia, Kovner, & Green, 1970). In that regard, it would be valuable to determine whether instrumental responding would have been acquired more readily after CS-shock pairings if the instrumental reinforcers were delivered to an alternate location, and whether the results of the two consumption tests would remain the same if their order was counterbalanced.
General Discussion
Under the experimental conditions used here, as with real foods (e.g. Garcia & Koelling, 1966), associatively-activated representations of food were more readily linked with illness than with shock, regardless of the manner in which the aversion was assessed. Administration of LiCl after the presentation of an auditory or visual CS that was presumed to activate a representation of a food, produced a selective reduction in both the consumption of that CS’s food associate and in its ability to reinforce the acquisition of an instrumental response. By contrast, we found no evidence that CS-shock pairings could selectively affect either measure of the establishment of a food aversion. Furthermore, although we found general reductions in both consumption and instrumental learning after CS-shock pairings, the results of Experiment 2 suggest that these deficits were mediated by fear conditioning to the food cup or punishment of food cup approach responses consequent to the CS-shock pairings. Thus, we found no support for the hypothesis that pairings of an associatively-activated food representation with illness would especially reduce consumption of that food whereas pairings with shock would especially reduce its ability to serve as a reinforcer. Likewise, although these experiments were not designed to compare the sensitivity of these two measures across a range of conditions, no evidence was found to support the notion that instrumental responding for food is a more sensitive measure of its value than consumption. Indeed, a great deal of evidence suggests that those two measures are often quite dissociated (e.g. Morgan, 1975).
This is not to say however that the auditory/visual CS-flavor, CS-illness combination is uniquely capable of producing learning that is mediated by associatively-activated event representations. First, we explored only a small parameter space in attempting to form aversions after CS-flavor, CS-shock procedures. Hall (1996) described an experiment conducted with Ward-Robinson in which the reinforcing power of a food was apparently reduced in a flavor-specific manner after tone-shock pairings. Rats received two auditory cues, each paired with a different flavored food, delivered to the same food cup. Then one tone was paired with shock, and the two foods were used as reinforcers for two different instrumental responses. One of the foods was a significantly poorer instrumental reinforcer when its tone partner had been paired with shock. Unfortunately, the reinforcing power of the other food was not affected in that manner, and the overall ANOVA did not show a significant effect of tone-shock pairing. But this result suggests the possibility that such mediated aversions might be formed, despite our observations. Second, there is ample evidence that associations can be formed between auditory and visual cues (e.g., Holland & Sherwood, 2008; Wheeler, Sherwood, & Holland, 2008) and between auditory and visual cues and shock, when the auditory and visual cues are activated associatively. For example, Ward-Robinson and Hall (1996) provided examples of “backward sensory preconditioning” in which A→X, A→shock combinations produced X-shock associations, when A and X were auditory and/or visual stimuli. Similarly, Wheeler et al. (2008) found evidence for X–Y associations after A→X, B→Y and A→B training. Thus, the inability of the directly-presented cue to effectively overshadow conditioning of the associatively-activated stimulus is probably not crucial to the observation of mediated learning.
In most mammals, pairing a flavored food with gastrointestinal malaise results in the subsequent avoidance and reduction of consumption of that food (e.g., Garcia & Kimeldorf, 1957). Although there is substantial evidence that such flavor aversion learning shares many features with other examples of associative learning (Rescorla & Holland, 1976), many researchers agree that it reflects a privileged system with impressive capabilities and unique specializations. For example, in rats, flavor aversions may be learned even when the flavor and illness experiences are separated by hours, and illness-induced aversions are much more readily acquired to flavor cues than to auditory, visual or olfactory stimuli. Nevertheless, it is clear that such exteroceptive cues may gain access to this privileged system in rats through associative learning. For example, Gustavson, Garcia, Hankins and Rusiniak (1974), in a project designed to selectively reduce predatory behavior in captive coyotes by poisoning them after they consumed the meat of a prey animal, suggested that the coyotes first learned an aversion to the taste of the prey and then associated the prey’s olfactory and visual characteristics with the now-aversive flavor, causing them to avoid that prey animal in the future.
Similarly, we found that pairing auditory and visual cues with flavored foods endowed those cues with the ability to substitute for their flavor referents in both the control of action and the acquisition of new learning about those flavors (e.g., Holland & Wheeler, 2008). Experiments such as the present ones show that rats can learn an aversion to a flavored food if they become ill after presentations of an auditory or visual cue that, days before, had been paired with that food. Moreover, the results of recent reinforcer devaluation experiments (Holland, 1990, Exp. 1–3; Kerfoot et al., 2007; Holland et al., 2008) suggest that auditory cues can gain access to taste-illness systems in the control of evaluative aspects of consummatory behavior. For example, Kerfoot et al. (2007) first paired a tone with intraoral delivery of liquid sucrose. Then, some rats received a single sucrose-LiCl pairing in the absence of the tone, whereas others received those events unpaired. Finally, a range of behaviors was assessed in the presence of the tone alone and in the presence of tone and unflavored water. Rats that had received sucrose and LiCl unpaired engaged in vigorous licking and consummatory behaviors during tone presentations, whereas rats that had received those events paired did not. More important, rats that had received sucrose-LiCl pairing also showed aversive taste reactivity responses (e.g., gapes, chin rubs, paw flailing; Berridge, 2000) in the presence of the tone, which they had never previously displayed in the presence of either the tone or sucrose. By contrast, rats that received the sucrose and LiCl unpaired displayed hedonic (positive) responses (e.g., broad tongue protrusions). Thus, the auditory cues had gained access to a system that responds to the current evaluation of foodstuffs and controls consummatory responses accordingly. This conclusion is further supported by the results of Kerfoot et al.’s (2007) assessment of brain FOS (the protein product of the activity-dependent immediate early gene, c-fos) after these tone test presentations. Rats that had previously received sucrose-LiCl pairings showed greater FOS, and by inference greater neural activity, in brain regions implicated in processing of aversive substances and those that had received those events unpaired showed greater FOS in regions associated with processing of positive substances. Further study of such brain-behavior relations may lead to better understanding of how, through associative learning, exteroceptive cues may be integrated into specialized flavor aversion processing systems.
Acknowledgements
This research was supported in part by grant MH65879. I thank Vanessa McKenna for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neuroscience and Biobehavioral Reviews. 2000;24:173–198. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Grill HJ, Norgren R. Relation of consummatory responses and preabsorptive insulin release to palatability and learned taste aversions. Journal of Comparative and Physiological Psychology. 1981;95:363–382. doi: 10.1037/h0077782. [DOI] [PubMed] [Google Scholar]
- Garcia J, Kimeldorf DJ. Temporal relationships within the conditioning of a saccharine aversion through radiation exposure. Journal of Comparative and Physiological Psychology. 1957;50:180. doi: 10.1037/h0046326. [DOI] [PubMed] [Google Scholar]
- Garcia J, Koelling RA. Relation of Cue to Consequence in avoidance Learning. Psychonomic Science. 1966;4:123–124. [Google Scholar]
- Garcia J, Kovner R, Green KS. Cue properties versus palatability of flavors in avoidance learning. Psychonomic Science. 1970;20:313–314. [Google Scholar]
- Gustavson CR, Garcia J, Hankins WG, Rusiniak KW. Coyote predation control by aversive conditioning. Science. 1974;184:581–583. doi: 10.1126/science.184.4136.581. [DOI] [PubMed] [Google Scholar]
- Hall G. Learning about associatively-activated stimulus representations: Implications for acquired equivalence and perceptual learning. Animal Learning & Behavior. 1996;24:233–255. [Google Scholar]
- Holland PC. Conditioned stimulus as a determinant of the form of the Pavlovian conditioned response. Journal of Experimental Psychology: Animal Behavior Processes. 1977;3:77–104. doi: 10.1037//0097-7403.3.1.77. [DOI] [PubMed] [Google Scholar]
- Holland PC. Acquisition of representation mediated conditioned food aversions. Learning and Motivation. 1981;12:1–18. doi: 10.1016/j.lmot.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC. Event representation in Pavlovian conditioning: Image and action. Cognition. 1990;37:105–131. doi: 10.1016/0010-0277(90)90020-k. [DOI] [PubMed] [Google Scholar]
- Holland PC, Lasseter H, Agarwal I. Amount of training and cue-evoked taste-reactivity responding in reinforcer devaluation. Journal of Experimental Psychology: Animal Behavior Processes. 2008 doi: 10.1037/0097-7403.34.1.119. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Sherwood A. Formation of excitatory and inhibitory associations between absent events. Journal of Experimental Psychology: Animal Behavior Processes. 2008 doi: 10.1037/0097-7403.34.3.324. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Straub JJ. Differential effects of two ways of devaluing the unconditioned stimulus after Pavlovian appetitive conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1979;5:65–78. doi: 10.1037//0097-7403.5.1.65. [DOI] [PubMed] [Google Scholar]
- Holland PC, Wheeler DS. Representation-mediated food aversions. In: Reilly S, Schachtman T, editors. Conditioned Taste Aversion: Behavioral and Neural Processes. Oxford: Oxford University Press; 2008. in press. [Google Scholar]
- Kerfoot EC, Agarwal I, Lee HJ, Holland PC. Control of appetitive and aversive taste-reactivity responses by an auditory conditioned stimulus in a devaluation task: a FOS and behavioral analysis. Learning and Memory. 2007;14:581–589. doi: 10.1101/lm.627007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MJ. Resistance to satiation. Animal Behavior. 1974;22:449–466. [Google Scholar]
- Pickens CL, Saddoris MP, Setlow B, Gallagher M, Holland PC, Shoenbaum G. Different roles for orbitofrontal cortex and basolateral amygdala in a reinforcer devaluation task. Journal of Neuroscience. 2003;23:11078–11084. doi: 10.1523/JNEUROSCI.23-35-11078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA, Holland PC. Some behavioral approaches to the study of learning. In: Bennett E, Rozensweig MR, editors. Neural mechanisms of learning and memory. Cambridge, Mass: MIT Press; 1976. pp. 165–192. [Google Scholar]
- Ward-Robinson J, Hall G. Backward sensory preconditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1996;22:395–404. [Google Scholar]
- Ward-Robinson J, Hall G. The role of mediated conditioning in acquired equivalence. Quarterly Journal of Experimental Psychology. 1999;52B:335–350. doi: 10.1080/027249999393031. [DOI] [PubMed] [Google Scholar]
- Wheeler DS, Sherwood A, Holland PC. Excitatory and inhibitory learning with absent stimuli. Journal of Experimental Psychology: Animal Behavior Processes. 2008 doi: 10.1037/0097-7403.34.2.247. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]