Abstract
Panaxytriol is a nutraceutical-based active constituent of Korean red ginseng and is reported to exhibit potent anti-tumor properties. Its activity may be in part due to its induction of phase 2 chemoprotective enzymes. Its unique properties may have important implications in cancer therapeutics.
Ginseng is a commonly used nutraceutical. It has attracted the attentions of health advocates and scientists alike, generating one of the most extensive bodies of scientific literature of any medicinal herb.i Ginseng belongs to botanical species of genus Panax, indigenous to Asia. The English word “ginseng” is a literal translation from Chinese meaning “man root.” Panax ginseng is regarded as a panacea, partly perpetuated by its name Panax meaning “cure all” derived from Greek. As such Panax ginseng became a much sought after medicine throughout the world, with annual sales of 0.3 billion in the United States alone. Panax ginseng generally refers to Asian ginseng (Chinese or Korean) and is commonly known as red ginseng. This herbal root is widely used throughout Asia as folk medicine for a variety of maladies for over 2000 years. In Chinese medicine, ginseng is used with the intention of increasing the body’s resistance to physical, chemical and biological stress. The therapeutic benefits of Panax ginseng are described in the pharmacopeia of several countries such as China, Germany, Japan, United Kingdom and France, possible applications are for cardiovascular concerns, diabetes and cancer.ii The latter attribute, in particular, has been supported through a number of in vitro and in vivo biological studies of red ginseng extract.iii
The active principal chemical components of red ginseng are ginsenosides (plant steroids of the saponin class), polysaccharides (ginsanonans), peptidoglycans (panaxans), and volatile oils, all of which may, in part or cooperatively, stimulate its reported pharmacological effects. In 1983, (3R,9R,10R)-panaxytriol was isolated as a characteristic constituent of red ginseng (from the steamed and dried root of Panax ginseng C. A. Meyer).iv was shown to exhibit in vitro inhibitory activity against a range of tumor cells, including human gastric carcinoma (MK-1),v mouse lymphoma (P388D1),vi and human breast carcinoma (Breast M25-SF).vii In addition, an in vivo study of panaxytriol reported the suppression of the growth of B16 melanoma cells.viii
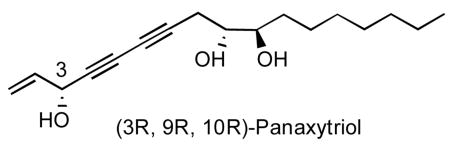
Mindful of pananxytriol’s potentially important properties, we began to take an interest in its synthesis. In 2003 we disclosed a concise enantioselective synthesis of (3R, 9R, 10R)-panaxytriol that enabled access to multigram quantities of the pure agent to allow for biological evaluations.ix Subsequently through diverted total synthesis, SAR studies uncovered several analogs that are more potent than the natural product with little or no toxicity. These congeners appeared not to be vulnerable to the phenomenon of multidrug resistance.x
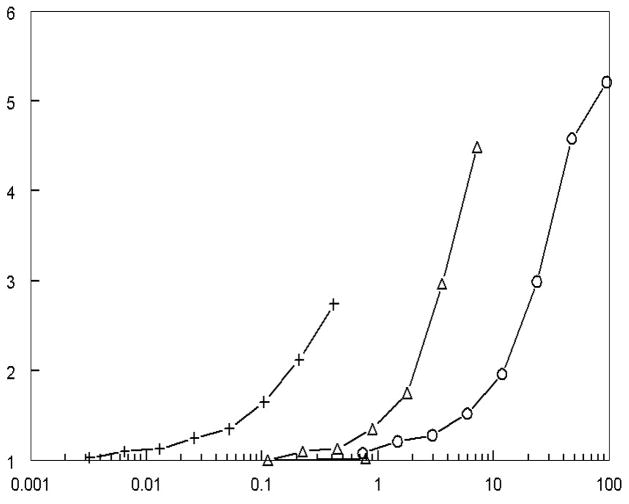
In the course of these pursuits, we became interested in exploring the mechanism of action of panaxytriol in the context of cancer therapeutics. The preliminary findings from the Johns Hopkins based studies, appear to indicate that panaxytriol exhibits cancer prevention activity in part by the induction of phase 2 enzymes. These phase 2 enzymes promote detoxification reactions. These chemoprotective enzymes provide a major mechanism to protect cells against the toxicities of reactive electrophiles and reactive oxygen species. Their induction may serve to modulate carcinogen metabolism. Induction of these enzymes may serve to neutralize reactive electrophiles that might otherwise emerge as ultimate carcinogens or mutagents.xi The study reported herein was initiated to evaluate the phase 2 enzyme inducer activities of different types of Panax ginseng extracts and the various phytochemical components of the ginseng extracts. The method employed to detect and quantify the inducer potencies of the pure components and ginseng extracts utilized an established rapid assay, directed to measuring the output of quinone reductase. This is a convenient enzyme target due to its coordinate induction with other phase 2 enzymes, its large inducer response, and its ease of quantification by a coupled tetrazolium dye reduction assay.xii As condensed in Figure 1, homogeneous and total synthesis-derived panaxytriol, otherwise an active constituent of Korean red ginseng, registered significant induction of phase 2 enzyme activity, as well as Korean ginseng and protopanaxatriol (a deglycosylated derivative of the ginsenosides). This induction study is in tune with other studies of the cancer preventive effects of ginseng.xiii It established that the activity of panaxytriol is based on the agent itself and does not depend on synergism with other components of ginseng.
Figure 1.
Induction of quinone reductase (NQO1) by Korean red ginseng extract, protopanaxatriol and panaxytriol. NQO1 inducer activity was assayed as described in the text, and reported here as treated/control at different concentrations of ginseng extract or component per well. Significant cytotoxicity was observed for panaxytriol above 0.5 μg/well and protopanaxadiol above 5 μg/well. (+) Panaxytriol (molecular weight 267), (▵) Protopanaxatriol (molecular weight 461), (○) Korean red Sun ginseng extract.
This finding is not without potentially significant implications in the context of cancer therapeutics. Panaxytriol stands out as a rare instance of an anti-tumor agent with documented anti-cancer properties that is found in a widely consumable food product. As such even a moderate cytotoxic effect can be valuable since its side effects are clearly accommodable. Through molecular editing,10 we have developed analogs with enhanced potency, and with little or no toxicity. Happily its activity is not compromised by multidrug resistance. These attributes begin to approach the embodiment of a successful cancer drug for long-term chemotherapy. Furthermore, since panaxytriol and selected analogs appear to operate through a unique mechanism of induction of phase 2 enzymes,xiv this may render it compatible with other cancer drugs in synergy, in this instance to better target specific cancer cells with lower dosage regimens. We close by noting that an increasingly multifaceted aspect of cancer care is the disparate chemotolerance between cell lines which might enhance the benefits of chemotherapy. As such a drug discovery program through molecular editingxv of nutraceutical panaxytriol may be a satisfactory approach to effective cancer care.
Acknowledgments
This work was supported by the National Institutes of Health grant HL25848 and a pilot grant from the National Center for Complementary and Alternative Medicine, National Institutes of Health, USA to Johns Hopkins University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- i.www.nlm.nih.gov for ginseng related literature.
- ii.Helms S. Altern Med Rev. 2004;9:259. [PubMed] [Google Scholar]
- iii.Saito T, Matsunaga H, Yamamoto H, Nagumo F, Fujito H, Mori M, Katano M. Biol Pharm Bull. 1994;17:798. doi: 10.1248/bpb.17.798. [DOI] [PubMed] [Google Scholar]
- iv.Kitagawa I, Yoshikawa M, Yoshihara M, Hayashi T, Taniyama T. Yakugaku Zasshi. 1983;103:612. [PubMed] [Google Scholar]
- v.Saita T, Katano M, Matsunaga H, Kouno I, Fujito H, Mori M. Biol Pharm Bull. 1995;18:933. doi: 10.1248/bpb.18.933. [DOI] [PubMed] [Google Scholar]
- vi.Kim JY, Lee KW, Kim SH, Wee JJ, Kim YS, Lee HJ. Planta Med. 1992;68:119. doi: 10.1055/s-2002-20240. [DOI] [PubMed] [Google Scholar]
- vii.Matsunaga H, Saita T, Naguo F, Mori M, Katano M. Cancer Chemother Pharmacol. 1995;35:291. doi: 10.1007/BF00689447. [DOI] [PubMed] [Google Scholar]
- viii.Katano M, Yamamoto H, Matsunaga H, Mori M, Takata K, Nakamura M. Gan to Kagaku Ryoho. 1990;17:1045. [PubMed] [Google Scholar]
- ix.Yun H, Danishefsky SJ. J Org Chem. 2003;68:4519. doi: 10.1021/jo0341665.For other syntheses of panaxytriol see: Cho EJ, Lee D. Org Lett. 2008;10:257. doi: 10.1021/ol702651s.Yadav JS, Maiti A. Tetrahedron. 2002;58:4955.Mayer SF, Steinreiber A, Orru RVA, Faber K. J Org Chem. 2002;67:9115. doi: 10.1021/jo020073w.Lu W, Zheng GR, Cai JC. Synlett. 1998:737.Satoh M, Takeuchi N, Fujimoto Y. Chem Pharm Bull. 1997;45:1114.
- x.Yun H, Chou T-C, Dong H, Tian Y, Li Y-m, Danishefsky SJ. J Org Chem. 2005;70:10375. doi: 10.1021/jo0515475. [DOI] [PubMed] [Google Scholar]
- xi.Talalay P. BioFactors. 2000;12:5. doi: 10.1002/biof.5520120102. [DOI] [PubMed] [Google Scholar]
- xii.Prochaska HJ, Santamaria AB. Anal Biochem. 1988;169:328. doi: 10.1016/0003-2697(88)90292-8. [DOI] [PubMed] [Google Scholar]
- xiii.Halim M, Yee D, Sames D. An independent study from the laboratories of Dr. Dalibor Sames at Columbia University supports the phase 2 enzyme induction mode of action of panaxytriol. J Am Chem Soc. 2008 (in press) [Google Scholar]
- xiv.Unpublished results in collaboration with Dr. Dalibor Sames of Columbia University.
- xv.Wilson RM, Danishefsky SJ. J Org Chem. 2006;71:8329. doi: 10.1021/jo0610053. [DOI] [PubMed] [Google Scholar]