Abstract
Background & Aims
Several lines of evidence support a role for TLR signaling to protect the intestine from pathogenic infection. We hypothesize that TLR signaling at the level of the intestinal epithelium is critical for mucosal immune responses.
Methods
We generated transgenic mice that express a constitutively active form of TLR4 in the intestinal epithelium (V-TLR4 mice). Lamina propria cellularity was evaluated by immunostaining and flow cytometry. IgA levels in the stool and serum were measured by ELISA. Chemokine and cytokine expression were analyzed by quantitative PCR and ELISA.
Results
V-TLR4 transgenic mice reproduced normally and had a normal lifespan. Constitutive activity of TLR4 in the intestinal epithelium promoted recruitment of B cells and an increase in fecal IgA levels. Intestinal epithelial cells of V-TLR4 mice expressed higher levels of CCL20 and CCL28, chemokines known to be involved in B cell recruitment, and of APRIL, a cytokine that promotes T cell-independent class switching of B cells to IgA. The changes in B cell numbers and IgA levels were blocked by simultaneous expression in intestinal epithelial cells of M3, a herpesvirus protein that binds and inhibits multiple chemokines.
Conclusion
TLR signaling in the intestinal epithelial cells significantly elevated the production of IgA in the intestine. This effect was mediated by TLR-induced expression of a specific set of chemokines and cytokines that promoted both recruitment of B cells into the lamina propria and IgA class switching of B cells.
Keywords: TLR4, B cells recruitment, chemokine, IgA, Class switching
Introduction
The gastrointestinal tract hosts a complex community of microorganisms, which may be both beneficial and potentially pathogenic. To coexist with this microbiota, and to respond properly to pathogenic bacteria, the body has evolved a variety of mechanisms including physical barriers and a complex array of cellular and humoral immune mechanisms. The intestinal epithelium, in addition to its nutrient absorptive functions, is a selective barrier between the luminal bacteria and underlying lamina propria, which is replete with immune responsive cells. The intestinal epithelial cells (IECs) also produce cytokines in response to pathogenic infection1, 2, and respond to signals from immune cells in the lamina propria. More recently, IECs have been shown to participate in the generation of IgA-secreting lamina propria B cells through expression of cytokines responsible for local class switching3, 4. These data interpose IECs between innate and adaptive immune responses in the gut.
TLRs are pattern-recognition receptors that function as sensors of microbial infection and are critical for the initiation of innate inflammatory and adaptive immune defense responses5, 6. Activation TLR4 by LPS, a membrane component of all Gram-negative bacteria, triggers the production by inflammatory cells of inflammatory cytokines and IFN-β. Furthermore, TLR expression on IECs is increased in inflammatory bowel disease7–11. In particular, functional TLR4 has been described on epithelial cells of the small intestine11–14. After birth, TLR4 signaling is downregulated to cope with colonization of the intestine by the microbiota10. However, TLR4 signaling in response to pathogenic infection results in intestinal epithelial chemokine expression and the recruitment of inflammatory cells and intraepithelial dendritic cells15–17.
We and others have explored the role of TLR4 in intestinal epithelial homeostasis and defense against pathogens. TLR4 knockout mice develop worse clinical symptoms of colitis following injury with dextran sodium sulfate (DSS)18, 19. Underlying this phenotype, TLR4 knockout mice have decreased intestinal epithelial cell proliferation, decreased recruitment of inflammatory cells, and increased bacterial translocation to mesenteric lymph nodes18. Moreover, TLR4 regulates defensin expression in the intestine20. These data all point to a critical role for TLR4 in intestinal epithelial protection against pathogenesis by luminal bacteria.
To understand more fully the biological role(s) of TLR4 in the intestine, we generated transgenic mice (V-TLR4 mice) that express a constitutively active form of TLR4 under the control of the villin promoter. Expression of this constitutively active TLR4 on IECs mimicked signaling by Toll ligands and upregulated the expression of a select group of chemokines that drive the recruitment of B cells to the lamina propria. It also promoted upregulation of the TNF family member, a proliferation-inducing ligand (APRIL), by IECs and increased secretion of IgA into the intestinal lumen of V-TLR4 mice. These data support a model in which TLR signaling by the intestinal epithelium protects the mucosa by directing a program designed to increase IgA secretion and promote immune exclusion of pathogens.
Materials and Methods
Mice
Plasmids containing the mouse villin promoter (pBS-Villin)21 and mCD4-hTLR4 fusion gene have been previously described22. The mCD4-hTLR4 was PCR amplified and cloned into pBS-Villin. The sequences were verified by sequencing. The transgene was gel purified, microinjected into fertilized eggs from [C57BL/6J ×DBA/2]F2 mice and the eggs transferred into oviducts of ICR foster mothers. Identification of the transgenic mice was done by PCR amplification using the primers: 5′-GGA GGG AGG GGT ATG TTT TA -3′ and 5′-TCC CTT GAG TGA CAG CTA GG -3′. The resulting transgenic mice were kept under pathogen-free conditions. All experiments involving animals were performed following the guidelines of Animal Care and Use Committee of Mount Sinai School of Medicine.
Immunostaining
Immunostaining of OCT mounted fresh tissue was performed as previously described23. Primary Abs used were anti-CD3 (no. 550275), anti-CD4 (no. 550278) and anti-IgA (no. 556969) from BD Biosciences. Secondary antibodies used were Alexa Fluor 488 and 594 goat anti-rat IgG (#A-11006, and #A-11007) from Molecular Probes (Eugene, OR), and Cy3 goat anti-Armenian hamster (#127-165-160) from Jackson ImmunoResearch (West Grove, PA).
Flow cytometry
Lamina propria cells were resuspended in FACS staining buffer (PBS containing 0.5% BSA) and then incubated for 20 min at 4°C with 5 µg/ml Fc block (BD PharMingen, San Diego, CA) and then stained with directly conjugated primary mAbs. Monoclonal antibodies to the following mouse leukocyte surface markers were purchased from BD PharMingen: CD45 (30-F11), CD3 (145-2C11), CD19 (1D3). To determine viability, samples were subsequently stained with 20 µl of 5 µg/ml propidium iodide (Calbiochem, La Jolla, CA). Samples were analyzed in a FACSCanto instrument (Becton Dickinson, San Jose, CA). Data were analyzed using the FlowJo software (Tree Star).
RNA extraction, RT-PCR and Q-PCR analysis
Total RNA from intestine epithelial cells or different segments of the intestine was extracted using the RNeasy mini Kit (Qiagen) according to the manufacturer's instructions. Reverse transcription was performed using 2 µg of total RNA. Quantitative PCR was conducted in duplicate. Relative expression levels were calculated as 2^ (Ct Ubiquitin - Ct gene) using Ubiquitin RNA as the endogenous control. If not specified, every sample represented the pool of cDNA from 4 mice of the same age, sex and genotype. The data in figures was representative of three independent experiments. RT-PCR analysis for α-CT was performed as described previously24.
Western blotting
Proteins were extracted from intestinal epithelial cells (please see Supp Methods) and analyzed using 4–20% pre-casted gradient gel (Biorad). After transferred onto a PVDF membrane (Biorad), blots were incubated with antibodies against APRIL (no. 14–6138, eBioscience) and β-actin (Abcam) and then with a peroxidase-conjugated anti-rabbit IgG (Abcam Inc).
NF-κB p65 Assay
For measurement of NF-κB activation, nuclear protein was extracted from freshly isolated duodenal epithelial cells using a nuclear extraction kit (Active Motif, Carlsbad, CA) according to the manufacturer’s instructions. 5µg of nuclear extract was used to measure the DNA-binding activity of NF-κB p65 using the TransAM NF-κB p65 Chemiluminescent kit (Active Motif, Carlsbad, CA) according to the manufacturer’s instructions. Chemiluminescent intensities were normalized with the standard curve created by a gradient of Jurkat cell DNA (µg of corresponding Jurkat cell extract).
Measurement of immunoglobulin and chemokine levels by ELISA
Fecal pellets were disrupted in PBS/0.02% sodium azide by vortexing on high for 5 min, and insoluble material was removed by centrifugation at 10,000 RCF for 10 min at room temperature. Total IgA levels in serum and fecal extracts, were assessed by ELISA as described25. The values were expressed as ng per mg feces.
CCL20 and CCL28 concentrations were determined using ELISA kits from R & D Systems (Minneapolis, MN) following the manufacturer’s instructions. The amount of chemokine assayed in each intestinal sample is expressed as pg of chemokine per mg of tissue protein.
Statistical analysis
Statistical analysis of the data was performed using Prism 2.0c. Data in the text are given as means ± SD unless otherwise stated. Unpaired Student’s t test was used to determine statistical significance. Differences were considered significant when P < 0.05.
Results
Generation of transgenic mice expressing mCD4-hTLR4
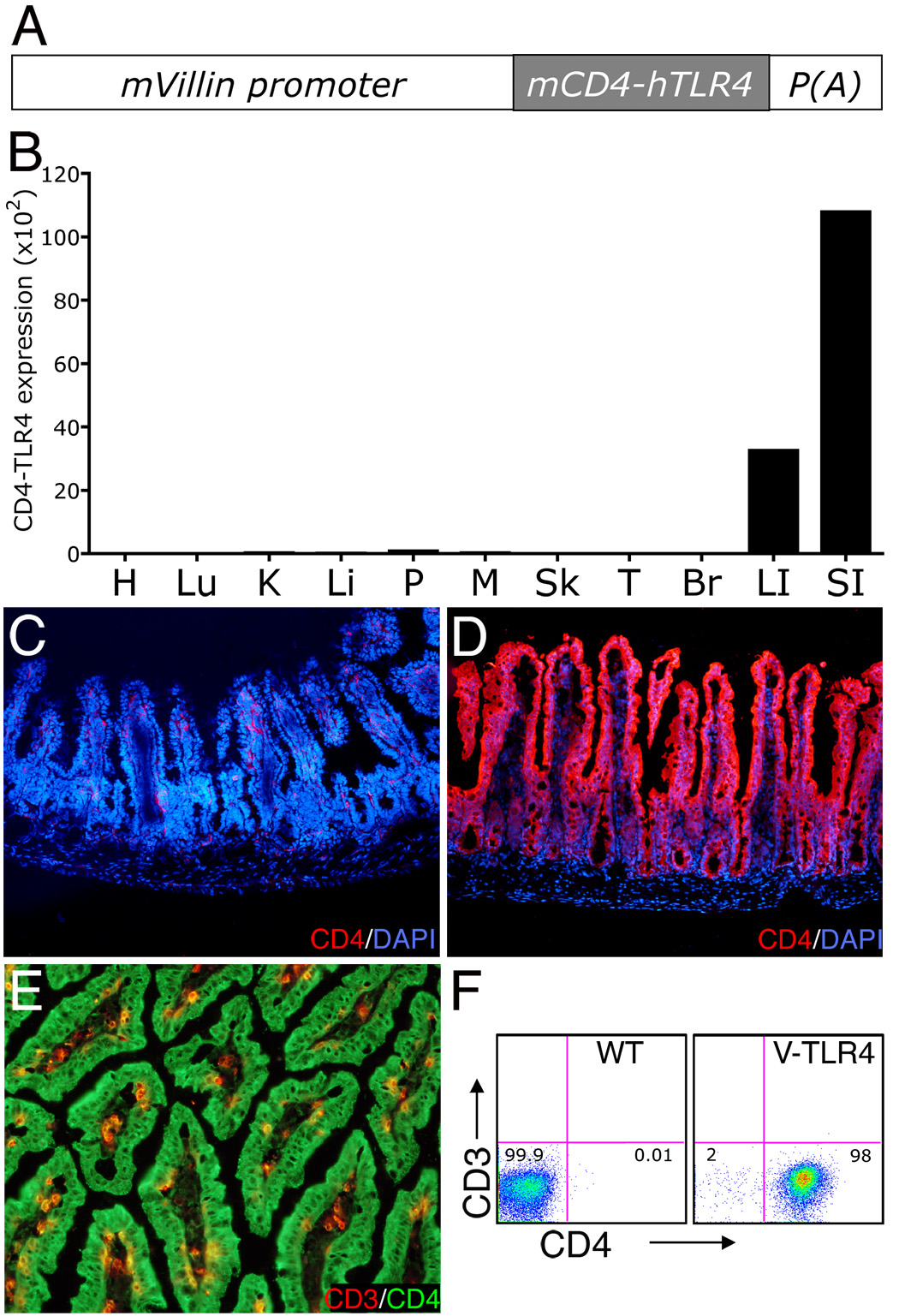
To study the biological effects of TLR signaling in the intestinal epithelium in vivo, we generated transgenic mice expressing a constitutively active form of TLR4 on IECs. The mice carry a mCD4-hTLR4 transgene that consists of the human TLR4 transmembrane and intracellular domains fused to the extracellular domain of murine CD4. This construct has been shown to signal similarly to endogenous TLR4 both in vitro and in vivo 22, 26. The transgene was driven by the villin promoter (Figure 1A), which has been previously shown to target transgene expression predominately to IECs of both small and large intestine21. Four transgenic lines were established from five founders and are referred to as V-TLR4 mice. For further study we selected by quantitative-PCR (Q-PCR) the transgenic line expressing the highest level of the transgene in the small intestine. In this line the transgene was expressed predominantly in the large and small intestine (Figure 1B). Immunostaining with mouse CD4 antibody revealed the fusion protein in epithelial cells of the small intestine of transgenic (Figure 1D) but not of control mice (Figure 1C). Flow cytometric (FACS) analysis and immunostaining confirmed that the transgene was expressed exclusively by intestinal epithelial cells (Figure 1, E and F).
Figure 1. Expression of the CD4-TLR4 transgene in V-TLR4 mice.
A) Diagram of the V-TLR4 transgene. The transgene encodes a fusion protein containing the mouse CD4 (mCD4) extracellular domain and human TLR4 signaling domain and is driven by the mouse villin promoter (mVillin). p(A) represents SV40 poly A sequences. B) CD4-TLR4 mRNA expression in different tissues of V-TLR4 mice. The values were standardized to ubiquitin levels in each sample. H: Heart; Lu: Lung; K: kidney; Li: liver; P: Pancreas; M: Skeletal Muscle; Sk: Skin; T: Testis; Br: Brain; LI: Large intestine; SI: Small intestine. C–D). Representative immunostaining for CD4 (red) and DAPI (blue) in small intestine of WT (C) and V-TLR4 (D) mice. E) Representative immunostaining for CD3 (red) and CD4 (green) in small intestine of V-TLR4 mice. Note that the transgene expression is restricted to epithelial cells. F) FACS analysis of CD4-TLR4 expression in epithelial cells isolated from small intestine. Cells were gated on the CD45−/PI−.
Activation of TLR signaling pathways in IECs of V-TLR4 mice
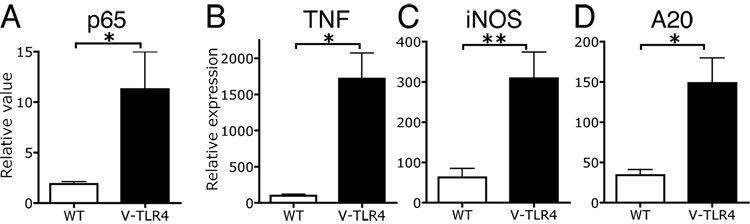
NF-κB activation is a major downstream pathway of TLR signaling. To confirm that the TLR4 fusion protein was functional, we prepared nuclear protein extracts from IECs isolated from the small intestine of transgenic and control mice and analyzed p65 activation by ELISA. IECs from V-TLR4 mice showed 4–5 fold higher levels in NF-κB p65 activation than controls (Figure 2A). We also measured the expression of several TLR target genes by Q-PCR in IECs isolated from the small intestine. V-TLR4 transgenic mice expressed 15 fold more TNFα mRNA in IECs than WT littermates (Figure 2B). iNOS, another proinflammatory gene induced by TLR4 activation in macrophages, was also upregulated in small intestinal IECs of V-TLR4 mice (Figure 2C). Finally, A20, an NF-κB signaling pathway feedback regulator that is induced by TLR4 activation, was also upregulated in V-TLR4 IECs (Figure 2D). Taken together, these results indicate that the fusion gene was functional in IECs of V-TLR4 mice.
Figure 2. Activation of the TLR signaling pathway in IECs of V-TLR4 mice.
A) DNA binding ELISA analysis of p65 in IECs isolated from the small intestine of WT and V-TLR4 mice (n=10/group, *p<0.05). B–D). Relative mRNA expression of TNFα (B), iNOS (C), and A20 (D) in IECs isolated from small intestine of WT and V-TLR4 mice (n=3/group; * p<0.05, ** p<0.01).
Expression of TLR4 in IEC results in increased numbers of B cells and increased levels of B cell-tropic chemokines
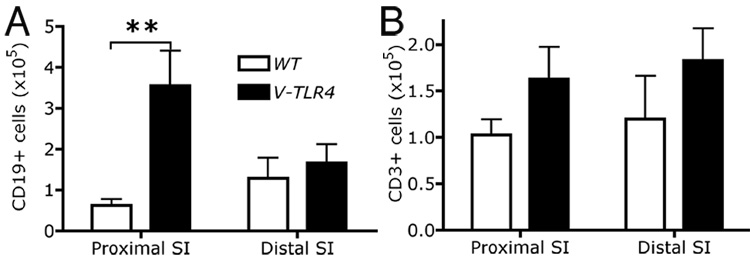
The V-TLR4 transgenic mice reproduced normally and had a normal lifespan. To investigate if transgene expression in IECs induced changes in the cellularity of the lamina propria, we performed FACS analysis. The V-TLR4 mice had significantly higher numbers of B cell in the lamina propria of the proximal small intestine than control mice (Figure 3A), but the difference in B cells in the distal small intestine was not significant. No difference was observed in T cell numbers between transgenic and WT mice (Figure 3B).
Figure 3. Transgene expression in IECs increases the number of B cells in the lamina propria.
A–B) FACS analysis of CD19+ B cells (A) and CD3+ T cells (B) in lamina propria of WT and V-TLR4 mice. Values indicate the absolute number of each subset (n=4/group). Note the increased number of B cells in the proximal small bowel of V-TLR4 mice (** p<0.01).
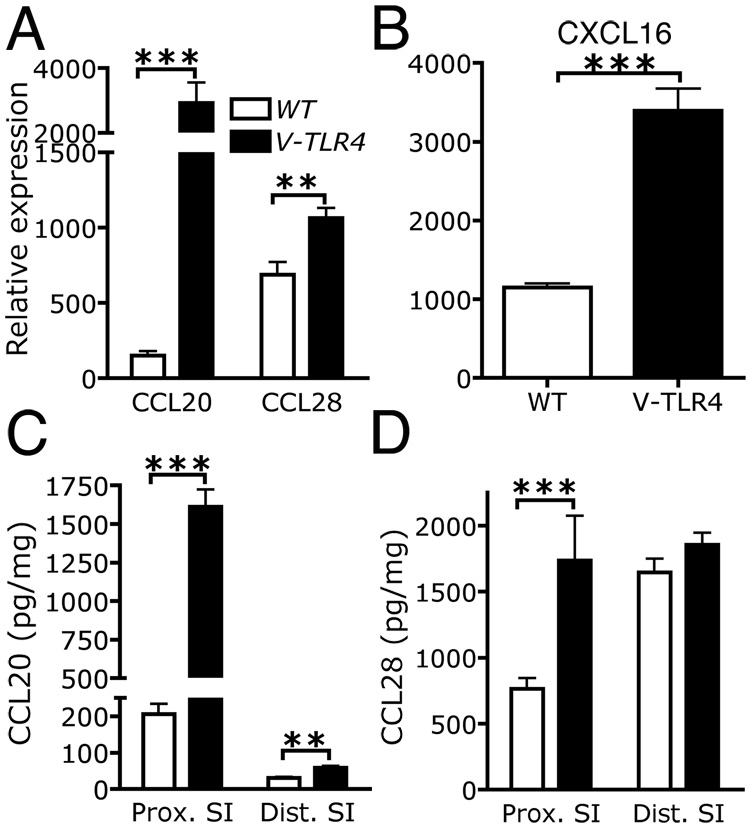
The influx of B cells into lymphoid structures and the lamina propria of the intestine is dependent on chemokines27, 28. To investigate if chemokine expression was altered in the small intestine of V-TLR4 mice, we determined mRNA levels of all known murine chemokines by Q-PCR. As expected, CCL25 (not shown) and CCL28 (Figure 4A) were expressed in the small intestine 29, 30. We found that expression of CCL28, CCL20, and CXCL16 mRNA levels were significantly upregulated in V-TLR4 mice (Figure 4A and B). CCL20 protein levels were found to be increased more than 5 fold in proximal small intestine of V-TLR4, whereas CCL28 protein levels were on average 1.6 fold higher (Figure 4C and D). However, in the distal small intestine CCL20 levels were increased only two-fold and CCL28 levels were not different from controls (Figure 4C and D). Confirming that these changes were likely due to expression of the TLR4 chimera in IECs we found corresponding increases in CCL20, CCL28, and CXCL16 mRNA levels in purified IECs from V-TLR4 mice (Supplemental Figure 1).
Figure 4. Increased expression of chemokines in the proximal small intestine of V-TLR4 mice.
A–B) Q-PCR analysis of CCL20, CCL28 (A) and CXCL16 (B) expression in the proximal small intestine of WT and V-TLR4 mice (n=7/group; ** p<0.01, *** p<0.001). C–D) CCL20 (C) and CCL28 (D) protein levels in small intestine of V-TLR4 mice and WT mice (WT n=12, V-TLR4 n=15; *** p< 0.001).
V-TLR4 mice have both increased numbers of IgA+ cells in the lamina propria and higher fecal IgA levels compared to control mice
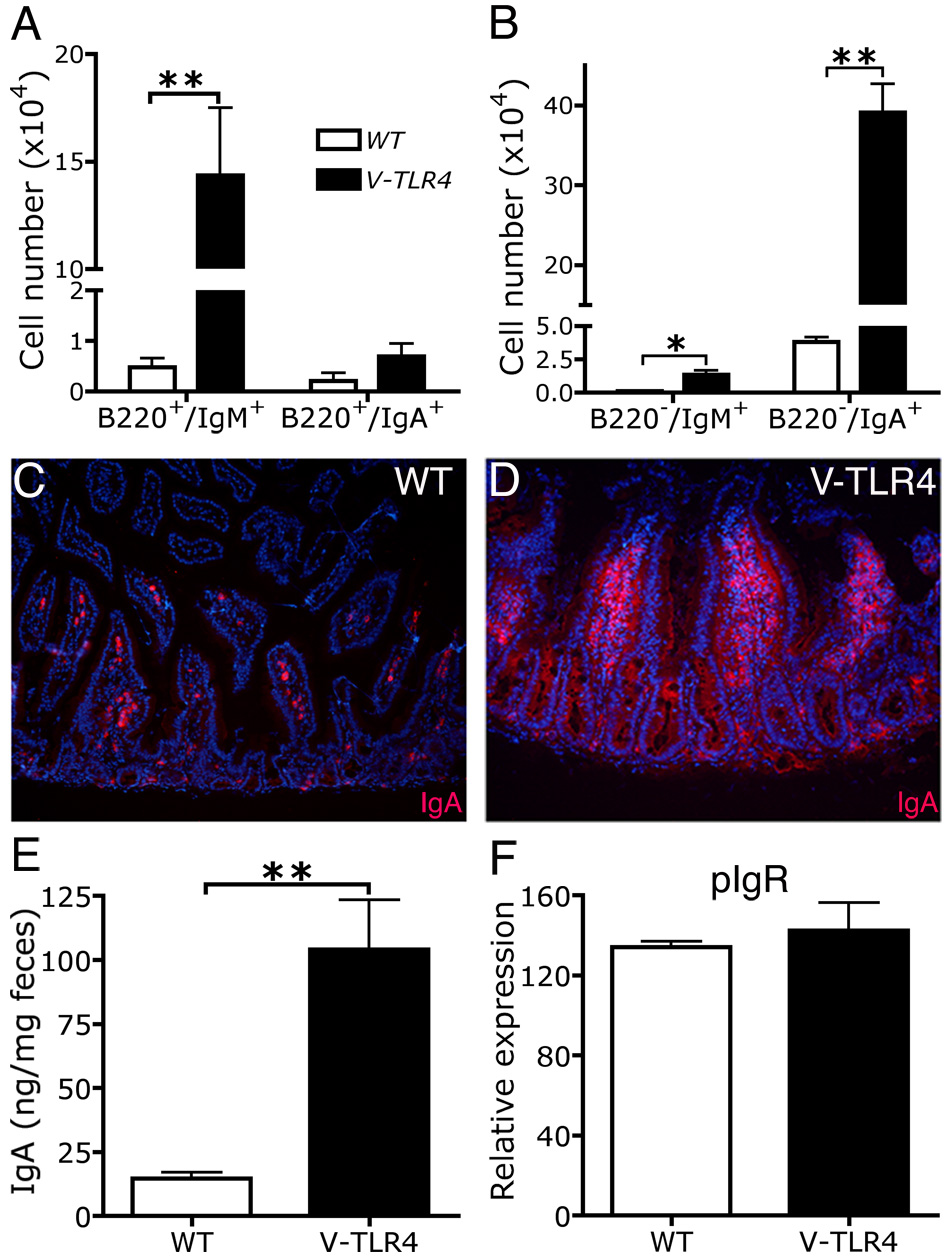
Next we examined the B cell subsets in the lamina propria. FACS analysis showed that the V-TLR4 mice had a ten fold increase in the number of B220+/IgM+ naïve B cells in the proximal small intestine compared to controls. The number of B220+/IgA+ memory B cells did not differ from that of controls (Figure 5A), but the number of B220−/IgA+ plasma cells was markedly increased (8 fold higher than controls). The number of IgA producing plasma cells vastly exceeded that of plasma cells producing IgM or IgG (Figure 5B and Supplemental Figure 2). Immunostaining analysis confirmed that the number of surface IgA+ cells in lamina propria of the proximal small intestine was dramatically elevated relative to controls (Figure 5C and D). Consistent with the increased number of IgA+ cells in the lamina propria, the IgA levels in the stools were 5 times higher in V-TLR4 mice compared to controls (Figure 5E). Since increased fecal IgA could be due to increased production of IgA and/or to increased transport of IgA from the lamina propria across the epithelium, we measured the expression of the major IgA transporter, pIgR, on IECs by Q-PCR. We found that mRNA levels of pIgR were unchanged by expression of the TLR4 transgene (Figure 5F). These results indicate that expression of TLR4 in IECs led to accumulation of IgA producing cells in the small intestine and increased secretion of IgA into the intestinal lumen.
Figure 5. Increased IgA production in the lamina propria of V-TLR4 mice.
A–B). FACS analysis of B cell (A) and plasma cell subsets (B) in lamina propria of proximal small intestine of WT and V-TLR4 mice (WT n=3, V-TLR4 n=4; * p< 0.05, ** p< 0.01). C & D). Immunostaining of IgA+ cells in duodenal lamina propria of WT (C) and V-TLR4 (D) mice. E). IgA levels in feces of WT (n=24) and V-TLR4 (n=50) mice (** p<0.01). F). Q-PCR analysis of pIgR expression in IECs of WT and V-TLR4 mice (n=4/group).
TLR4 signaling in intestinal epithelial cells enhances B cell class switching to IgA in lamina propria
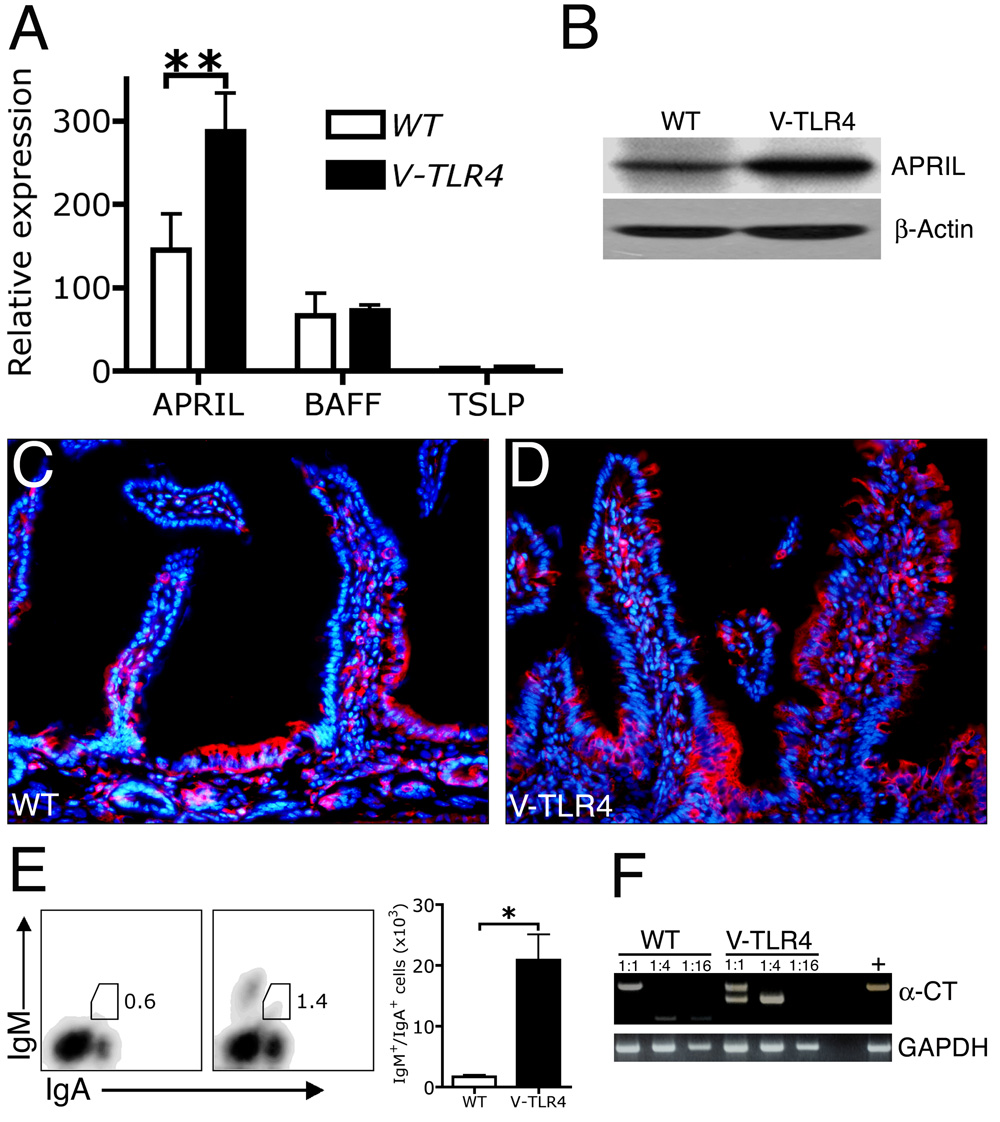
Increased IgA secretion into the intestinal lumen could be due to increased recruitment of IgA+ cells to the gut, increased B cell class switching to IgA in the gut, or both. To test whether expression of TLR4 in the intestine led to increased IgA isotype switching, we examined the expression of three factors recently reported to promote T cell-independent IgA class switching: APRIL, BAFF, and TSLP4, 31. Q-PCR analysis in isolated IECs from proximal small intestine revealed that the expression of BAFF and TSLP did not differ between V-TLR4 and WT mice, but that the expression of APRIL was twofold higher in the transgenic IECs (Figure 6A). No changes were detected in the expression of other cytokines implicated in class switch including IL-6, IL-10 and TGF-β (data not shown). Western blot analysis confirmed that IECs of V-TLR4 mice produced higher levels (1.6 fold) of APRIL than WT littermates (Figure 6B). APRIL expression was detected in epithelial cells and cells of the lamina propria of both V-TLR4 and WT mice throughout the small intestine (Figure 6C, Figure 6D and data not shown).
Figure 6. Increase in APRIL expression in IECs and B cell class switching to IgA in V-TLR4 mice.
A). Q-PCR analysis of APRIL, BAFF and TSLP expression in proximal small intestinal epithelial cells of WT and V-TLR4 mice (n=3/group; **, p<0.01). B). Western blot of APRIL protein in proximal small intestinal epithelial cells of WT and V-TLR4 mice. C & D). Immunostaining of APRIL in duodenum of WT (C) and V-TLR4 (D) mice (red, APRIL; blue, DAPI). E). FACS analysis of IgM+/IgA+ cells in lamina propria of proximal small intestine of WT and V-TLR4 mice (WT n=3, V-TLR4 n=4; * p< 0.05). F). RT-PCR analysis of post switch circular transcript (α-CT) expression in lamina propria B cells from WT and V-TLR4 mice. Data represented pooled B cells from 20 WT or 10 V-TLR4 mice. Different bands represent different splice variants of α-CT as previously described 24.
To determine whether the increased expression of APRIL was associated with augmented IgA class switching, we first examined the number of IgM+/IgA+ cells in lamina propria of V-TLR4 mice. IgM+/IgA+ cells are thought to represent a subset of newly switched B cells. We detected an increase in both relative and absolute number of IgM+/IgA+ cells in the lamina propria of V-TLR4 mice (Figure 6E). We also prepared mRNA from B220+ B cells sorted from the lamina propria. First we examined the presence of activation-induced cytidine deaminase (AID), a critical enzyme involved in class switching32. We found equivalent AID mRNA expression in purified lamina propria B cells from both WT and transgenic mice (Supplemental Figure 3). Next, we examined the presence of post-switching circular transcripts (α-CT) by RT-PCR analysis. We observed that B cells from the lamina propria of V-TLR4 mice showed more post-switching circular transcripts than WT (Figure 6F). As α-CT exists only transiently after class switching, the results suggest that the class switching occurred in situ. Together, these data indicate that increased TLR signaling by IECs is associated with increased immunoglobulin class switch to IgA in the lamina propria, which may account in part for the increase in IgA in feces.
Increased B cell numbers and fecal IgA levels in V-TLR4 mice are blocked by co-expression of the chemokine binding protein M3 in IECs
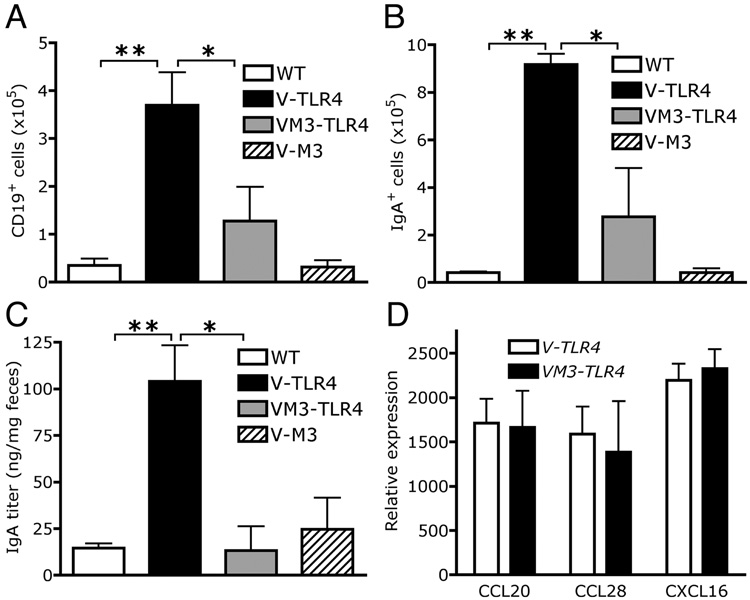
The results presented above suggested that the major factor underlying increased secretion of IgA into the intestinal lumen was the increased numbers of B cells, since the magnitude of the change in B cell number was so much greater than the increase in APRIL synthesis. To discriminate between these possibilities we devised a strategy to block multiple chemokines at once in the intestine, by generating mice expressing in IECs the MHV-68-encoded chemokine binding protein M3, a pan-chemokine inhibitor (Shang, L and Lira ,SA, manuscript in preparation). Double transgenic V-M3/TLR4 mice were produced by intercrossing Villin-M3 (V-M3) with V-TLR4 mice. FACS analysis of lamina propria cells from small intestine of V-M3 mice did not show major differences in the numbers of lymphocytes. Double transgenic V-M3/TLR4 mice showed significantly fewer B cells than V-TLR4 mice (Figure 7A). Moreover, VM3-TLR4 mice had significantly fewer IgA+ cells than the V-TLR4 mice (Figure 7B), and the same low level of fecal IgA as WT mice (Figure 7C). Importantly, M3 expression per se did not alter the mRNA expression of CCL20, CCL28, CXCL16 or APRIL (Figure 7D and data not shown). Together the results suggest that the main factor contributing to increased secretion of IgA into the lumen is the increased production of chemokines triggered by expression of constitutively active TLR4 in IECs.
Figure 7. Blockade of B cell recruitment and IgA production in the lamina propria of V-TLR4 mice by co-expression of chemokine binding protein M3.
A). Absolute number of CD19+ B cells in the lamina propria of WT, V-TLR4, V-M3 and double transgenic mice (n=4/group; ** p<0.01, * p<0.05). B). Absolute number of IgA+ cells in the lamina propria of the proximal small intestine of WT, V-TLR4, V-M3 and double transgenic mice (n=4/group; ** p<0.01, * p<0.05). C). IgA levels in feces of WT, V-TLR4, V-M3 and double transgenic mice (WT n=36, V-TLR4 n=51, V-M3 n=18, V-M3/V-TLR4 double TG n=11; ** p<0.01, * p<0.05). D). Q-PCR analysis of CCL20, CCL28 and CXCL16 expression in the proximal small intestine of WT, V-TLR4, V-M3 and double transgenic mice (n=4/group).
Discussion
Here we report that increased activation of the TLR signaling in IECs induces a remarkable and unsuspected phenotype: the accumulation of large numbers of B cells and IgA+ cells in the intestine. This response reveals an important role for the IEC compartment in response to environmental signals and suggests a novel mechanism for maintenance of intestinal homeostasis. By mediating an increase in luminal IgA in response to TLR activation, IECs may prevent attachment and possibly invasion of pathogenic organisms.
Expression of the CD4-TLR4 fusion gene in mouse IECs activated NF-κB signaling, and increased expression of several NF-κB-dependent genes including TNFα, iNOS and A20. This result is consistent with previous studies showing that the intracellular domain of the TLR4 molecule activates NF-κB in a ligand-independent fashion22, 26. TLRs signal through two major pathways, the MyD88-dependent activation of the NF-κB pathway and the TRIF-dependent IRF3 pathway6, 33. The NF-κB pathway is important for the upregulation of proinflammatory cytokines including TNFα and iNOS. The IRF3 pathway is important for activation of the IFN-β response as well as the upregulation of TNFα. It is important to stress that the fusion protein used in our study activates pathways used by other TLRs, and therefore it is likely that the phenotypes observed reflect activation of one or more TLRs in IECs.
Constitutive activation of TLR signaling in IECs significantly increased the expression of several chemokines, most notably CCL20, CCL28 and CXCL16. We hypothesize that this altered chemokine expression pattern contributed to the recruitment of B cells to the intestine and their subsequent differentiation into IgA producing cells. These changes were more apparent in the proximal small intestine, despite the fact that the transgene was expressed at equal levels in both proximal and distal segments (data not shown). These differences may be due to a higher responsiveness of the proximal small intestine to TLR signaling. LPS treatment leads to NF-kB activation selectively in duodenum and proximal jejunum, but not in the ileum or colon34.
The increased level of CCL20 driven by the constitutively active TLR4 chimera may contribute to the increased numbers of B cells in the lamina propria, because CCL20, through its interaction with CCR635, is chemotactic for naïve and memory B cells, immature dendritic cells, and effector memory T cells. We suggest that TLR activation promoted CCL20 expression via NF-KB activation, as has been shown in human IECs36.
CCL28, a chemokine modestly elevated in V-TLR4 mice, is expressed by epithelia in diverse mucosal tissues, and selectively attracts IgA-secreting B cells from both intestinal and non-intestinal lymphoid and effector tissues37. Studies with animals infected with rotavirus suggest that recruitment of IgA+ plasmablasts into the lamina propria is highly dependent on both CCL28 and CCL25 28. We suggest that the increased levels of CCL28 secreted by the IECs in the V-TLR4 mice acted synergistically with the homeostatically high levels of CCL25 to drive the increased accumulation of IgA-secreting B cells in the lamina propria29, 38, 39.
Finally, we observed a threefold upregulation of CXCL16 mRNA by proximal intestine of V-TLR4 mice. Membrane expression of CXCL16 by stromal cells has been implicated in the homing of plasma cells, and the expression of its receptor (CXCR6) has been documented in human bone marrow plasma cells, along with CXCR4, CCR10 and CCR340. Collectively the results suggest that V-TLR4 expression induced the synthesis of a specific set of chemokines with the ability to recruit B cells and plasma cells into the lamina propria.
To test the functional relevance of the elevated secretion of chemokines found in V-TLR4 mice, we generated transgenic mice expressing both the TLR4 chimera and M3, a chemokine binding protein that binds and inhibits many chemokines41. We have shown that expression of M3 in the pancreatic β-cells blocks recruitment of leukocytes promoted by the chemokines CCL2, CXCL13 and CCL21 and dramatically inhibits development of inflammatory and autoimmune diabetes23, 42–44. Here we show that expression of M3 in IECs almost totally inhibited the recruitment of B cells promoted by the expression of the TLR4 chimera. The possibility that the M3 transgene expression could have interfered with the chemokine expression pattern in double transgenic mice, was ruled out by our demonstration that the levels of expression of these molecules in the double transgenic mice was similar to those of V-TLR4 mice. Therefore, we suggest that the reduction of CD19+ B cells in the lamina propria is a direct consequence of blockade of the induced chemokines by M3. Coexpression of M3 also reduced the number of lamina propria IgA+ cells as well as IgA levels. In vitro, M3 binds murine CCL20 and CCL25 with high affinity, (Kd= 0.4 nM and 30 nM respectively) but the affinities for CXCL16 or CCL28 have not yet been defined (Antonio Alcami, personal communication). Studies to assess directly the contribution of individual chemokines and their receptors to the observations reported here are currently underway.
IgA is the most abundant immunoglobulin in the body and plays an important role in mucosal immunity. Secretory IgA is constantly released to mucosal surfaces where, in addition to controlling pathogenic microorganisms and dietary toxins, it controls the growth of commensal bacteria and regulates their interaction with IECs45, 46. IgA class switch can occur via T-cell dependent mechanisms involving CD40L and TGF-β within germinal centers of mucosal inductive sites 47–49. IgA class switch can also proceed via a T-cell independent mechanism. B cells present in the lamina propria express AID50 and respond to factors produced by DC and epithelial cells 4, 32, 51, 52. Among the well characterized factors involved in this process are APRIL, BAFF53–55 and iNOS56.
Although it is well-established that IECs play a major role in IgA transportation to the intestinal lumen by expression of pIgR3, their role in IgA class switching was only recognized recently4. Cerutti and colleagues demonstrated that APRIL is expressed by human IECs and that APRIL expression is induced by TLR ligands in IEC cell lines. They also demonstrated that APRIL production by IECs plays a major role in controlling B cell class switch to IgA4. Our results indicate that activation of TLR signaling in IECs in vivo is sufficient to induce class switching locally. Whether the increased expression of APRIL by IECs is directly mediated by TLR signaling or indirectly mediated by iNOS is unclear at this point. It is also unclear if the production of these and other molecules such as TNFα will modify the function of the resident lamina propria lymphocytes and dendritic cells and contribute to the changes reported here. Of note, we have observed a small but significant increase in the number of CD11c+ cells in the lamina propria of the V-TLR4 mice, compared to controls (data not shown), but it is unclear if this cell population is contributing to the changes seen here. Finally we should point out that besides affecting class switch, APRIL can also promote plasma cell survival and differentiation57 and thus contribute to the increase in the number of IgA+ cells in V-TLR4 mice.
Local generation of IgA helps in the maintenance of systemic immune tolerance to commensal bacteria58 and prevention of pathogenic infection59. Local generation of IgA differs from the conventional models involving immunization and does not depend on prior exposure to the pathogen. Our results suggest that TLR signaling in the small intestine triggers increased recruitment of B cells from the circulation into the LP and their conversion into plasma cells expressing IgA. This response to luminal bacteria may serve to minimize bacterial concentrations in the small intestine. Interestingly, we observed that in the steady state, TLR4-deficient mice have normal levels of stool IgA, but that mice in which TLR signaling has been compromised (i.e. Myd88-deficient mice) have much lower levels of IgA in fecal pellets than controls (supplemental Figure 4). At present, however, it is unclear if this defect is caused by the deficiency of Myd88 at the level of the epithelium or at the level of hematopoietic cells. Other mechanisms that could potentiate this anti-bacterial response include the increased secretion of chemokines by IECs. CCL20 and CCL28 have been shown to have direct antimicrobicidal activity60, 61, and their increased production could contribute to the elimination of bacterial pathogens.
In summary, activation of TLR signaling in IECs increases recruitment of B cells to the lamina propria and results in increased secretion of IgA into the intestinal lumen. We suggest that the combination of the effector mechanisms identified here (chemokines/recruitment; cytokines/class switch) ultimately result in increased production of IgA, which may be needed to neutralize of pathogenic bacteria and control commensal bacteria. Our results have implications for the use of probiotic agents, which may enhance local IgA production and thereby decrease intestinal inflammation.
Isolation of intestinal epithelial cells
Segments of the proximal (8 cm away from stomach) and distal (10 cm away from cecum) small intestine were obtained and Peyer’s patches were removed. Adipose tissue and mesentery were dissected out and the intestine was cut open along the mesenteric side. Tissues were washed three times in HBSS with 2% FBS and incubated in HBSS with 5% FBS and 1 mM DTT at 37°C for 20 min. Epithelial cells were then removed from the tissues by digestion with 1 mg/ml dispase I in RPMI 1640 supplied with 5 % FBS. After two washes in RPMI 1640, single cell suspensions were labeled with anti-CD45 antibodies linked to MACS beads (Miltenyi Biotec Inc., Auburn, CA) and passed through MACS column to eliminate leukocytes. The purity of the epithelial cells was checked by FACS analysis with anti-CD45 Ab. IECs preparations containing less than 3% CD45+ cells were used for RNA or protein analysis.
Isolation of lamina propria leukocytes
Lamina propria leukocytes were isolated as described previously 26. Briefly, the small intestine was removed, flushed with ice-cold calcium- and magnesium-free HBSS supplemented with 2% FBS and freed of fat, mesentery and Peyer's patches. The tissue was then cut into small pieces about 0.5 cm in length and incubated at 37°C with shaking for 20 min in HBSS containing 1 mM DTT, followed by PBS with 1 mM EDTA for 1 h at 37°C. Fragments of intestine were further incubated in 5 mL of 1 mg/mL collagenase D in RPMI for 1 h at 37°C and lamina propria leukocytes were isolated on a 44/66% Percoll density gradient. To sort B cells, single cell suspensions were incubated with an anti-B220 Ab conjugated with MACS beads (Miltenyi Biotec Inc., Auburn, CA) and passed them over a MACS column following the manufacturer’s instructions. Purity of the preparation was confirmed to be more than 90% by FACS analysis.
Supplementary Material
Acknowledgments
We thank Claudia Canasto-Chibuque for technical help, Sylvie Robine and Ruslan Medzhitov for reagents, and Julie Magarian Blander for comments to the manuscript. We thank Tom Moran for comments, suggestions and for technological support from CIVIA.
Supported by NIH grants P01 DK072201 (SAL and LM), AI052266 (MTA), DK069594 (MTA), Career Development Award from CCFA (MF), Uehara Memorial Foundation Research Fellowship (MF), New York Crohn’s Foundation and CCFA (LM) and support from the Center for Investigating Viral Immunity and Antagonism (CIVIA).
Nonstandard Abbreviations
- IECs
Intestinal epithelial cells.
- APRIL
a proliferation-inducing ligand.
- FACS
flow cytometry.
- BAFF
B cell-activation factor of the tumor necrosis factor family.
- TSLP
thymic stroma lymphopoietin.
- AID
activation-induced cytidine deaminase.
- α-CT
α-circular transcripts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement: There is no conflict of interest to disclose
References
- 1.Jung HC, Eckmann L, Yang SK, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff MF. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kagnoff MF, Eckmann L. Epithelial cells as sensors for microbial infection. J Clin Invest. 1997;100:6–10. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimada S, Kawaguchi-Miyashita M, Kushiro A, Sato T, Nanno M, Sako T, Matsuoka Y, Sudo K, Tagawa Y, Iwakura Y, Ohwaki M. Generation of polymeric immunoglobulin receptor-deficient mouse with marked reduction of secretory IgA. J Immunol. 1999;163:5367–5373. [PubMed] [Google Scholar]
- 4.He B, Xu W, Santini PA, Polydorides AD, Chiu A, Estrella J, Shan M, Chadburn A, Villanacci V, Plebani A, Knowles DM, Rescigno M, Cerutti A. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 6.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 7.Abreu MT, Arnold ET, Thomas LS, Gonsky R, Zhou Y, Hu B, Arditi M. TLR4 and MD-2 expression is regulated by immune-mediated signals in human intestinal epithelial cells. J Biol Chem. 2002;277:20431–20437. doi: 10.1074/jbc.M110333200. [DOI] [PubMed] [Google Scholar]
- 8.Bogunovic M, Dave SH, Tilstra JS, Chang DT, Harpaz N, Xiong H, Mayer LF, Plevy SE. Enteroendocrine cells express functional Toll-like receptors. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1770–G1783. doi: 10.1152/ajpgi.00249.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010–7017. doi: 10.1128/iai.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lotz M, Gutle D, Walther S, Menard S, Bogdan C, Hornef MW. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J Exp Med. 2006;203:973–984. doi: 10.1084/jem.20050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortega-Cava CF, Ishihara S, Rumi MA, Kawashima K, Ishimura N, Kazumori H, Udagawa J, Kadowaki Y, Kinoshita Y. Strategic compartmentalization of Toll-like receptor 4 in the mouse gut. J Immunol. 2003;170:3977–3985. doi: 10.4049/jimmunol.170.8.3977. [DOI] [PubMed] [Google Scholar]
- 12.Frolova L, Drastich P, Rossmann P, Klimesova K, Tlaskalova-Hogenova H. Expression of Toll-like Receptor 2 (TLR2), TLR4, and CD14 in Biopsy Samples of Patients With Inflammatory Bowel Diseases: Upregulated Expression of TLR2 in Terminal Ileum of Patients With Ulcerative Colitis. J Histochem Cytochem. 2007 doi: 10.1369/jhc.7A7303.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hornef MW, Frisan T, Vandewalle A, Normark S, Richter-Dahlfors A. Toll-like receptor 4 resides in the Golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J Exp Med. 2002;195:559–570. doi: 10.1084/jem.20011788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hornef MW, Normark BH, Vandewalle A, Normark S. Intracellular recognition of lipopolysaccharide by toll-like receptor 4 in intestinal epithelial cells. J Exp Med. 2003;198:1225–1235. doi: 10.1084/jem.20022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furuta T, Kikuchi T, Akira S, Watanabe N, Yoshikawa Y. Roles of the small intestine for induction of toll-like receptor 4-mediated innate resistance in naturally acquired murine toxoplasmosis. Int Immunol. 2006;18:1655–1662. doi: 10.1093/intimm/dxl099. [DOI] [PubMed] [Google Scholar]
- 17.Khan MA, Ma C, Knodler LA, Valdez Y, Rosenberger CM, Deng W, Finlay BB, Vallance BA. Toll-like receptor 4 contributes to colitis development but not to host defense during Citrobacter rodentium infection in mice. Infect Immun. 2006;74:2522–2536. doi: 10.1128/IAI.74.5.2522-2536.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukata M, Michelsen KS, Eri R, Thomas LS, Hu B, Lukasek K, Nast CC, Lechago J, Xu R, Naiki Y, Soliman A, Arditi M, Abreu MT. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1055–G1065. doi: 10.1152/ajpgi.00328.2004. [DOI] [PubMed] [Google Scholar]
- 19.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Vora P, Youdim A, Thomas LS, Fukata M, Tesfay SY, Lukasek K, Michelsen KS, Wada A, Hirayama T, Arditi M, Abreu MT. Beta-defensin-2 expression is regulated by TLR signaling in intestinal epithelial cells. J Immunol. 2004;173:5398–5405. doi: 10.4049/jimmunol.173.9.5398. [DOI] [PubMed] [Google Scholar]
- 21.Pinto D, Robine S, Jaisser F, El Marjou FE, Louvard D. Regulatory sequences of the mouse villin gene that efficiently drive transgenic expression in immature and differentiated epithelial cells of small and large intestines. J Biol Chem. 1999;274:6476–6482. doi: 10.1074/jbc.274.10.6476. [DOI] [PubMed] [Google Scholar]
- 22.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 23.Martin AP, Canasto-Chibuque C, Shang L, Rollins BJ, Lira SA. The chemokine decoy receptor M3 blocks CC chemokine ligand 2 and CXC chemokine ligand 13 function in vivo. J Immunol. 2006;177:7296–7302. doi: 10.4049/jimmunol.177.10.7296. [DOI] [PubMed] [Google Scholar]
- 24.Bergqvist P, Gardby E, Stensson A, Bemark M, Lycke NY. Gut IgA class switch recombination in the absence of CD40 does not occur in the lamina propria and is independent of germinal centers. J Immunol. 2006;177:7772–7783. doi: 10.4049/jimmunol.177.11.7772. [DOI] [PubMed] [Google Scholar]
- 25.Kato H, Fujihashi K, Kato R, Yuki Y, McGhee JR. Oral tolerance revisited: prior oral tolerization abrogates cholera toxin-induced mucosal IgA responses. J Immunol. 2001;166:3114–3121. doi: 10.4049/jimmunol.166.5.3114. [DOI] [PubMed] [Google Scholar]
- 26.Qureshi ST, Zhang X, Aberg E, Bousette N, Giaid A, Shan P, Medzhitov RM, Lee PJ. Inducible activation of TLR4 confers resistance to hyperoxia-induced pulmonary apoptosis. J Immunol. 2006;176:4950–4958. doi: 10.4049/jimmunol.176.8.4950. [DOI] [PubMed] [Google Scholar]
- 27.Forster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- 28.Feng N, Jaimes MC, Lazarus NH, Monak D, Zhang C, Butcher EC, Greenberg HB. Redundant role of chemokines CCL25/TECK and CCL28/MEC in IgA+ plasmablast recruitment to the intestinal lamina propria after rotavirus infection. J Immunol. 2006;176:5749–5759. doi: 10.4049/jimmunol.176.10.5749. [DOI] [PubMed] [Google Scholar]
- 29.Kunkel EJ, Campbell JJ, Haraldsen G, Pan J, Boisvert J, Roberts AI, Ebert EC, Vierra MA, Goodman SB, Genovese MC, Wardlaw AJ, Greenberg HB, Parker CM, Butcher EC, Andrew DP, Agace WW. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: Epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med. 2000;192:761–768. doi: 10.1084/jem.192.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan J, Kunkel EJ, Gosslar U, Lazarus N, Langdon P, Broadwell K, Vierra MA, Genovese MC, Butcher EC, Soler D. A novel chemokine ligand for CCR10 and CCR3 expressed by epithelial cells in mucosal tissues. J Immunol. 2000;165:2943–2949. doi: 10.4049/jimmunol.165.6.2943. [DOI] [PubMed] [Google Scholar]
- 31.Fagarasan S, Honjo T. Intestinal IgA synthesis: regulation of front-line body defences. Nat Rev Immunol. 2003;3:63–72. doi: 10.1038/nri982. [DOI] [PubMed] [Google Scholar]
- 32.Fagarasan S, Kinoshita K, Muramatsu M, Ikuta K, Honjo T. In situ class switching and differentiation to IgA-producing cells in the gut lamina propria. Nature. 2001;413:639–643. doi: 10.1038/35098100. [DOI] [PubMed] [Google Scholar]
- 33.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 34.Magness ST, Jijon H, Van Houten Fisher N, Sharpless NE, Brenner DA, Jobin C. In vivo pattern of lipopolysaccharide and anti-CD3-induced NF-kappa B activation using a novel gene-targeted enhanced GFP reporter gene mouse. J Immunol. 2004;173:1561–1570. doi: 10.4049/jimmunol.173.3.1561. [DOI] [PubMed] [Google Scholar]
- 35.Krzysiek R, Lefevre EA, Bernard J, Foussat A, Galanaud P, Louache F, Richard Y. Regulation of CCR6 chemokine receptor expression and responsiveness to macrophage inflammatory protein-3alpha/CCL20 in human B cells. Blood. 2000;96:2338–2345. [PubMed] [Google Scholar]
- 36.Izadpanah A, Dwinell MB, Eckmann L, Varki NM, Kagnoff MF. Regulated MIP-3alpha/CCL20 production by human intestinal epithelium: mechanism for modulating mucosal immunity. Am J Physiol Gastrointest Liver Physiol. 2001;280:G710–G719. doi: 10.1152/ajpgi.2001.280.4.G710. [DOI] [PubMed] [Google Scholar]
- 37.Lazarus NH, Kunkel EJ, Johnston B, Wilson E, Youngman KR, Butcher EC. A common mucosal chemokine (mucosae-associated epithelial chemokine/CCL28) selectively attracts IgA plasmablasts. J Immunol. 2003;170:3799–3805. doi: 10.4049/jimmunol.170.7.3799. [DOI] [PubMed] [Google Scholar]
- 38.Kunkel EJ, Butcher EC. Chemokines and the tissue-specific migration of lymphocytes. Immunity. 2002;16:1–4. doi: 10.1016/s1074-7613(01)00261-8. [DOI] [PubMed] [Google Scholar]
- 39.Pabst O, Ohl L, Wendland M, Wurbel MA, Kremmer E, Malissen B, Forster R. Chemokine receptor CCR9 contributes to the localization of plasma cells to the small intestine. J Exp Med. 2004;199:411–416. doi: 10.1084/jem.20030996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakayama T, Hieshima K, Izawa D, Tatsumi Y, Kanamaru A, Yoshie O. Cutting edge: profile of chemokine receptor expression on human plasma cells accounts for their efficient recruitment to target tissues. J Immunol. 2003;170:1136–1140. doi: 10.4049/jimmunol.170.3.1136. [DOI] [PubMed] [Google Scholar]
- 41.Alcami A. Structural basis of the herpesvirus M3-chemokine interaction. Trends Microbiol. 2003;11:191–192. doi: 10.1016/s0966-842x(03)00077-5. [DOI] [PubMed] [Google Scholar]
- 42.Jensen KK, Chen SC, Hipkin RW, Wiekowski MT, Schwarz MA, Chou CC, Simas JP, Alcami A, Lira SA. Disruption of CCL21-induced chemotaxis in vitro and in vivo by M3, a chemokine-binding protein encoded by murine gammaherpesvirus 68. J Virol. 2003;77:624–630. doi: 10.1128/JVI.77.1.624-630.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin AP, Alexander-Brett JM, Canasto-Chibuque C, Garin A, Bromberg JS, Fremont DH, Lira SA. The chemokine binding protein M3 prevents diabetes induced by multiple low doses of streptozotocin. J Immunol. 2007;178:4623–4631. doi: 10.4049/jimmunol.178.7.4623. [DOI] [PubMed] [Google Scholar]
- 44.Martin AP, Grisotto MG, Canasto-Chibuque C, Kunkel SL, Bromberg JS, Furtado GC, Lira SA. Islet expression of M3 uncovers a key role for chemokines in the development and recruitment of diabetogenic cells in NOD mice. Diabetes. 2007 doi: 10.2337/db07-1309. [DOI] [PubMed] [Google Scholar]
- 45.Brandtzaeg P, Baekkevold ES, Morton HC. From B to A the mucosal way. Nat Immunol. 2001;2:1093–1094. doi: 10.1038/ni1201-1093. [DOI] [PubMed] [Google Scholar]
- 46.Czerkinsky C, Anjuere F, McGhee JR, George-Chandy A, Holmgren J, Kieny MP, Fujiyashi K, Mestecky JF, Pierrefite-Carle V, Rask C, Sun JB. Mucosal immunity and tolerance: relevance to vaccine development. Immunol Rev. 1999;170:197–222. doi: 10.1111/j.1600-065X.1999.tb01339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Castigli E, Alt FW, Davidson L, Bottaro A, Mizoguchi E, Bhan AK, Geha RS. CD40-deficient mice generated by recombination-activating gene-2-deficient blastocyst complementation. Proc Natl Acad Sci U S A. 1994;91:12135–12139. doi: 10.1073/pnas.91.25.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Coffman RL, Lebman DA, Shrader B. Transforming growth factor beta specifically enhances IgA production by lipopolysaccharide-stimulated murine B lymphocytes. J Exp Med. 1989;170:1039–1044. doi: 10.1084/jem.170.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fayette J, Dubois B, Vandenabeele S, Bridon JM, Vanbervliet B, Durand I, Banchereau J, Caux C, Briere F. Human dendritic cells skew isotype switching of CD40-activated naive B cells towards IgA1 and IgA2. J Exp Med. 1997;185:1909–1918. doi: 10.1084/jem.185.11.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crouch EE, Li Z, Takizawa M, Fichtner-Feigl S, Gourzi P, Montano C, Feigenbaum L, Wilson P, Janz S, Papavasiliou FN, Casellas R. Regulation of AID expression in the immune response. J Exp Med. 2007;204:1145–1156. doi: 10.1084/jem.20061952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, von Andrian UH. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 52.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 53.Castigli E, Scott S, Dedeoglu F, Bryce P, Jabara H, Bhan AK, Mizoguchi E, Geha RS. Impaired IgA class switching in APRIL-deficient mice. Proc Natl Acad Sci U S A. 2004;101:3903–3908. doi: 10.1073/pnas.0307348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castigli E, Wilson SA, Scott S, Dedeoglu F, Xu S, Lam KP, Bram RJ, Jabara H, Geha RS. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med. 2005;201:35–39. doi: 10.1084/jem.20032000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tezuka H, Abe Y, Iwata M, Takeuchi H, Ishikawa H, Matsushita M, Shiohara T, Akira S, Ohteki T. Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature. 2007;448:929–933. doi: 10.1038/nature06033. [DOI] [PubMed] [Google Scholar]
- 57.MacLennan I, Vinuesa C. Dendritic cells, BAFF, and APRIL: innate players in adaptive antibody responses. Immunity. 2002;17:235–238. doi: 10.1016/s1074-7613(02)00398-9. [DOI] [PubMed] [Google Scholar]
- 58.Konrad A, Cong Y, Duck W, Borlaza R, Elson CO. Tight mucosal compartmentation of the murine immune response to antigens of the enteric microbiota. Gastroenterology. 2006;130:2050–2059. doi: 10.1053/j.gastro.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 59.Wijburg OL, Uren TK, Simpfendorfer K, Johansen FE, Brandtzaeg P, Strugnell RA. Innate secretory antibodies protect against natural Salmonella typhimurium infection. J Exp Med. 2006;203:21–26. doi: 10.1084/jem.20052093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hieshima K, Ohtani H, Shibano M, Izawa D, Nakayama T, Kawasaki Y, Shiba F, Shiota M, Katou F, Saito T, Yoshie O. CCL28 has dual roles in mucosal immunity as a chemokine with broad-spectrum antimicrobial activity. J Immunol. 2003;170:1452–1461. doi: 10.4049/jimmunol.170.3.1452. [DOI] [PubMed] [Google Scholar]
- 61.Yang D, Chen Q, Hoover DM, Staley P, Tucker KD, Lubkowski J, Oppenheim JJ. Many chemokines including CCL20/MIP-3alpha display antimicrobial activity. J Leukoc Biol. 2003;74:448–455. doi: 10.1189/jlb.0103024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.