Abstract
To investigate the basis for impaired sentence comprehension in patients with frontotemporal dementia (FTD) we assessed grammatical comprehension and verbal working memory in 88 patients with three distinct presentations: progressive nonfluent aphasia (PNFA), semantic dementia (SD), and nonaphasic patients with a disorder of social comportment and executive processing (SOC/EXEC). We related sentence comprehension and working memory performance to regional cortical volume in a subgroup of 29 patients with structural MRI scans using voxel-based morphometry. PNFA patients exhibited the greatest difficulty with sentence comprehension and were especially impaired with grammatically complex sentences, which correlated with atrophy in left inferior frontal cortex. Working memory performance in these same patients correlated with a proximal but distinct left inferior frontal region. SD patients’ sentence comprehension scores correlated with left inferolateral temporal lobe damage, which we hypothesize and reflect impairments in lexical processing. We did not observe any consistent relationship between cortical atrophy and sentence comprehension impairment in SOC/EXEC patients, suggesting the deficits in this subgroup may be due to more variable declines in executive resources.
Keywords: progressive aphasia, semantic dementia, sentence comprehension, MRI, VBM (voxel-based morphometry)
1. Introduction
The complex task of comprehending a single word involves a large number of brain regions involved in phonological, lexical, and semantic processing. Above and beyond these demands, understanding a sentence requires a knowledge of rules governing the relationships between words (Chomsky, 1981) and executive resources used to apply these rules online during sentence comprehension (Cooke et al., 2002). In this study we look to three groups of patients with differing patterns of cortical atrophy for evidence regarding the neuroanatomical bases for sentence comprehension.
Frontotemporal dementia is a neurodegenerative disease that results in varying patterns of cognitive impairment (Grossman, 2002). Based on their behavioral presentation, patients with frontotemporal dementia can be clinically classified into at least three subgroups (Snowden, Neary, & Mann, 1996). Because the relative distributions of cortical atrophy differ among the three subgroups, analysis of language deficits in each group can provide insight into the neuroanatomical regions supporting various aspects of cognition, including language (Peelle & Grossman, In press). Briefly, progressive nonfluent aphasia (PNFA) is associated with effortful, agrammatic, and dysarthric speech. Patients with semantic dementia (SD), by comparison, display a fluent form of progressive aphasia associated with impaired naming and single word comprehension difficulty associated with a modality-neutral semantic memory deficit. Nonaphasic frontotemporal dementia patients (SOC/EXEC) have a disorder of social comportment and show executive dysfunction. Although these patients are not aphasic, diminished executive resources can result in language difficulty. Improved clinical diagnosis and knowledge about characteristic distribution of cortical atrophy in these patient groups has proved helpful in linking cortical atrophy to changes in language function; however, direct correlations between the two measures are rare. In this report, we used voxel-based morphometry (VBM) to directly correlate regional gray matter volume in PNFA, SD, and SOC/EXEC patients with behavioral measures of sentence comprehension and working memory.
A number of functional and structural neuroimaging studies have associated PNFA with left hemisphere disease in inferior frontal cortex and adjacent regions such as the frontal operculum, anterior insula, and anterior superior temporal cortex (Gorno-Tempini et al., 2004; Grossman et al., 2004; Grossman, Mickanin et al., 1996; Nestor et al., 2003). Left inferior frontal regions have been consistently implicated as being important for sentence comprehension in healthy adults in stroke (Zurif, 1996; Zurif, Caramazza, & Myerson, 1972) and functional imaging studies (Caplan, Alpert, & Waters, 1999; Heim, Opitz, & Friederici, 2003; Peelle, McMillan, Moore, Grossman, & Wingfield, 2004). Of particular interest is that inferior frontal regions have been linked to both processing the long-distance grammatical dependencies between words in a sentence (Kang, Constable, Gore, & Avrutin, 1999; Ni et al., 2000) and retaining information transiently in verbal working memory during ongoing sentence processing (Cooke et al., 2006; Cooke et al., 2002; Fiebach, Schlesewsky, Lohmann, von Cramon, & Friederici, 2005).
Consistent with these findings in healthy adults, several studies have found evidence for grammatical processing deficits in PNFA patients. These include both offline measures of sentence comprehension (Grossman, Mickanin et al., 1996; Thompson, Ballard, Tait, Weintraub, & Mesulam, 1997) and online measures using a target word monitoring paradigm (Grossman, Rhee, & Moore, 2005; Peelle, Cooke, Moore, Vesely, & Grossman, 2007). In an fMRI study of sentence comprehension, PNFA patients with grammatical comprehension difficulties showed reduced activation in the ventral portion of the left inferior frontal cortex, although they were able to activate the dorsal portion of the left inferior frontal cortex (Cooke et al., 2003). The opposite pattern was seen in SOC/EXEC patients with limited working memory, who showed reduced activation of the dorsal portion of left inferior frontal cortex, but relatively greater recruitment of the ventral portions. Together these studies are consistent in implicating ventral inferior frontal areas differentially affected in PNFA with these patients’ grammatical comprehension difficulties.
Cortical atrophy in SD typically includes anterior, lateral, and ventral portions of both temporal lobes, with left hemisphere atrophy being more prominent (Galton et al., 2001; Gorno-Tempini et al., 2004; Grossman et al., 2004; Mummery et al., 2000). Numerous studies have characterized the lexical comprehension difficulties of SD patients, which include particular difficulty with confrontation naming regardless of the modality of presentation (Bozeat, Lambon Ralph, Patterson, Garrard, & Hodges, 2000; Hodges, Graham, & Patterson, 1995; Hodges, Patterson, Oxbury, & Funnell, 1992). SD patients’ difficulties have been attributed to anterior temporal damage (Lambon Ralph, McClelland, Patterson, Galton, & Hodges, 2001), although a VBM study with SD patients correlated atrophy in left lateral inferotemporal regions with lexical access problems (Grossman et al., 2004).
SOC/EXEC patients tend to have frontal and temporal lobe atrophy that is more right lateralized, and less damage to left inferior frontal regions affected in PNFA (Grossman et al., 2004; Rosen et al., 2002; Williams, Nestor, & Hodges, 2005). SOC/EXEC patients typically have cognitive deficits in a variety of executive domains including selective attention, strategic planning, and working memory (Libon et al., 2007; Rahman, Sahakian, Hodges, Rogers, & Robbins, 1999). Subjectively the SOC/EXEC patients’ language difficulties present more variably than PNFA or SD patients, perhaps due to heterogeneous executive impairments. Even though these executive limitations have been shown to affect SOC/EXEC patients’ language processing (Ash et al., 2006; Murray, Koenig, Antani, McCawley, & Grossman, 2007; Peelle et al., 2007), their language impairments are generally less severe than those seen in PNFA or SD patients.
These three patient groups allowed us to examine neuroanatomically dissociable resource allocation during sentence comprehension. Our particular interest centered on the contribution of verbal working memory to this task. The nature and involvement of working memory in sentence comprehension has received considerable attention in the literature. While few dispute the need for the short-term storage of information during sentence processing, debate has centered on the issue of whether the resource involved is language-specific or more general in nature (Caplan, Alpert, Waters, & Olivieri, 2000; Caplan & Waters, 1999; Daneman & Carpenter, 1980; Fiebach et al., 2005). In the current study we choose to measure a more general form of working memory using a standard backward digit span task. We expected, first, that this measure would reflect SOC/EXEC patients’ general executive limitations, and be a way to parsimoniously characterize the role of executive resources in sentence comprehension. Second, this allowed us to test whether PNFA patients’ left inferior frontal disease forces these patients to compensate for their language limitations by relying on a domain-general form of working memory for the grammatically-demanding sentences. This hypothesis would be consistent with reduced left ventral frontal recruitment during sentence processing (Cooke et al., 2003) and slowed grammatical processing in a word monitoring task (Grossman et al., 2005).
Although sentence comprehension has been studied in frontotemporal dementia, direct correlations involving measures of sentence processing and regional cortical atrophy are rare. In the current study we use voxel-based morphometry (Ashburner & Friston, 2000) to determine regions of cortical atrophy that are significantly related to sentence comprehension and working memory in PNFA, SD, and SOC/EXEC patients. We sought to determine whether differing patterns of atrophy between patient groups are related to differing patterns of language comprehension difficulty, and also whether working memory deficits are related to sentence comprehension ability.
2. Method
2.1 Participants
We studied 88 right-handed native English speakers diagnosed with FTD in the Department of Neurology at the University of Pennsylvania. The clinical diagnoses were established using a modification of published criteria (McKhann et al., 2001; Neary et al., 1998). Exclusion criteria included the presence of other dementing conditions such as Alzheimer’s disease, history of stroke, or presence of primary psychiatric disorders or systemic illness that might interfere with cognitive functioning.
Patients were divided into subgroups through a consensus mechanism based on modifications of published criteria (Davis, Price, Moore, Campea, & Grossman, 2001; C. Price, Davis, Moore, Campea, & Grossman, 2001). Subgroup diagnosis resulted in 28 PNFA patients, 28 SD patients, and 32 SOC/EXEC patients. Demographic and clinical information for all patients is summarized in Table 1. The patient groups were matched for age, education, disease duration, and Mini-Mental State Exam score (Folstein, Folstein, & McHugh, 1975). All patients and their legal representatives participated in an informed consent procedure approved by the Institutional Review Board at the University of Pennsylvania.
Table 1.
Mean (+ SD) demographic, clinical, and cognitive scores for each patient group
| PNFA | SD | SOC/EXEC | ||||
|---|---|---|---|---|---|---|
| Cognitive | Imaging | Cognitive | Imaging | Cognitive | Imaging | |
| Number of patients | 28 | 7 | 28 | 8 | 32 | 14 |
| Age (years) | 66.04 (10.2) | 68.86 (11.4) | 68.39 (9.9) | 65.50 (13.0) | 65.47 (11.3) | 63.07 (12.2) |
| Education (years) | 14.29 (2.5) | 14.85 (1.9) | 15.54 (2.7) | 15.38 (2.3) | 15.09 (2.7) | 15.07 (2.3) |
| Duration (months) | 28.57 (19.1) | 39.00 (22.5) | 44.57 (24.4) | 41.50 (39.2) | 38.28 (45.6) | 42.43 (33.5) |
| MMSE (max=30) | 22.68 (6.5) | 21.85 (7.1) | 23.07 (5.5) | 23.75 (4.6) | 21.69 (5.4) | 18.00 (6.5) |
| Sentence comprehension (z score a) | −1.71 (1.2) | −1.61 (0.9) | −1.17 (1.4) | −1.03 (1.5) | −1.09 (1.2) | −0.95 (1.0) |
| Reverse digit span (z score a) | −1.44 (1.1) | −1.55 (1.0) | −1.09 (1.4) | −0.42 (1.4) | −1.04 (1.1) | −1.21 (1.3) |
| Boston naming test (z score a) | −1.91 (2.7) | −3.22 (3.9) | −3.49 (0.6) | −4.33 (2.4) | −1.71 (2.5) | −3.11 (2.6) |
Relative to age- and education-matched healthy adults.
Cognitive data in the patients were evaluated relative to 29 healthy adults matched for age and education. The performance of each patient was converted to a z score based on the performance of these healthy control participants.
Structural MRI scans were available for a subset of 29 patients, also listed in Table 1. The patient groups used in the imaging study were matched for age, education, disease duration, and MMSE. Imaging data in the patients were compared to structural MRI scans obtained from 12 healthy adults matched for age and education with the patients.
2.2 Cognitive Materials and Procedure
In order to characterize language comprehension ability in these patients, we administered several tasks testing sentence comprehension and verbal working memory. Tasks were administered among other measures during a 60-minute session containing a variety of additional tasks, and were presented in a fixed order, with sentence comprehension tasks occurring prior to working memory tasks. The cognitive measures included the following:
Sentence comprehension
Participants responded to a simple question about the agent of the action of short sentences, spoken by the researcher, containing familiar words (Grossman, D'Esposito et al., 1996). Two types of sentences were used: 4 simple sentences containing a subject-verb-object structure (e.g., “The nice, tall girl chased the friendly boy.”), and 8 complex sentences featuring a subject-relative or object-relative embedded clause (e.g., “The friendly boy that the girl chased was nice.”). We equated the sentences for length across the two types by including adjectives in the simple sentences. Sentences were read twice to each participant at a natural cadence with a normal stress pattern. If requested by the patient, the sentence could be repeated. Data for this test was not available in one SOC/EXEC patient who refused to complete this section of the protocol, and data for three SOC/EXEC patients and one PNFA patient were excluded from the correlation analysis because their sentence comprehension data was separated from their MRI by more than one year.
Working memory
We assessed verbal working memory using the backward digit span test from the Wechsler Adult Intelligence Scale (Wechsler, 1997). A sequence of digits was presented at a rate of 1/sec. Participants repeated the digits in the opposite order of presentation. The sequence length began at 2 digits, and was increased by one digit until a patient failed to repeat two trials of a sequence at a specific length. Data were not available in three PNFA patients, three SD patients, and six SOC/EXEC patients.
Boston naming test
Lexical access was investigated using an abbreviated version of the Boston Naming Test (Kaplan, Goodglass, & Weintraub, 1983), consisting of 15 line drawings selected from the full test. Data were not available in one SD patient.
2.3 Imaging Procedure
Structural MRI images were acquired on a GE Horizon Echospeed 1.5T scanner (GE Medical Systems, Milwaukee, WI). We obtained T1-weighted 3D spoiled gradient echo images (TR = 35 ms, TE = 6 ms, slice thickness = 1.3 mm, flip angle = 30°, matrix size of 128 × 256, in-plane resolution of 0.9 × 0.9 mm).
All processing was performed using SPM99 software (Wellcome Trust Centre for Neuroimaging, University College London, UK). We used voxel-based morphometry (Ashburner & Friston, 2000) to compare the gray matter of the patient groups to the healthy adults, and to correlate regional cortical atrophy with performance on the cognitive tasks. The structural images were normalized into a standard stereotactic space (Friston et al., 1995) using a standard T1-weighted template. We segmented each subject’s structural image into three tissue types: gray mater, white matter, and cerebrospinal fluid (Ashburner & Friston, 1997). We visually inspected each resulting gray matter volume to ensure no gross errors, such as voxels from the dural sinuses or other non-brain structures being misclassified as gray matter. The gray matter segmentations were then smoothed using a 12 mm FWHM Gaussian kernel.
Cortical volume in each patient group was compared to control participants using a two-sample t-test to identify regions of significant cortical atrophy in each group. A proportional analysis threshold was used to include only voxels with 40% or greater of the grand mean value. We set our voxelwise statistical threshold for patient groups relative to control participants at p < .0001, uncorrected for multiple comparisons. We accepted only clusters comprised of 100 or more adjacent voxels. Coordinates were transformed from MNI space into Talairach coordinates (Talairach & Tournoux, 1988) using a non-linear transformation (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach).
The correlation analysis involved a regression of comprehension accuracy for grammatically complex sentences on cortical volume. We masked these correlations by areas of significant atrophy for each patient group to determine regions of significant atrophy that were predictive of sentence comprehension accuracy. We used a more relaxed threshold of p < .001 for the correlation analyses to ensure the detection of subtle effects in these predefined locations.
We elected to restrict our correlation analysis to regions of significant atrophy because our primary interest in examining language processing in patients with neurodegenerative disease is to determine the effect of disease-related grey matter loss. These regions are defined by the initial t-test between gray matter density for patients and controls. Restricting our correlation analysis to these regions is therefore most in line with our theoretical interest in these populations. Other researchers have restricted analyses to pre-defined regions of interest (e.g., Amici, Brambati, Wilkins, Ogar, & Dronkers, 2007). Such an approach may miss regions of atrophy that do not correspond to the researchers’ notion of a core language processing network, which is likely to be influenced by a research group’s particular theoretical framework. Our approach, by contrast, is capable of detecting regions of cortical atrophy involved in sentence comprehension and working memory regardless of their anatomical location.
Finally, an important methodological decision in VBM centers on whether to take into account changes in apparent gray matter intensity due to the warping associated with the normalization process. This can be accounted for by scaling the intensity of each voxel in the normalized image by the Jacobian determinant of the deformation field, often referred to as “modulated” VBM (Mechelli, Price, Friston, & Ashburner, 2005). For the current study we examined the results of both modulated and unmodulated routines, but found no regional differences. We report the results of the unmodulated analysis.
3. Results
3.1 Cognitive Observations
We first examined sentence comprehension abilities in each FTD subgroup relative to healthy participants. Overall sentence comprehension scores are shown in Table 1. Although all patients showed some impairment in their sentence comprehension, PNFA patients’ difficulty was most pronounced. Using a criterion of z < −1.65 (equivalent to p < .05, one-tailed), PNFA patients were shown to perform significantly worse than controls. We investigated this effect further to see whether this difference could be explained by a relative impairment in PNFA patients’ grammatical processing, as would be indicated by greater difficulty comprehending the complex sentences. To do this we examined each individual’s sentence comprehension scores for the complex sentences, using a z score criterion of −1.65 to assess whether a patient significantly differed from the control participants. We found that 43% of the PNFA patients were significantly impaired in their comprehension of the grammatically complex sentences. By contrast, 21% of the SD patients and 28% of the SOC/EXEC patients were significantly impaired at understanding grammatically complex sentences relative to healthy adults.
We next investigated the relationship between working memory, assessed by backward digit span, and sentence comprehension ability. Working memory did not differ among the patient groups, F(2, 73) < 1. In PNFA, working memory correlated with comprehension of grammatically complex sentences, r(23) = 0.57, p < .005, but not simpler sentences, r(23) = 0.19, n.s.. There was no correlation between working memory and sentence comprehension in SD [simple: Pearson r(23) = 0.28; complex: Pearson r(23) = −0.06]. In SOC/EXEC patients, working memory correlated with both simple and complex sentence comprehension, but did not discriminate between these sentence types [simple: Pearson r(24) = 0.44, p < .05; complex: Pearson r(24) = 0.45, p < .05].
3.2 Imaging Observations
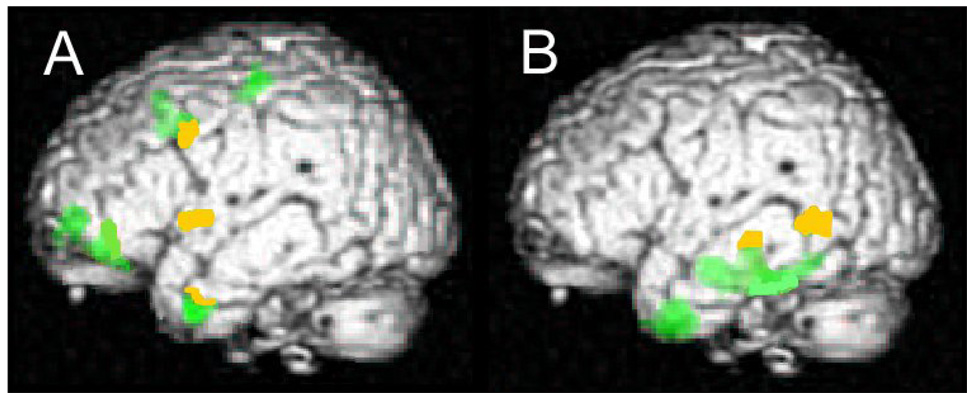
VBM results for PNFA and SD patients are shown in Figure 1. Colored regions (either green or yellow) are those that show significant atrophy in the patient group relative to healthy control participants. These findings have been previously published (Grossman et al., 2004) and are presented here for reference. PNFA patients, seen in Figure 1A, showed gray matter atrophy in inferior frontal, orbital frontal, frontal operculum, insula, premotor, and dorsolateral prefrontal regions of the left hemisphere. PNFA patients also had atrophy in anterior and ventral portions of the left temporal cortex. SD patients’ atrophy was seen in several left temporal regions, including anterior, ventral, posterolateral, and parahippocampal regions, displayed in Figure 1B. SOC/EXEC patients (not shown) had atrophy in the left anterior insula, left anterior temporal, left parahippocampal, right dorsolateral prefrontal, right anterior prefrontal, and right insular regions.
Figure 1.
Correlation between grammatically complex sentence comprehension and cortical atrophy in PNFA (A) and SD (B). All colored regions respond to significant cortical atrophy relative to healthy age-matched control participants. Yellow regions are areas of significant cortical atrophy that showed a significant correlation with sentence comprehension. There were no such regions found for SOC/EXEC patients.
Within these of significant patient atrophy we looked for regions that correlated with sentence comprehension scores. These regions are shown in Figure 1 in yellow. Correlations of gray matter atrophy with sentence comprehension difficulty in PNFA included left inferior frontal cortex and several adjacent regions. The anatomic distribution of peak voxels correlating with comprehension of grammatically complex sentences, where these regions corresponded to significant atrophy in PNFA patients, is summarized in Table 2. In PNFA, regions where cortical volume correlated with comprehension of grammatically complex sentences included inferior frontal, frontal opercular, insula, dorsolateral prefrontal, and anterior temporal cortices of the left hemisphere.
Table 2.
Correlations of cortical atrophy with comprehension of grammatically complex sentences
| Coordinates | ||||||
|---|---|---|---|---|---|---|
| Group | Anatomic locus (Brodmann area) | X | Y | Z | # Voxels | Z-Score |
| PNFA | Left inferior frontal (6) | −56 | 0 | 34 | 3501 | 3.15 |
| Left dorsolateral prefrontal (46) | −56 | 36 | 12 | 3501 | 3.30 | |
| Left superior temporal (22) | −34 | −26 | 4 | 3501 | 3.46 | |
| SD | Left inferior parietal/lateral temporal (40, 39, 21) | −38 | −58 | 42 | 17887 | 4.14 |
| SOC/EXEC | No significant correlations | |||||
Figure 1B shows the left lateral regions of significant correlation between sentence comprehension difficulty and cortical volume that correspond to areas of significant atrophy in SD, illustrated in yellow. In SD, correlations between comprehension of grammatically complex sentences and cortical volume include dorsolateral prefrontal, premotor, temporal, and parietal regions of the left hemisphere, as well as right temporal and right parietal cortex. Correspondence between this correlation and areas of atrophy in SD was seen in a large region with peak correlation in inferior parietal cortex that extended down into lateral temporal cortex, listed in Table 2.
There were no significant correlations between sentence comprehension difficulty and cortical atrophy in the SOC/EXEC patients.
Because there were relationships between cortical atrophy and sentence comprehension in PNFA and SD patients, we also investigated the relationship between working memory and cortical atrophy in these two groups. We did this to determine whether working memory deficits might explain sentence comprehension difficulties seen in these patients. To this end we performed direct contrasts of cortical atrophy associated with these two measures. In PNFA regions associated to a greater degree with sentence comprehension than working memory included inferior frontal, insula, and dorsolateral prefrontal regions of the left hemisphere, showing good correspondence to regions of cortical atrophy. Left prefrontal and left premotor cortical regions are associated more strongly with working memory than the sentence comprehension task. These results are listed in Table 3. There were no significant correlations between working memory and regions of significant atrophy for the SD patients.
Table 3.
Comparison of sentence comprehension and working memory correlations with regional gray matter volume in regions of significant atrophy in PNFA
| Coordinates | ||||||
|---|---|---|---|---|---|---|
| Contrast | Anatomic locus (Brodmann Area) | X | Y | Z | # Voxels | Z-score |
| Sentence comprehension > working memory | Left inferior frontal (47) | −64 | −8 | 16 | 1390 | 3.73 |
| Left dorsolateral prefrontal (9) | −28 | 42 | 36 | 322 | 3.87 | |
| Left insula | −36 | −26 | 4 | 354 | 3.50 | |
| Working memory > sentence comprehension | Left prefrontal (46) | −44 | 22 | 12 | 645 | 4.68 |
| Left premotor (6) | −28 | −4 | 44 | 274 | 4.07 | |
4. Discussion
Sentence comprehension requires both the processing of individual words and the rules governing their relationships. Because the words of a sentence emerge over several seconds, understanding these syntactic dependencies requires executive resources such as working memory that retain critical initial portions of a sentence required for understanding later portions of the sentence. In the current study we examined sentence processing, verbal working memory, and their relationship with cortical atrophy in three groups of FTD patients.
4.1 Sentence processing in PNFA
Several previous studies have reported grammatical comprehension difficulty in PNFA patients (Grossman, Mickanin et al., 1996; Peelle et al., 2007; Thompson et al., 1997). Consistent with these studies, we found that PNFA patients were significantly impaired in their sentence processing, especially for syntactically complex sentences. We observed a significant correlation between the comprehension of grammatically complex sentences and cortical volume in several regions of the left hemisphere, including inferior frontal cortex, frontal operculum, insula, dorsolateral frontal cortex, and anterior temporal cortex. These areas also had significant cortical atrophy relative to healthy control participants, supporting the inference that atrophy in these regions is causally related to impaired grammatical processing. The inferior frontal distribution of atrophy in these PNFA patients and the selective correlation of working memory scores with grammatically complex sentence comprehension suggests that working memory is compensating in part for the sentence processing limitations of PNFA patients.
This finding is in agreement with a considerable amount of indirect evidence supporting a relationship between grammatical comprehension difficulty and disease in left frontal cortex and adjacent regions as seen in PNFA (Gorno-Tempini et al., 2004; Grossman, Mickanin et al., 1996; Turner, Kenyon, Trojanowski, Gonatas, & Grossman, 1996). A recent VBM study also found that regions of left frontal atrophy were related to sentence comprehension difficulty (Amici et al., 2007).Given the prevalence of activation in these regions in healthy adults during grammatically complex sentence comprehension (Caplan et al., 1999; Cooke et al., 2002; Ni et al., 2000; Peelle et al., 2004), this adds further support for the broad involvement of left inferior frontal regions in processes supporting grammatical comprehension.
PNFA patients experienced greater difficulty with grammatically complex sentences. This difficulty was significantly correlated with their verbal working memory scores, assessed using a backward digit span task. The relationship of span measures to syntactic working memory is beyond the scope of this report (Caplan & Waters, 1999). However, there are two possible explanations for the relationship between digit span and syntactic processing in these PNFA patients. First, it is possible that backward digit span does indeed tap a resource important for grammatical processing. We find this unlikely, as this relationship was not seen in the other two patient groups studied. A second, more plausible, alternative is one that takes into account the relative distribution of cortical atrophy in the PNFA patients relative to the other patient groups. In this account, due to damage to inferior frontal regions particularly important for syntactic processing, PNFA patients are forced to rely on more general working resources to comprehend grammatically complex sentences. We believe this account best explains the results of the current study, particularly in the context of neuroimaging studies of sentence comprehension in healthy adults.
We found regions in the left inferior frontal cortex that correlated with working memory to a greater degree than sentence comprehension. This is consistent with fMRI findings in healthy adults that associate working memory demands with dorsal portions of left inferior frontal cortex and grammatical complexity with more ventral portions (Cooke et al., 2006; Cooke et al., 2002; Fiebach et al., 2005; Grossman et al., 2002). These findings suggest that sentence comprehension difficulty in PNFA is compromised in two related aspects: in the rules governing word relationships, and in the working memory required for the online processing of these rules. These processes are localized to ventral and dorsal portions of left inferior frontal cortex, respectively.
4.2 Sentence comprehension in SD
Although SD patients demonstrate some difficulty with sentence comprehension, their impairments are less severe than those seen in PNFA. The absence of a differential impairment for grammatically complex sentences is consistent with previous studies that fail to show a significant grammatical impairment in SD (Gorno-Tempini et al., 2004). Regional cortical volume correlating with verbal working memory did not correspond to regions of atrophy in SD patients, suggesting that working memory does not play a significant role in SD patients’ sentence comprehension. Rather, it is likely that sentence comprehension deficits in SD are due instead to difficulties accessing single word meaning.
SD patients’ difficulties with single word processing are well known (Reilly, Martin, & Grossman, 2005; Snowden, Goulding, & Neary, 1989; Warrington, 1975), and the SD patients in current study were more impaired in their naming than either PNFA or SOC/EXEC groups. Comprehension of both written and spoken words involves extensive activation of the left lateral temporal cortex (C. J. Price, Wise, & Frackowiak, 1996; C. J. Price, Wise, Warburton et al., 1996), and in a previous study looking at regional cortical volume correlated with confrontation naming, SD patients showed significant correlations in these left lateral temporal regions (Grossman et al., 2004). These results are consistent with lateral temporal damage affecting word processing in SD.
SD is commonly associated with atrophy in the anterior temporal lobes (Gorno-Tempini et al., 2004; Mummery et al., 2000), prompting many researchers to associate these regions with semantic processing (Lambon Ralph et al., 2001; Patterson, Nestor, & Rogers, 2007; Rogers et al., 2004). Imaging studies in healthy adults have also implicated anterior temporal involvement in semantic processing (Rogers et al., 2006; Tyler et al., 2004). However, it is lateral temporal regions, rather than anterior regions, that appear to be associated with both sentence comprehension (in the current study) and lexical access (Grossman et al., 2004) in SD patients. Additional evidence for the role of the lateral temporal lobes in semantic processing comes from Noppeney et al. (2007), who compared SD patients to a group of patients with herpes simplex virus encephalitis (HSVE). Unlike SD patients, who have a broad semantic memory impairment across categories, HSVE patients showed a category-specific deficit in which living things were significantly more impaired than nonliving things. The only region in which SD patients showed greater cortical atrophy than HSVE patients was on the lateral left temporal lobe, suggesting that this region may play a role in the impaired naming of SD patients. Together these findings suggest that the lateral temporal lobe plays an important role in lexical access, and that damage to this region contributes to lexical impairments in SD and leads to their word comprehension difficulty. We attribute sentence comprehension deficits seen in SD patients in the current study to these word-level impairments.
4.3 Sentence comprehension in SOC/EXEC
Like the SD patients tested, the SOC/EXEC patients’ sentence comprehension was moderately impaired relative to healthy adults, and they did not demonstrate a grammatical processing impairment. We found that SOC/EXEC patients’ working memory correlated with sentence comprehension. For the PNFA patients, working memory scores correlated with grammatically complex sentences but not grammatically simple sentences, implicating a deficit in working memory that is closely tied to the processing of long-distance relationships in sentence processing. By contrast, in the SOC/EXEC patients working memory correlated with sentence comprehension for both types of sentences. This suggests a more general impairment in executive resources, consistent with previous studies of these patients (Hodges et al., 1995). The lack of any significant correlation between regional atrophy and sentence comprehension for the SOC/EXEC patients, coupled with the clinical observations of greater variability, suggests that the causes of SOC/EXEC patients’ sentence comprehension difficulties may be less homogeneous than the other patient groups tested, reflecting differentially affected executive resources. This interpretation must be viewed with caution given the limited number of patients available for the current study. Future work examining possible subdivisions within the SOC/EXEC patients would provide stronger evidence regarding this hypothesis.
5. Conclusions
We examined sentence comprehension and regional cortical atrophy in three subtypes of FTD. Damage to inferior frontal regions in PNFA affected grammatical processing and working memory resources important for sentence comprehension, leading to a differential impairment with grammatically complex sentences. SD patients exhibited atrophy in lateral temporal regions that correlated with sentence comprehension, most likely due to its impact upon single word processing. Finally, SOC/EXEC patients did not show a consistent correlation between any region of cortical atrophy and sentence comprehension. We attribute the mild sentence comprehension deficits in this group to more general executive resource declines. These patient groups thus illustrate at least three neuroanatomically separable components of a sentence processing network that can be disrupted in frontotemporal dementia.
Acknowledgements
This work was supported by the US Public Health service (AG17586, AG15116, and NS44266, NS54575). Portions of this study were presented at the 56th annual meeting of the American Academy of Neurology, San Francisco, June 2004. We thank Jamie Reilly for helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amici S, Brambati SM, Wilkins DP, Ogar J, Dronkers NL. Anatomical correlates of sentence comprehension and verbal working memory in neurodegenerative disease. Journal of Neuroscience. 2007;27(23):6282–6290. doi: 10.1523/JNEUROSCI.1331-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash S, Moore P, Antani S, McCawley G, Work M, Grossman M. Trying to tell a tale: Discourse impairments in progressive aphasia and frontotemporal dementia. Neurology. 2006;66:1405–1413. doi: 10.1212/01.wnl.0000210435.72614.38. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Multimodal image coregistration and partitioning - A unified framework. NeuroImage. 1997;6(3):209–217. doi: 10.1006/nimg.1997.0290. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry–The methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson K, Garrard P, Hodges JR. Non-verbal semantic impairment in semantic dementia. Neuropsychologia. 2000;38:1207–1215. doi: 10.1016/s0028-3932(00)00034-8. [DOI] [PubMed] [Google Scholar]
- Caplan D, Alpert N, Waters G. PET studies of syntactic processing with auditory sentence presentation. NeuroImage. 1999;9(3):343–351. doi: 10.1006/nimg.1998.0412. [DOI] [PubMed] [Google Scholar]
- Caplan D, Alpert N, Waters G, Olivieri A. Activation of Broca's area by syntactic processing under conditions of concurrent articulation. Human Brain Mapping. 2000;9(2):65–71. doi: 10.1002/(SICI)1097-0193(200002)9:2<65::AID-HBM1>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan D, Waters GS. Verbal working memory and sentence comprehension. Behavioral and Brain Sciences. 1999;22(1):77–126. doi: 10.1017/s0140525x99001788. [DOI] [PubMed] [Google Scholar]
- Chomsky N. Lectures on government and binding. Foris: Dordrecht; 1981. [Google Scholar]
- Cooke A, DeVita C, Gee J, Alsop D, Detre J, Chen W, et al. Neural basis for sentence comprehension deficits in frontotemporal dementia. Brain and Language. 2003;85(2):211–221. doi: 10.1016/s0093-934x(02)00562-x. [DOI] [PubMed] [Google Scholar]
- Cooke A, Grossman M, DeVita C, Gonzalez-Atavales J, Moore P, Chen W, et al. Large-scale neural network for sentence processing. Brain and Language. 2006;96(1):14–36. doi: 10.1016/j.bandl.2005.07.072. [DOI] [PubMed] [Google Scholar]
- Cooke A, Zurif EB, DeVita C, Alsop D, Koenig P, Detre J, et al. Neural basis for sentence comprehension: Grammatical and short-term memory components. Human Brain Mapping. 2002;15(2):80–94. doi: 10.1002/hbm.10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman M, Carpenter P. Individual differences in working memory and reading. Journal of Verbal Learning and Verbal Behavior. 1980;19:450–466. [Google Scholar]
- Davis KL, Price C, Moore P, Campea S, Grossman M. Evaluating the clinical diagnosis of Frontotemporal degeneration: A re-examination of Neary et al., 1998. Neurology. 2001;56:A144–A145. [Google Scholar]
- Fiebach CJ, Schlesewsky M, Lohmann G, von Cramon DY, Friederici AD. Revisiting the role of Broca's area in sentence processing: Syntactic integration versus syntactic working memory. Human Brain Mapping. 2005;24:79–91. doi: 10.1002/hbm.20070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental state: A practical method for grading the state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline J-B, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Human Brain Mapping. 1995;2:165–189. [Google Scholar]
- Galton CJ, Patterson K, Graham K, Lambon Ralph MA, Williams G, Antoun N, et al. Differing patterns of temporal atrophy in Alzheimer's disease and semantic dementia. Neurology. 2001;57:216–225. doi: 10.1212/wnl.57.2.216. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology. 2004;55(3):335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M. Frontotemporal dementia: A review. Journal of the International Neuropsychological Society. 2002;8(4):566–583. doi: 10.1017/s1355617702814357. [DOI] [PubMed] [Google Scholar]
- Grossman M, Cooke A, DeVita C, Alsop D, Detre J, Chen W, et al. Age-related changes in working memory during sentence comprehension: an fMRI study. NeuroImage. 2002;15(2):302–317. doi: 10.1006/nimg.2001.0971. [DOI] [PubMed] [Google Scholar]
- Grossman M, D'Esposito M, Hughes E, Onishi K, Biassou N, White-Devine T, et al. Language comprehension profiles in Alzheimer's disease, multi-infarct dementia, and frontotemporal degeneration. Neurology. 1996;47(1):183–189. doi: 10.1212/wnl.47.1.183. [DOI] [PubMed] [Google Scholar]
- Grossman M, McMillan C, Moore P, Ding L, Glosser G, Work M, et al. What's in a name: voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer's disease, frontotemporal dementia and corticobasal degeneration. Brain. 2004;127(3):628–649. doi: 10.1093/brain/awh075. [DOI] [PubMed] [Google Scholar]
- Grossman M, Mickanin J, Onishi K, Hughes E, D'Esposito M, Ding X-S, et al. Progressive non-fluent aphasia: Language, cognitive and PET measures contrasted with probable Alzheimer's disease. Journal of Cognitive Neuroscience. 1996;8(2):135–154. doi: 10.1162/jocn.1996.8.2.135. [DOI] [PubMed] [Google Scholar]
- Grossman M, Rhee J, Moore P. Sentence processing in frontotemporal dementia. Cortex. 2005;41(6):764–777. doi: 10.1016/s0010-9452(08)70295-8. [DOI] [PubMed] [Google Scholar]
- Heim S, Opitz B, Friederici AD. Distributed cortical networks for syntax processing: Broca's area as the common denominator. Brain and Language. 2003;85(3):402–408. doi: 10.1016/s0093-934x(03)00068-3. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Graham N, Patterson K. Charting the progression in semantic dementia: implications for the organisation of semantic memory. Memory. 1995;3:463–495. doi: 10.1080/09658219508253161. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic Dementia: Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115(6):1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Kang AM, Constable RT, Gore JC, Avrutin S. An event-related fMRI study of implicit phrase-level syntactic and semantic processing. NeuroImage. 1999;10(5):555–561. doi: 10.1006/nimg.1999.0493. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston naming test. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- Lambon Ralph MA, McClelland JL, Patterson K, Galton CJ, Hodges JR. No right to speak? The relationship between object naming and semantic impairment: Neuropsychological evidence and a computational model. Journal of Cognitive Neuroscience. 2001;13(3):341–356. doi: 10.1162/08989290151137395. [DOI] [PubMed] [Google Scholar]
- Libon DJ, Xie SX, Moore P, Farmer J, Antani S, McCawley G, et al. Patterns of neuropsychological impairment in frontotemporal dementia. Neurology. 2007;68:369–375. doi: 10.1212/01.wnl.0000252820.81313.9b. [DOI] [PubMed] [Google Scholar]
- McKhann G, Trojanowski JQ, Grossman M, Miller BL, Dickson D, Albert M. Clinical and pathological diagnosis of frontotemporal dementia: Report of a work group on frontotemporal dementia and Pick's disease. Archives of Neurology. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Friston KJ, Ashburner J. Voxel-based morphometry of the human brain: Methods and applications. Current Medical Imaging Reviews. 2005;1(2):105–113. [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RSJ, Hodges JR. A voxel-based morphometry study of Semantic Dementia: Relationship between temporal lobe atrophy and semantic memory. Annals of Neurology. 2000;47:36–45. [PubMed] [Google Scholar]
- Murray R, Koenig P, Antani S, McCawley G, Grossman M. Lexical acquisition in progressive aphasia and frontotemporal dementia. Cognitive Neuropsychology. 2007;24(1):48–69. doi: 10.1080/02643290600890657. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Graham NL, Fryer TD, Williams GB, Patterson K, Hodges JR. Progressive non-fluent aphasia is associated with hypometabolism centred on the left anterior insula. Brain. 2003;126(11):2406–2418. doi: 10.1093/brain/awg240. [DOI] [PubMed] [Google Scholar]
- Ni W, Constable RT, Mencl WE, Pugh KR, Fulbright RK, Shaywitz SE, et al. An event-related neuroimaging study distinguishing form and content in sentence processing. Journal of Cognitive Neuroscience. 2000;12(1):120–133. doi: 10.1162/08989290051137648. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Patterson K, Tyler LK, Moss H, Stamatakis EA, Bright P, et al. Temporal lobe lesions and semantic impairment: A comparison of herpes simplex virus encephalitis and semantic dementia. Brain. 2007;130:1138–1147. doi: 10.1093/brain/awl344. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in teh human brain. Nature Reviews Neuroscience. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Peelle JE, Cooke A, Moore P, Vesely L, Grossman M. Syntactic and thematic components of sentence processing in progressive nonfluent aphasia and nonaphasic frontotemporal dementia. Journal of Neurolinguistics. 2007;20:482–494. doi: 10.1016/j.jneuroling.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, Grossman M. Langauge processing in frontotemporal dementia: A brief review. Langauge and Linguistics Compass. (In press) [Google Scholar]
- Peelle JE, McMillan C, Moore P, Grossman M, Wingfield A. Dissociable patterns of brain activity during comprehension of rapid and syntactically complex speech: Evidence from fMRI. Brain and Language. 2004;91:315–325. doi: 10.1016/j.bandl.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Price C, Davis KL, Moore P, Campea S, Grossman M. Clinical diagnosis of Frontotemporal dementia (FTD) Neurology. 2001;56:A176. [Google Scholar]
- Price CJ, Wise RJS, Frackowiak RSJ. Demonstrating the implicit processing of visually presented words and pseudowords. Cerebral Cortex. 1996;6:62–70. doi: 10.1093/cercor/6.1.62. [DOI] [PubMed] [Google Scholar]
- Price CJ, Wise RJS, Warburton EA, Moore CJ, Howard D, Patterson K, et al. Hearing and saying: The functional neuro-anatomy of auditory word processing. Brain. 1996;119(3):919–931. doi: 10.1093/brain/119.3.919. [DOI] [PubMed] [Google Scholar]
- Rahman S, Sahakian BJ, Hodges JR, Rogers RD, Robbins TW. Specific cognitive deficits in mild frontal variant frontotemporal dementia. Brain. 1999;122:1469–1493. doi: 10.1093/brain/122.8.1469. [DOI] [PubMed] [Google Scholar]
- Reilly J, Martin N, Grossman M. Verbel learning in semantic dementia: Is repetition priming a useful strategy? Aphasiology. 2005;19:329–339. [Google Scholar]
- Rogers TT, Hocking J, Noppeney U, Mechelli A, Gorno-Tempini ML, Patterson K, et al. Anterior temporal cortex and semantic memory: Reconciling findings from neuropsychology and functional imaging. Cognitive, Affective, and Behavioral Neuroscience. 2006;6(3):201–203. doi: 10.3758/cabn.6.3.201. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, McClelland JL, Hodges JR, et al. Structure and deterioration of semantic memory: A neuropsychological and computational investigation. Psychological Review. 2004;111(1):205–235. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58(2):198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Goulding PJ, Neary D. Semantic dementia: A form of circumscribed cerebral atrophy. Behavioural Neurology. 1989;2:167–182. [Google Scholar]
- Snowden JS, Neary D, Mann DM. Fonto-temporal lobar degeneration: Fronto-temporal dementia, progressive aphasia, semantic dementia. New York: Churchill Livingstone; 1996. [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Thompson CK, Ballard KJ, Tait ME, Weintraub S, Mesulam M. Patterns of language decline in non-fluent primary progressive aphasia. Aphasiology. 1997;11:297–331. [Google Scholar]
- Turner RS, Kenyon LC, Trojanowski JQ, Gonatas N, Grossman M. Clinical, neuroimaging, and pathologic features of progressive nonfluent aphasia. Annals of Neurology. 1996;39(2):166–173. doi: 10.1002/ana.410390205. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Stamatakis EA, Bright P, Acres K, Abdallah S, Rodd JM, et al. Processing objects at different levels of specificity. Journal of Cognitive Neuroscience. 2004;16(3):351–362. doi: 10.1162/089892904322926692. [DOI] [PubMed] [Google Scholar]
- Warrington EK. The selective impairment of semantic memory. Quarterly Journal of Experimental Psychology. 1975;27:635–657. doi: 10.1080/14640747508400525. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3rd ed. New York: Psychological Corporation; 1997. [Google Scholar]
- Williams GB, Nestor PJ, Hodges JR. Neural correlates of semantic and behavioural deficits in frontotemporal dementia. NeuroImage. 2005;24(4):1042–1051. doi: 10.1016/j.neuroimage.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Zurif EB. Brain regions of relevance to syntactic processing. In: Osherson D, editor. An invitation to cognitive science. 2nd ed. Cambridge: MIT Press; 1996. pp. 381–397. [Google Scholar]
- Zurif EB, Caramazza A, Myerson R. Grammatical judgments of agrammatic aphasics. Neuropsychologia. 1972;10(4):405–417. doi: 10.1016/0028-3932(72)90003-6. [DOI] [PubMed] [Google Scholar]